Octacalcium Phosphate Bone Substitute (Bontree®): From Basic Research to Clinical Case Study
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Basic Research
2.1.1. Material Characterization
2.1.2. In Vitro Study
2.1.3. In Vivo Study
2.1.4. Statistical Analysis
2.2. Clinical Case Study
Patient Selection
3. Results
3.1. Basic Research
3.1.1. Material Characterization
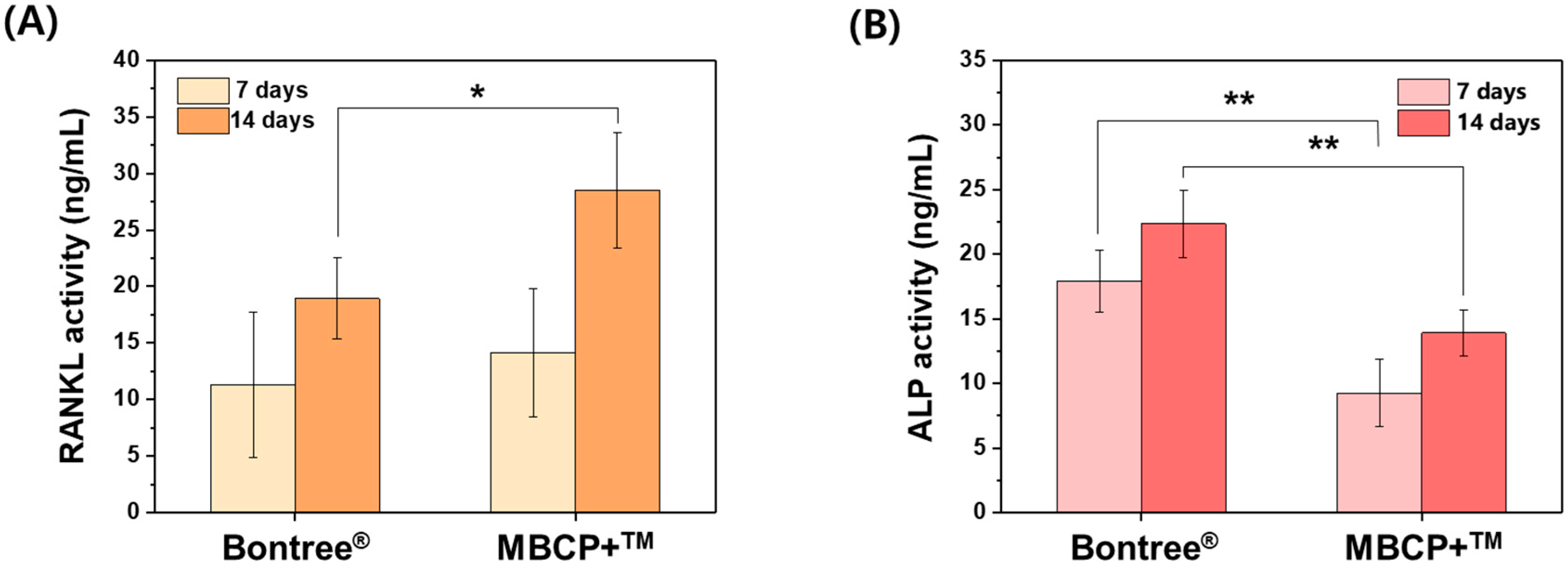
3.1.2. In Vitro Study
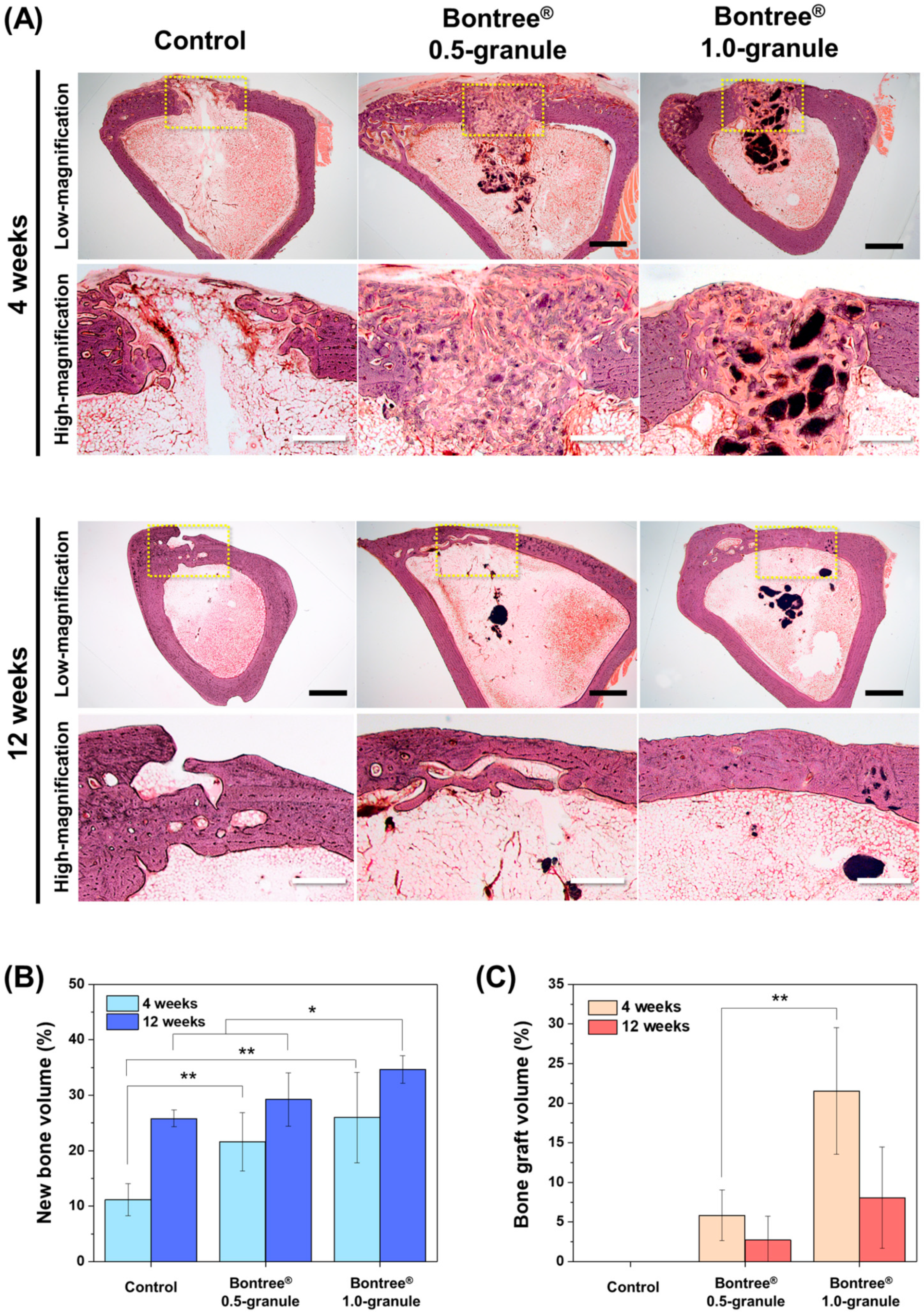
3.1.3. In Vivo Study
3.2. Clinical Case Study
3.2.1. Description of Three Cases
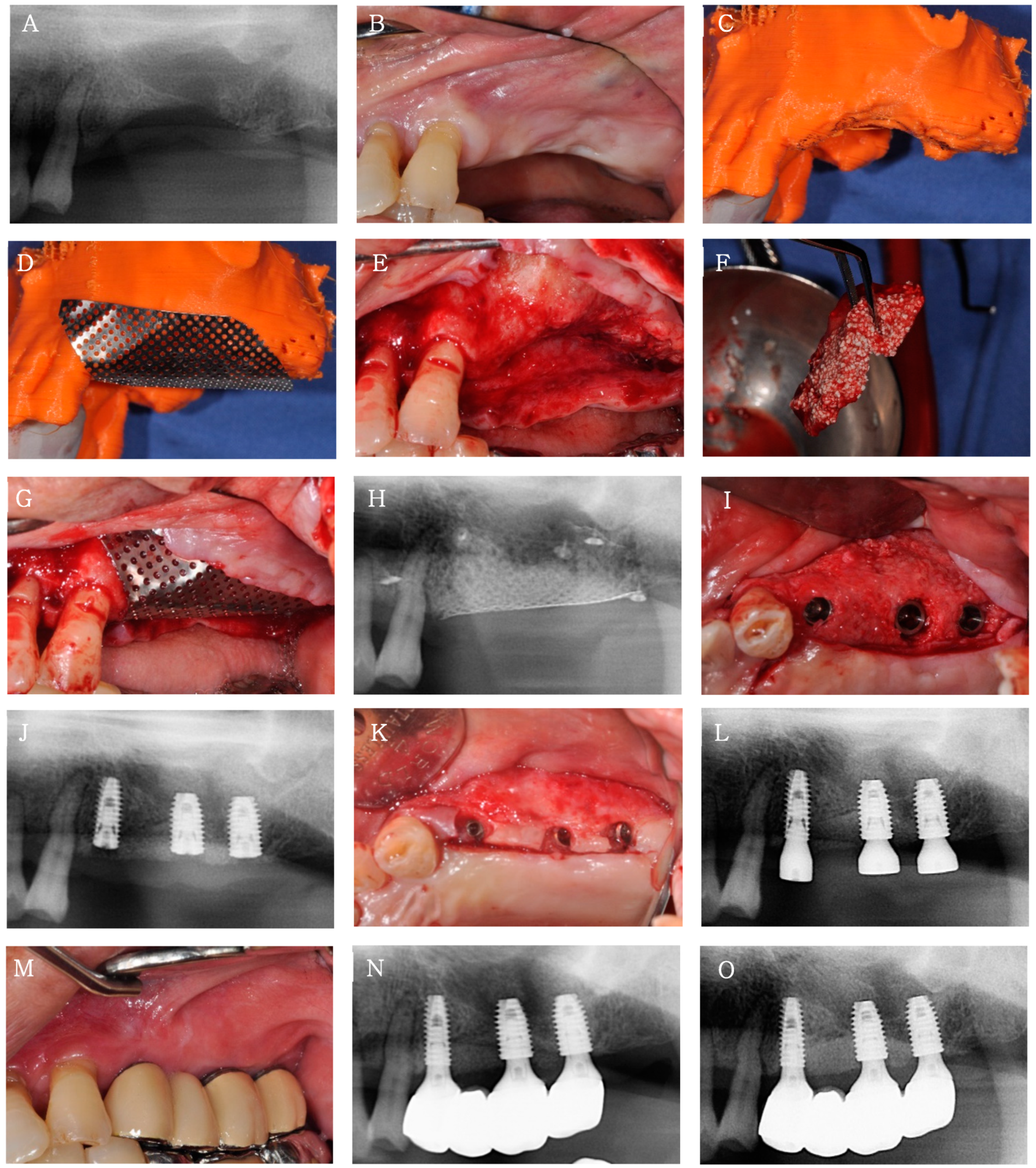
Case 1
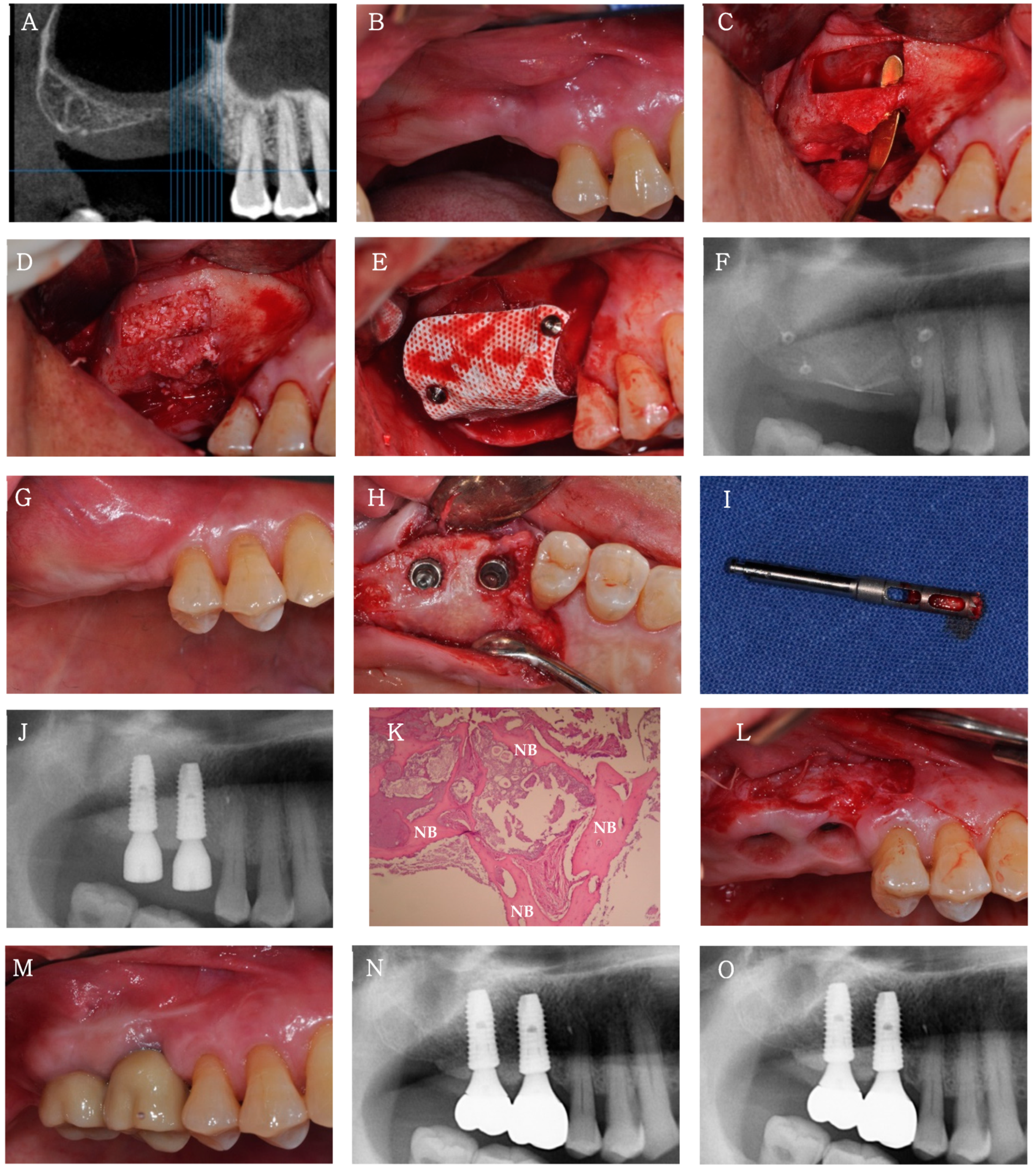
Case 2
Case 3
3.2.2. Clinical and Radiological Findings
3.2.3. Histological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent statement
Data Availability Statement
Conflicts of Interest
References
- Couso-Queiruga, E.; Stuhr, S.; Tattan, M.; Chambrone, L.; Avila-Ortiz, G. Post-extraction dimensional changes: A systematic review and meta-analysis. J. Clin. Periodontol. 2021, 48, 127–145. [Google Scholar] [CrossRef]
- Jambhekar, S.; Kernen, F.; Bidra, A.S. Clinical and histologic outcomes of socket grafting after flapless tooth extraction: A systematic review of randomized controlled clinical trials. J. Prosthet. Dent. 2015, 113, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Breine, U.; Brånemark, P.-I. Reconstruction of alveolar jaw bone. Scand. J. Plast. Reconstr. Surg. 1980, 14, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, V.; Vamsi, A.R.; Shenoy, S.; Ashok, K.; Thomas, B. Guided Bone Regeneration-A Comprehensive Review. J. Clin. Diagn. Res. 2021, 15, 1–4. [Google Scholar]
