Abstract
Much of the complex medical physics work requires radiation dose delivery, which requires dosimeters to accurately measure complex three-dimensional dose distribution with good spatial resolution. MAGIC-f polymer gel is one of the emerging new dosimeters widely used in medical physics research. The purpose of this study was to present an overview of polymer gel dosimetry, using MAGIC-f gel, including its composition, manufacture, imaging, calibration, and application to medical physics research. In this review, the history of polymer gel development is presented, along with the applications so far. Moreover, the most important experiments/applications of MAGIC-f polymer gel are discussed to illustrate the behavior of gel on different conditions of irradiation, imaging, and manufacturing techniques. Finally, various future works are suggested based on the past and present works on MAGIC-f gel and polymer gel in general, with the hope that these bits of knowledge can provide important clues for future research on MAGIC-f gel as a dosimeter.
1. Introduction
Gel dosimeters are composed of radiation-sensitive chemicals that experience a fundamental change in their properties following ionizing radiation, due to the absorbed dose [1,2]. These features of the polymeric gel dosimeters can store dose distribution information in three dimensions, an additional benefit compared to other dosimeters that provide only one-dimensional dose distribution, such as ionization chambers and films in a point, or two-dimensional dose distribution [2,3]. This benefit is especially important for emerging technologies relating to radiation, where there is a substantial incidence of high-dose gradients [4]. There are various kinds of gel dosimeters that have been discovered so far, but two types, with the names Fricke and polymer gel, have been extensively used. These polymer gels are usually analyzed by using various diagnostic imaging techniques, such as sonography, optical computer tomography (OCT), X-ray computer tomography (X-ray CT), and magnetic resonance imaging (MRI) [5].
A radiation dosimetry process with MAGIC-f (Methacrylic Ascorbic Acid in Gelatin Caused by Copper-Added Formaldehyde) gel consists of steps involving gel preparation, irradiation with ionizing radiation, taking images of irradiated gels using some imaging techniques and finally analyzing the images to calculate the dose distribution. A typical polymer gel dosimetry process involves three stages known as fabrication, irradiation, and evaluation stages, as shown in Figure 1. Therefore, development process of MAGIC-f gel dosimetry consists of development of all these three processes as well. Thus, in this review, besides the discussion of development trends of MAGIC-f gel from the development of a precursor form of polymer gel such as dye made up of methylene, development of each step of MAGIC-f dosimetry is also discussed. Hence, this review briefly examines the current knowledge of dosimetry using MAGIC-f gel to emphasize its structure, manufacture, imaging, calibration, and application to medical physics research.
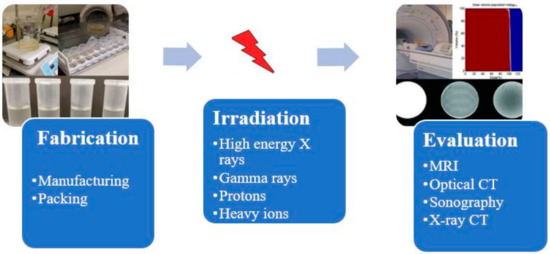
Figure 1.
The process of obtaining dose distribution, using MAGIC-f. The figures were adapted from difference cited sources [6,7].
2. Development of MAGIC-f Gel
The development trends of MAGIC-f gel can be described from the development of a precursor form of polymer gel, such as dye made up of methylene, to the latest important innovation in the polymer gel dosimetry using MAGIC-f gel. Before the polymer gel dosimeter’s innovation, dosimetry calculations were made only through ion chambers and film, which only gives point dose and two-dimensional dose distribution, respectively [3]. The use of a sensitive gel to irradiation was first proposed by Day and Stein in 1950, where the gel containing dye such as methylene blue gives color changes when subjected to radiation [8]. However, the use of polymer system/gel for radiation dosimetry was first initiated by Alexander et al. in 1954 [9]; Hoecker et al., in 1958, worked on radiation dosimetry using liquid polymer [10]; and Boni. Et al. used polyacrylamide in 1961 [11]. Audet et al. found improvements in measurements of irradiated polyethylene oxide transverse relaxation by NMR [12]. The credit of discovery of the currently used radiation-sensitive polymer gel for radiation dosimetry goes to Gore et al. [13], who investigated the relaxation properties after irradiation of previously developed ferrous sulfate chemical dosimeter by Fricke et al. [14,15], by nuclear NMR. Gore et al. showed that radiation-induced shifts could be quantified by using NMR relaxation steps where irradiation causes iron ions to gain a higher oxidation state, i.e., from ferrous (Fe2+) into ferric (Fe3+) ions [13]. Later, Kennan et al. observed increased relaxation rates with the absorbed dose from the longitudinal relaxation studies of NMR conducted on irradiated N,N′-methylene-bis-acrylamide form of aqueous solution [16].