- Springfield, D. Autograft reconstructions. Orthop. Clin. N. Am. 1996, 27, 483–492. [Google Scholar] [CrossRef]
- Betz, R.R. Limitations of autograft and allograft: New synthetic solutions. Orthopedics 2002, 25, S561–S570. [Google Scholar] [CrossRef]
- Dos Santos Canellas, J.V.; Drugos, L.; Ritto, F.G.; Fischer, R.G.; Medeiros, P.J.D.A. Xenograft materials in maxillary sinus floor elevation surgery: A systematic review with network meta-analyses. Br. J. Oral Maxillofac. Surg. 2021, 59, 742–751. [Google Scholar] [CrossRef]
- Lu, J.; Descamps, M.; Dejou, J.; Koubi, G.; Hardouin, P.; Lemaitre, J.; Proust, J.P. The biodegradation mechanism of calcium phosphate biomaterials in bone. J. Biomed. Mater. Res. 2002, 63, 408–412. [Google Scholar] [CrossRef]
- Kawai, T.; Tanuma, Y.; Matsui, K.; Suzuki, O.; Takahashi, T.; Kamakura, S. Clinical safety and efficacy of implantation of octacalcium phosphate collagen composites in tooth extraction sockets and cyst holes. J. Tissue Eng. 2016, 7, 2041731416670770. [Google Scholar] [CrossRef]
- Brown, W.E.; Smith, J.P.; Lehr, J.R.; Frazier, A.W. Octacalcium phosphate and hydroxyapatite: Crystallographic and chemical relations between octacalcium phosphate and hydroxyapatite. Nature 1962, 196, 1050–1055. [Google Scholar] [CrossRef]
- Kamakura, S.; Sasano, Y.; Shimizu, T.; Hatori, K.; Suzuki, O.; Kagayama, M.; Motegi, K. Implanted octacalcium phosphate is more resorbable than β-tricalcium phosphate and hydroxyapatite. J. Biomed. Mater. Res. 2002, 59, 29–34. [Google Scholar] [CrossRef]
- Kamakura, S.; Sasaki, K.; Homma, T.; Honda, Y.; Anada, T.; Echigo, S.; Suzuki, O. The primacy of octacalcium phosphate collagen composites in bone regeneration. J. Biomed. Mater. Res. Part A 2007, 83, 725–733. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, W.-H.; Lee, S.-H.; Ju, K.W.; Jang, E.-H.; Kim, S.-O.; Kim, B.; Lee, J.-H. Evaluation of New Octacalcium Phosphate-Coated Xenograft in Rats Calvarial Defect Model on Bone Regeneration. Materials 2020, 13, 4391. [Google Scholar] [CrossRef]
- Kawai, T.; Matsui, K.; Ezoe, Y.; Kajii, F.; Suzuki, O.; Takahashi, T.; Kamakura, S. Efficacy of octacalcium phosphate collagen composite for titanium dental implants in dogs. Materials 2018, 11, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robin, M.; Von Euw, S.; Renaudin, G.; Gomes, S.; Krafft, J.-M.; Nassif, N.; Azaïs, T.; Costentin, G. Insights into OCP identification and quantification in the context of apatite biomineralization. CrystEngComm 2020, 22, 2728–2742. [Google Scholar] [CrossRef]
- Marycz, K.; Smieszek, A.; Trynda, J.; Sobierajska, P.; Targonska, S.; Grosman, L.; Wiglusz, R.J. Nanocrystalline hydroxyapatite loaded with resveratrol in colloidal suspension improves viability, metabolic activity and mitochondrial potential in human adipose-derived mesenchymal stromal stem cells (hASCs). Polymers 2019, 11, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toker, H.; Ozdemir, H.; Ozer, H.; Eren, K. A