The polymer gel proposed by Gore et al. is a precursor version of the currently utilized polymer gel dosimeter for radiation dosimetry, which requires significant improvements due to many difficulties encountered during use. The polymer-gel dosimeter is based on radiation-induced polymerization and crosslinking of acrylic monomers [17]. In contrast, Fricke gel is not a polymer-based dosimeter; it is based on radiation-induced oxidation of ferrous ions which modifies NMR relaxation rates. In 1986, Appleby et al.’s research showed that a gel matrix with Fricke dosimetry solutions could be used to obtain spatial three-dimensional dose distribution using magnetic resonance imaging [18]. Further, opposing the claim of previous researchers, failure of irradiated gel dosimeters of Fricke-type to maintain stable distribution of dose in three-dimensional space was shown for the purpose of ion diffusion in the dosimeters that are irradiated [19,20], and several authors’ publications also support this claim. As diffusion was the big problem in the innovation in polymer gel dosimetry, attempts made by Baldock et al. to investigate the Fricke solution with different gelling agents, such as gelatin, agarose, Sephadex, polyvinyl alcohol (PVA), and xylenol orange (XO), where xylenol orange gives the best result, but performance is minimal, and the diffusion cannot be omitted and remain as a problem [21]. Prior to Baldock et al., Tomas et al. also investigated to cope up with diffusion effect, where they proposed to use fast T1 imaging sequence to reduce the acquisition time and, ultimately, diffusion effect; they also found that diffusion can be lowered by adding chelating agents, such as xylenol orange to the gel, and found that diffusion was slower in gelatin gels [22]. Another system of polymer gel dosimetry that goes by the acronym BANANA polymer gel, due to the use of Bis (N,N-methylene-bis-acrylamide), AAm (acrylamide), nitrous oxide, and agarose, was formulated in 1992 by Maryanski et al., without having the diffusion problem [23]. Later, agarose in the BANANA gel was replaced by gelatin to form BANG (Bis, AAm, nitrogen, and aqueous gelatin) gel, which was patented for commercial use [24,25], and its in-house version is known as PAG gel [26]. Various compositions and formulations of polymer gel dosimeter have been studied, which brings significant innovation in polymer gel proposed by Gore et al.
The interest in the development of polymer gel dosimeters with different compositions and formulations continued. The Fricke-type gel dosimeter’s limitation has been addressed by several studies [5,27]; one major limitation of the Fricke-type dosimeter was the atmospheric oxygen inhibiting polymerization, as the radiation-induced polymerization is ineffective in those parts of the dosimeter contaminated by air. Hence, an oxygen-free environment should be maintained to manufacture the gel dosimeter. Fong et al. addressed this issue by developing a polymer gel dosimeter acronym as MAGIC due to its composition (Methacrylic and Ascorbic acid in Copper-Initiated Gelatin) [28]. The MAGIC gel can be manufactured in a normal laboratory environment, as the problem of oxygen inhibition was resolved by binding atmospheric oxygen in an organic complex (free oxygen found in the matrix of gelatin is bound by ascorbic acid to metallic–organic complexes with the help of metal-composed copper sulfate) [29]. Later, other authors proposed different kinds of antioxidants to manufacture such a class of polymer gel known as normoxic polymer gel [30]; as an example, tetrakis (hydroxymethyl) phosphonium chloride (THPC) was proposed by De Deene et al. [31].
Another issue that occurred in polymer gel dosimetry was the melting of the gel samples when kept at average room temperature, which resulted in a lack of knowledge on the dose distribution, hence restricting its use. Fernandes et al. solved this issue in 2008 by adding formaldehyde to the existing MAGIC formulation, which raised the melting point of gel to 69 °C and named it MAGIC-f gel [32]. Formaldehyde was applied to the MAGIC gel to increase the matrix’s stability through the development of the high number of hydrogen bonds between the gelatin and formaldehyde. The fusion point of MAGIC-f gel doubles if 1% of formaldehyde is used [28,32]. Hence, the MAGIC-f gel dosimeter is the latest innovation in the field of three-dimensional radiation dosimetry.