comparative evaluation of the systemic and local alendronate treatment in synthetic bone graft: A histologic and histomorphometric study in a rat calvarial defect model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, S146–S152. [Google Scholar] [CrossRef]
- Ling, L.; Murali, S.; Stein, G.S.; Van Wijnen, A.J.; Cool, S.M. Glycosaminoglycans modulate RANKL-induced osteoclastogenesis. J. Cell. Biochem. 2010, 109, 1222–1231. [Google Scholar] [CrossRef] [Green Version]
- Yeo, M.; Jung, W.-K.; Kim, G. Fabrication, characterisation and biological activity of phlorotannin-conjugated PCL/β-TCP composite scaffolds for bone tissue regeneration. J. Mater. Chem. 2012, 22, 3568–3577. [Google Scholar] [CrossRef]
- Xie, L.; Liu, N.; Xiao, Y.; Liu, Y.; Yan, C.; Wang, G.; Jing, X. In vitro and in vivo osteogenesis induced by icariin and bone morphogenetic protein-2: A dynamic observation. Front. Pharmacol. 2020, 11, 1058. [Google Scholar] [CrossRef]
- Park, D.Y.; Lee, W.P. Vestibuloplasty around teeth and dental implants using simplified periosteal fenestration (sPF): Case Reports. J. Korean Dent. Assoc. 2020, 59, 20–27. [Google Scholar]
- Lee, W.P.; Kwon, Y.S. Vestibuloplasty around Dental Implants Using Modified Periosteal Fenestration (mPF): Case Series. Implantology 2020, 24, 22–30. [Google Scholar] [CrossRef]