3. MAGIC-Gel Composition and Fabrication
It is important to understand the fundamental chemistry and physics of response before understanding the fabrication process. To understand the fundamental chemistry and physics of response, the composition and benefits of using each element of the composition should be known. A typical polymer gel should have a composition that gives a shape, ingredients that polymerize when subjected to radiation, and ingredients that retain the gel’s polymerization ability. A typical polymer gel consists of five different compositions to use as a dosimeter and give three-dimensional dose distribution when irradiated, as shown in Figure 2. The first two components are water and gelatin, which form the block structure of the dosimeter. Two active ingredients are monomers and crosslinker, which polymerize when exposed to ionizing radiation, i.e., with more ionizing radiation, more polymerizations occur. The last ingredient is oxygen scavenger. Too much free oxygen in a dosimeter makes it inert; thus, an oxygen scavenger is used to remove chemically active dissolved oxygen, which suppresses the radical initiated polymerization.
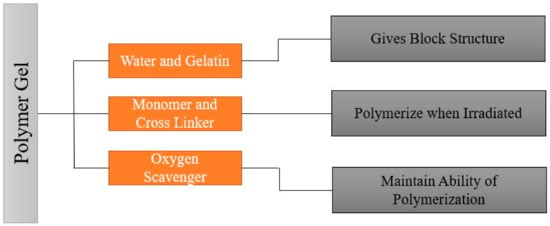
Figure 2.
General composition of the polymer gel.
Since 1954, different changes in composition have been made to make the polymer gel more accurate in three-dimensional dose distribution and easy to manufacture, handle, and use. Early polymer gel proposed by Gore et al., consisting of ferrous sulfate, and later used by Appleby et al., consisting of ferrous sulfate dispersed in a gel matrix, could not give a spatially stable distribution of dose because of diffusion of ion [13,18]. After these, several studies used not only ferrous solutions with various gelling agents, such as gelatin, agarose, Sephadex, and polyvinyl alcohol (PVA), but also chelating agents, such as xylenol orange (XO), to reduce ion diffusion [21]. However, there was an issue in manufacture because the polymerization can be disturbed due to the oxygen presence; hence, hypoxic environment is necessary to manufacture it. To address this issue, Fong et al. developed a novel polymer gel, acronym as MAGIC gel, which is formed by mixing methacrylate-based materials, ascorbic acid, and salt copper [28]. Ascorbato–copper complex provides oxygen absorption, allowing for the manufacturing of polymer gels under standard atmospheric conditions in 2001 [33]. Another issue was the melting of samples when kept at room temperature, creating a problem for its use and handle. Fernandes et al. gave the solution to this issue in 2008 by incorporating formaldehyde to the earlier MAGIC formulation, raising the melting point of gel to 69 °C, and labeling the new gel as MAGIC-f gel [32]. The formaldehyde improved the stability of the matrix as a result of the high number of hydrogen bonds formed between the formaldehyde and gelatin. Note that the MAGIC-f gel fusion point doubles if 1% of formaldehyde is used [28,33]. After 2008, the MAGIC-f gel with the atomic and chemical composition shown in Figure 3 was proposed and used by most of the studies, including that by T. Marques et al. [34]. In the atomic composition (w/w), H (10.33%), O (62.68%), C (23.52%), N (2.52%), and others (0.81%) are used. Chemical composition: w/w of 82.7% of Milli-q water (water that has been purified using resin filters and deionized to a high degree by a water purification system), 8.4% of gelatin, 0.02% of copper, 0.03% of ascorbic acid, 5.9% of methacrylic acid, and 1% of formaldehyde. Although the composition proposed by Fernandes et al. has been adopted in several studies, a few studies done by Pavoni et al. increased the composition of formaldehyde in the gel. They used a greater weight of formaldehyde in the gel mixture, i.e., 3.3% by weight in mixture, where most of the studies just used 1% by weight in gel mixture [35,36].