- Baek, J.-H.; Kim, B.-O.; Lee, W.-P. Implant Placement after Closure of Oroantral Communication by Sinus Bone Graft Using a Collagen Barrier Membrane in the Shape of a Pouch: A Case Report and Review of the Literature. Medicina 2021, 57, 626. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E.; Perel, M.L.; Wang, H.-L.; Sammartino, G.; Galindo-Moreno, P.; Trisi, P.; Steigmann, M.; Rebaudi, A.; Palti, A.; Pikos, M.A. Implant success, survival, and failure: The International Congress of Oral Implantologists (ICOI) pisa consensus conference. Implant Dent. 2008, 17, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Kishikawa, A.; Ogawa, S.; Shen, W.-R.; Qi, J.; Noguchi, T.; Nara, Y.; Mizoguchi, I. TNF-α directly enhances osteocyte RANKL expression and promotes osteoclast formation. Front. Immunol. 2019, 10, 2925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.-K.; Lee, H.; Kim, H.-E.; Lee, E.-J.; Jang, T.-S.; Jung, H.-D. Nano-Topographical Control of Ti-Nb-Zr Alloy Surfaces for Enhanced Osteoblastic Response. Nanomaterials 2021, 11, 1507. [Google Scholar] [CrossRef]
- Chou, Y.-C.; Lee, D.; Chang, T.-M.; Hsu, Y.-H.; Yu, Y.-H.; Liu, S.-J.; Ueng, S.W.-N. Development of a three-dimensional (3D) printed biodegradable cage to convert morselized corticocancellous bone chips into a structured cortical bone graft. Int. J. Mol. Sci. 2016, 17, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otero-Pérez, R.; Permuy, M.; López-Senra, E.; López-Álvarez, M.; López-Peña, M.; Serra, J.; González-Cantalapiedra, A.; Muñoz, F.M.; González, P. Preclinical Evaluation of an Innovative Bone Graft of Marine Origin for the Treatment of Critical-Sized Bone Defects in an Animal Model. Appl. Sci. 2021, 11, 2116. [Google Scholar] [CrossRef]