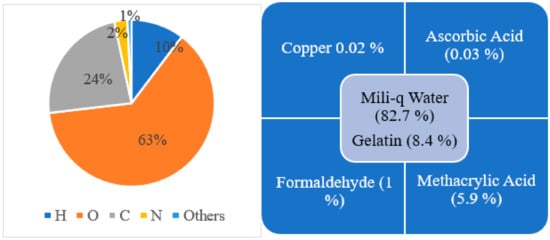
Figure 3.
Atomic (left side) and chemical (right side) composition for MAGIC-f gel.
4. Irradiation of MAGIC-f Gel
The dosimetry analysis using MAGIC-f gel is based on the chemical reaction induced by irradiation. The study of three-dimensional dose distribution in MAGIC-f gel is performed by using different radiation sources. As polymer gel dosimetry has been studied and used in various clinical applications since the 1950s; most studies use radiation of gamma rays and high-energy X-ray photons from different clinical linear accelerator sources [3]. Another form of irradiation is protons and heavy ions, but due to the saturation effect, the dosimetric reaction is lower than that from using gamma and X-rays [37,38,39,40,41,42,43]. Most of the studies on MAGIC-f gel also used gamma rays and high-energy X-ray photons from different clinical linear accelerator sources; however, only one study by Pianoschi et al. used an electron beam [44].
Figure 4 summarizes the basic chemical reaction on the MAGIC-f gel dosimeter after irradiation. In this reaction, several different free radicals are produced as a result of radiolysis of water molecule. The free radicals then react with the vinyl group (double bond between carbon atoms C = C) of the monomers, i.e., methacrylic acid. Moreover, there will be successive propagation and crosslinking reaction until all the radicals are consumed by the chain transfer reaction to form the polymer. This reaction is directly proportionate to the amount of the irradiation on the MAGIC-f gel dosimeter.
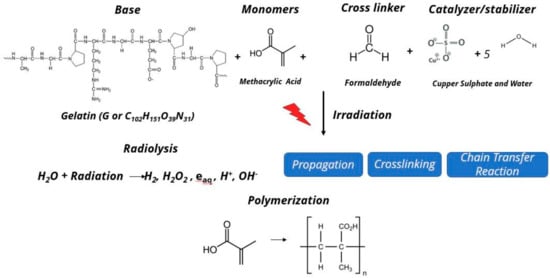
Figure 4.
Chemical reaction after irradiation on MAGIC-f gel.
5. Evaluation of MAGIC-f Gel Dosimeter
The MAGIC-f gel is analyzed by using the same diagnostic imaging techniques as other polymer gels, such as OCT, X-ray CT [12,44,45,46], magnetic resonance imaging (MRI) [3,27,47,48], and ultrasound (sonography) [49,50,51]. Out of these techniques OCT and MRI are most popular based on the physical change occurring in gel due to irradiation [52]. Ultrasound is the least-preferred method in recent practice of dosimetry evaluation, as after 2002, there was no significant study with ultrasound for imaging gel dosimeter, except for one in 2016, by Masoumi et al. [53].
Maryanski et al. introduced a new approach to three-dimensional dosimetry, using the MRI techniques, based on the principle that mapping dose distribution is given by any MRI contrast parameter that alters after polymerization in the gel. They found that the spin–lattice relaxation rate (R1) changes only slightly upon irradiation-induced polymerization. The most widely used MRI property is the spin–spin relaxation rate (R2) due to its wide dynamic range and sensitivity to polymerization caused by the absorbed dose from radiation exposure. After the discovery of MAGIC-f gel in 2008, the MRI has been extensively used in the experiment by Pavoni et al., Schwarcke et al., Quevedo et al., and Alva et al. [21,24,25]. R2 values can be calculated by using different MR imaging sequences. The image intensity of each MRI sequence should be calculated. The intensity ratio between MRI sequences is used to derive the R2 value for the gel composition. The simplest MRI sequencing method uses only two echoes to determine R2 value. The following formula is used to calculate R2 related to two consecutive echoes:
where E1 is the intensity of the gel dosimeter for the first echo, E2 is the intensity for the second echo, and ∆TE is the echo time between the first and second echo. The R2 value can be calculated for the whole MR image, which is called the R2 map. Moreover, more than two echoes of MRI sequences can be used to calculate the R2 value. Increasing the number of sequences in MRI sequences will result in better accuracy in R2 value.
The calculated R2 value can be used to determine the dose distribution in gel dosimeter. In this step, a determined dose is given to the gel dosimeter, and then this determined dose is related to the calculated R2 value; the calibration curve which relates the calculated R2 value to the given dose is acquired. The acquired calibration curve can be used to determine the delivered dose to the gel dosimeter for unknown dose values [54].
As an alternative to MRI technique, other types of techniques (OCTs) were used, based on the alternation of the absorption coefficient due to polymerization induced from the irradiation of polymer gel, and the ultrasonic which is based on the change of the sound speed due to the change in density and viscosity of the irradiated gel [53]. When the gel is not irradiated, it is transparent to light, but the irradiated MAGIC-f gel becomes darker and the amount of visible light which can pass through the gel decreases; thus, this feature can be used for gel dosimetry purposes. To calculate the dose delivered to a gel dosimeter in OCT method, a laser beam is projected to the gel and some detectors, such as charge-coupled device (CCD), or a complementary metal oxide semiconductor (CMOS) is used to get the required OCT image. The intensity ratio of the OCT images for irradiated and unirradiated gels is presented as follows [3]:
where I is the measured intensity for the irradiated gel dosimeter, Io is the intensity for the not irradiated gel, μ is the optical attenuation coefficient, and s is the distance that the laser beam travels through the sample. Then μ should be calculated. After μ is calculated, it can be related to a fitting curve. Knowing the fitting curve for μ, the delivered dose for unknown amount of irradiation can be measured by OCT method.
6. Application of MAGIC-f Gel
The gel dosimetry field is rapidly growing [54]. Gel dosimetry is applied in various applications for radiation dose measurement where the conventional dosimeter will not give an accurate measurement [54]; the application of polymer gel which was developed before MAGIC-f gel is discussed in several works of the literature [3,8,55,56,57,58]. The polymer gel dosimeter replaces the conventional dosimeter from the very basic dosimetry parameters such as percentage depth dose (PDD) in photons and electron beams, to the complex dosimetry parameters, due to its ability to demonstrate the dose distribution in three dimensions [25,44,58,59]. Moreover, the polymer gel has been applied to the dosimetry analysis in simple arrangements, such as nine-field tomotherapy [60], to a more complex anatomical situation, such as tangential breast cancer therapy [61,62] and scalp treatment with electron beams [63].
Lately, several advancements in the field of polymer gel dosimetry utilized to demonstrate dose distribution from imaging procedure have been made; the benefits are that the dose distribution throughout the desired volume of a patient can be evaluated without using point dosimeters, and the dose averaging along the line or volume is avoided [64]. Medical science has developed various new techniques/therapies for the treatment of various diseases for complicated dose distributions. On the one hand, ion chamber and thin film fail in the measurement of the complex dose distribution, whereas hand gel dosimeters are useful in the measurement of dose for complex dose distribution produced by such treatments as 3D conformal radiation therapy (3D CRT), intensity-modulated radiation therapy (IMRT), and brachytherapy [25,55,65,66,67]. The complexity of dose distribution in brachytherapy is due to the high dose rate produced by the therapy, which introduces the temperature gradients influencing the polymerization of acrylamide monomer in polymer gel [31,68,69,70]. The application area of the polymer gel broadens amid the rapid growing medial technology; these applications apply to several other functions, such as internal dosimetry, calculation of neutron [71,72,73,74], particle dose distribution [38,43,75,76,77], and in the evaluation of tissue heterogeneities [55,78,79,80,81,82].
The trend of interest in polymer gel application continues with more innovation in the polymer gel dosimetry by making it more efficient and easier to use. As an outcome of the innovation MAGIC-f gel is most suitable for a wide range of applications; many types of research have been conducted on the application of MAGIC-f gel to determine the dose distribution at different conditions for various applications, Pavoni et al. applied MAGIC-f gel to measure of three-dimensional dose distribution from a tomotherapy device. In this research, the gel phantom was simulated with a prostate intensity-modulated radiation therapy (IMRT), a tomotherapy device was used for treatment. Then, the spin-spin relaxation rate (R2) distribution measured by magnetic resonance imaging was evaluated for the dose distribution; a high similarity was found in the comparison of dose distribution measured from the gel and calculated one from the treatment planning system (TPS), the comparison was based on two quantities, the relative dose profile and gamma index analysis. Hence, this work concludes that the MAGIC-f gel is a promising gel for three-dimensional dose distribution measurement [36].