- Choi, S.W.; Bae, J.Y.; Shin, Y.H.; Song, J.H.; Kim, J.K. Treatment of forearm diaphyseal non-union: Autologous iliac corticocancellous bone graft and locking plate fixation. Orthop. Traumatol. Surg. Res. 2021, 102833. [Google Scholar] [CrossRef]
- Kikawa, T.; Kashimoto, O.; Imaizumi, H.; Kokubun, S.; Suzuki, O. Intramembranous bone tissue response to biodegradable octacalcium phosphate implant. Acta Biomater. 2009, 5, 1756–1766. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, K.; Jiang, X.; Xie, J.; Cai, P.; Li, F.; Liang, R.; Zhao, J.; Zheng, L. Electrospun poly (3-hydroxybutyrate-co-4-hydroxybutyrate)/octacalcium phosphate nanofibrous membranes for effective guided bone regeneration. Mater. Sci. Eng. C 2020, 112, 110763. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Liang, J.; Song, R.; Zhang, Y.; Ren, L.; Zhang, L.; Tang, P.; Lin, C. Effect of octacalcium-phosphate-modified micro/nanostructured titania surfaces on osteoblast response. ACS Appl. Mater. Interfaces 2015, 7, 14384–14396. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Jian, C.; Jianming, R.; Zhongcheng, Z.; Jianpeng, Z. Synthesis and degradation properties of beta-TCP/BG porous composite materials. Bull. Mater. Sci. 2011, 34, 357–364. [Google Scholar] [CrossRef]
- Sakai, S.; Anada, T.; Tsuchiya, K.; Yamazaki, H.; Margolis, H.C.; Suzuki, O. Comparative study on the resorbability and dissolution behavior of octacalcium phosphate, β-tricalcium phosphate, and hydroxyapatite under physiological conditions. Dent. Mater. J. 2016, 35, 216–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Case | Age | Sex | PMH | Procedure | Position of Implant Placement | ISQ (B/L) | Implant Prognosis |