The advancements in nuclear medicine and cancer treatment using radionuclides require a specific dosimetric knowledge; Monte Carlo simulations are used extensively for experimental study to verify the dose distribution in the new polymer gel dosimeter (MAGIC-f) that is used in nuclear medicine [3]. Schreck et al. obtained a dose-response in MAGIC-f gel after exposing it to 131I source [83]; the sensitivity of transversal relaxation rate (R2) value versus absorbed dose in gel obtained was 0.23 s−1Gy−1; this observation was verified by comparison with Monte Carlo simulation using PENELOPE code and thermoluminescent dosimeter (TLD), the Monte Carlo simulation result was agreed more to MAGIC-f gel dosimetry than TLD, which showed the potential of using MAGIC-f gel in nuclear medicine when three-dimensional dose distribution is required [83]. Silveira et al. used MAGIC-f gel for nuclear medicine application along with MRI, where it was used to calculate complex three-dimensional dose distributions of two prostate intensity-modulated radiation therapy (IMRT) treatments which required high-quality assurance [84]. This study shows that MAGIC-f gel qualifies for the quality assurance standard of the IMRT treatment system as the gamma index obtained from the dose distribution using MAGIC-f gel is higher (95.5%) in comparison with the dose distribution using treatment planning system (94.0%) [84]. The electron beam therapy treatment in nuclear medicine is used to deliver a high dose rate. Pianoschi et al. show that MAGIC-f gel can be utilized as a feasible dosimeter to measure the percentage depth dose (PDD) and beam profile in treatment planning as it has strong spatial resolution characteristics and can create three-dimensional dose distribution [44]. In that research, the response of MAGIC-f gel for a 6 MeV electron beam is studied using different doses and levels of dose along with the determination of dose-metric parameters for clinical beam such as PDDs and beam profile [44]. Quevedo et al. showed that the use of MAGIC-f gel with few modifications in composition is promising in dosimetry of high-gradient sources used in clinical brachytherapy. In this study, phantom of 5 × 5 × 7 cm3 of MAGIC-f gel manufactured by modifying the concentrations of the reagents, i.e., 11 g of gelatin, 2 mL of copper sulfate solution, 50.2 mg of ascorbic acid, 5.9 mL of acid methacrylic, and 4 mL of formaldehyde, was irradiated with a widely used dose of 7.5 Gy in gynecological treatment, using the clinical source 192Ir (Varian, GammaMed Plus model), and the measurement of dose distribution was taken by using MRI after 24 h of irradiation [85]. For the same configuration using the Monte Carlo PENELOPE code, whereby, for both the experimental and simulated studies, energy is kept limited at 100 keV and primary condensation parameters of particles is kept to 0.3 (0.3% of the particles enables the formation of droplets), the comparison of the experimental and simulated dose distributions obtained in two perpendicular sources showed an average variation of 12.5% and 6.4% [4].
Radiotherapy is the most common cancer therapy treatment. The dosimetric properties of various energy beams used in the therapy need to be studied for the efficient cancer treatment. Marques et al. used MAGIC-f gel to calculate the absorbed doses from these energy beams and theoretically verified them by using the PENELOPE Monte Carlo code. Moreover, a dependency of energy and dose rate was obtained in a range from 60 kV to 10 MeV and from 0.44 to 10 Gy/min, respectively. The MAGIC-f shows a linear relationship to all energies used in the study where the maximum and average variation was 8.6% and 7.7%, respectively. Moreover, the research on dose rate dependency reveals that the MAGIC-f gel shows no major variations of responses in different clinical dose rates which vary from 0.44 to 10 Gy/min, i.e., lower than 0.7% when X-rays are considered for evaluation and lower than 1.9% when all high- and low-energy beams (X-ray, γ-rays, photons, etc.) are produced from different accelerators [34]. Alva et al. showed the effectiveness of MAGIC-f gel by using small fields in the dosimetric calculation in conformal radiation therapy; this cancer treatment shapes the radiation beam to tally the tumor shape; the effectiveness of MAGIC-f gel is predicted by Monte Carlo simulation, using PENELOPE, and experimentally by irradiating a MAGIC-f gel in phantom of 12 cm diameter and 18 cm height with 9 fields of 6 MV photon beam, each one of 1 × 1 cm2 with a total dose of 16 Gy, and reading the result via MRI [86]. In another study by Alva et al., a comparison of the simulated and experimented dose distributions with the treatment planning system (TPS) gave the mean differences of 2.88% and 3.75%; these low percentages indicate that the response of the MAGIC-f polymeric gel could be suitable to use as a dosimeter in clinical procedures using small fields, especially in radiosurgery. Moreover, PENELOPE code can be used to simulate the MAGIC-f gel components for analysis and predict the gel response [87].