|---|---|---|---|---|---|---|---|
| 1 | 77 | Male | HTN | Bone augmentation of peri-implant defects | #44i | 78/84 | Success |
| #45i | 80/79 | Success | |||||
| #46i | 85/85 | Success | |||||
| 2 | 69 | Male | HTNCVD | Vertical ridge augmentation | #24i | 75/76 | Success |
| #26i | 71/71 | Success | |||||
| #27i | 75/73 | Success | |||||
| 3 | 63 | Male | HTNDM | Sinus and ridge augmentation | #16i | 62/75 | Success |
| #17i | 61/71 | Success |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-S.; Jang, T.-S.; Kim, S.-Y.; Lee, W.-P. Octacalcium Phosphate Bone Substitute (Bontree®): From Basic Research to Clinical Case Study. Appl. Sci. 2021, 11, 7921. https://doi.org/10.3390/app11177921
Kim J-S, Jang T-S, Kim S-Y, Lee W-P. Octacalcium Phosphate Bone Substitute (Bontree®): From Basic Research to Clinical Case Study. Applied Sciences. 2021; 11(17):7921. https://doi.org/10.3390/app11177921
Chicago/Turabian StyleKim, Joo-Seong, Tae-Sik Jang, Suk-Young Kim, and Won-Pyo Lee. 2021. "Octacalcium Phosphate Bone Substitute (Bontree®): From Basic Research to Clinical Case Study" Applied Sciences 11, no. 17: 7921. https://doi.org/10.3390/app11177921
APA StyleKim, J.-S., Jang, T.-S., Kim, S.-Y., & Lee, W.-P. (2021). Octacalcium Phosphate Bone Substitute (Bontree®): From Basic Research to Clinical Case Study. Applied Sciences, 11(17), 7921. https://doi.org/10.3390/app11177921