7. Discussion and Future Works
MAGIC-f gel dosimeters, with the ability to uniquely measure the distribution of radiation dose in three dimensions, have various specific benefits over other polymer gel dosimeters because of their high thermal stability, low dependence of varying dose rate, energy, and fractionation [35]. Other existing dosimeters which are specially considered in routine clinic, such as ionization chamber, thin-film, and diode arrays, have limited dose-verification application, as they record only in point, one and two dimensions. The MAGIC-f gel has been presented as a prominent method of evaluating dose in various applications, such as tomotherapy devices, intensity-modulated radiation therapy (IMRT) treatments, clinical brachytherapy, electron beam therapy, and conformal radiation therapy, where simple to complex dose distribution persisted (Table 1). MRI has been mostly used for the evaluation of the irradiated gel for the absorbed dose measurement. Few studies have been made to enhance the thermal stability, dependence of varying dose rate, energy, and percentage depth dose.

Table 1.
Summary of studies on MAGIC-f gel.
There has been some advancement in the dosimetry using MAGIC-f gel. In 2012, Pavoni et al. conducted experiments proposing different weights of the ingredients in MAGIC-f gel, as proposed by Farnandes et al. They manufactured the MAGIC-f gel with 3% formaldehyde and showed it as being characterized by high thermal stability, low dependence of varying dose rate, energy, and fractionation. Hence, suitable for three-dimensional dosimetry analysis [35]. Although this study does not discuss the effect of adding more formaldehyde, it would be interesting to know in future the effect of each ingredient’s composition in MAGIC-f gel.
In another study, Khosravi et al. showed an increase in the percentage depth dose (PDDs) by embedding gold nanoparticles (GNPs) in the MAGIC-f gel when exposed to high-energy photon beams. In this study, PDDs of the tube containing MAGIC-f gel fabricated based on its standard composition and after adding GNPs with an average diameter 15 nm and concentration of 0.1 mM, when irradiated with 18 Mev photons beams of an Elekta LINAC accelerator, was measured experimentally; the same configuration is made in Monte Carlo code Geant4 and computed. The result of the study of the MAGIC-f gel tube embedded with GNPs characterized with a dose-enhancement factor of 1.12 ± 0.08 and 1.13 ± 0.04 from Geant4 and experimental measurement, respectively, shows that MAGIC-f gel can be a suitable dosimeter for three-dimensional dose distribution in high-energy radiotherapy procedures where GNPs are used [88].
Although this field rapidly growing, with several improvements in dosimetry using MAGIC-f gel, many aspects of its application still need to be addressed, including the problem associated with the most efficient way to read dose where imaging techniques have imaging artifacts. As discussed by Geoffrey S. Ibbott [54], such artifacts are susceptible to interference artifacts in MRI, and streak and ring artifacts in CT. The susceptibility artifacts in MRI can most probably result from variations in the gel volume’s conductivity whose image is captured, or by the presence of air having low density, and the interference artifacts due to the multiplanar imaging of adjacent planes [89]. Streak artifacts are caused by the blocking of lights beam by any structures, whereas ring artifacts are due to the refraction of light at interference between gel and other materials [52]. Hilts et al. discuss the technical consideration for using X-ray CT for dosimetric evaluation: the low signal-to-noise ratio that occurs because of the very small density differences present in the gel may cause artifacts [90]. Thus, mitigating the imaging artifacts while reading the dose from imaging techniques would be a current research interest in the field of dosimetry, using MAGIC-f gel.
Although the MAGIC-f gel shows low dependence on varying dose rates and energy, several studies using other polymer gels show the dependence at a low-dose-rate therapy such as LDR brachytherapy [78,91]. Hence, a detailed investigation of dependence at low dose rate and energy is required to validate the claim. Similarly, the dependence on the temperature during the MAGIC-f gel irradiation has not been investigated so far. The increase in temperature during irradiation may affect gel polymerization, which further leads to affect the dose measurement. Another area that has not been studied in detail so far is a simulation of low-density tissue, such as lungs, by making gel, which is equivalent to low-density tissue. However, small efforts have been made by Haraldsson et al. in regard to this subject [92].
Radiotherapy dosimetry needs to achieve a dosimetric uncertainty better current result, which is up to 5% [84]. Therefore, a detailed investigation to find a more critical approach is required to enhance the dosimetric uncertainty. Hence, the polymer gel dosimetry, which is now at a promising phase of use in various applications, needs more investigation, which will create more confidence in routine use in clinical applications.
8. Conclusions
The development of MAGIC-f gel is a milestone in polymer gel application for three-dimensional dose measurement, due to its high thermal stability, low dependence of varying dose rate, energy, and fractionation. The MAGIC-f gel presents a prominent method for evaluating simple-to-complex dose-distribution applications, such as tomotherapy devices, intensity-modulated radiation therapy (IMRT) treatments, clinical brachytherapy, electron beam therapy, and conformal radiation therapy. From many studies, our understanding of different physical and chemical processes inside the polymer gel dosimeter has been extended. Very few attempts have been made so far to optimize the composition of MAGIC-f gel to obtain a highly efficient dosimeter that satisfies the dosimetric criteria and can achieve high dose sensitivity. Although Monte Carlo methods and comparison with TPS calculation are present gold standards for comparing the 3D dosimeter so far, future studies should focus on optimizing MAGIC-f gel capacity to give high spatial dose accuracy by optimizing its composition and developing more accurate evaluation-method parameters.
Author Contributions
Conceptualization, R.D. and H.M.; methodology, R.D.; investigation, R.D. and M.Y.; resources, H.M.; writing—original draft preparation, R.D. and M.Y.; writing—review and editing, R.D.; visualization, M.Y. and H.M.; supervision, H.M.; project administration, R.D.; funding acquisition, H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by proposal assisted program funding of Texas Tech University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We would like to acknowledge and extend our gratitude to the Fiber and Biopolymer Research Institute (FBRI) at Texas Tech University, for preparing MAGIC-f gel.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baldock, C. (Ed.) Historical Overview of the Development of Gel Dosimetry: A Personal Perspective; IOP Publishing: Bristol, UK, 2006; Volume 56, p. 002. [Google Scholar]
- Watanabe, Y.; Warmington, L.; Gopishankar, N. Three-dimensional radiation dosimetry using polymer gel and solid radiochromic polymer: From basics to clinical applications. World J. Radiol. 2017, 9, 112. [Google Scholar] [CrossRef]
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.; Oldham, M.; Schreiner, L.J. Polymer gel dosimetry. Phys. Med. Biol. 2010, 55, R1. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, A.L.; Nicolucci, P.; Borges, L.F. Evaluation of the response of modified MAGIC-f polymeric gel using a clinical brachytherapy source and Monte Carlo simulation with package PENELOPE. Rev. Bras. de Fis. Med. (Online) 2016, 10, 2–6. [Google Scholar] [CrossRef]
- Lepage, M.; Jayasakera, P.; Bäck, S.Å.J.; Baldock, C. Dose resolution optimization of polymer gel dosimeters using different monomers. Phys. Med. Biol. 2001, 46, 2665. [Google Scholar] [CrossRef]
- Shevtsov, M.A.; Nikolaev, B.P.; Yakovleva, L.Y.; Marchenko, Y.Y.; Dobrodumov, A.V.; Mikhrina, A.L.; Martynova, M.G.; Bystrova, O.A.; Yakovenko, I.V.; Ischenko, A.M. Superparamagnetic iron oxide nanoparticles conjugated with epidermal growth factor (SPION–EGF) for targeting brain tumors. Int. J. Nanomed. 2014, 9, 273. [Google Scholar] [CrossRef] [Green Version]
- Ab Razak, N.N.A.N.; Abd Rahman, A.; Kandaiya, S.; Shahrim, I.; Zakiah, N.; Yahaya, N.F.Z.; Yann, E.K. Role of anti-oxidants on normoxic methacrylic acid gelatin (MAG) polymer gel dosimeter at 6-MV photon beam using single spin echo MRI. Malays. J. Anal. Sci. 2014, 18, 423–433. [Google Scholar]
- Day, M.; Stein, G. Chemical effects of ionizing radiation in some gels. Nature 1950, 166, 146–147. [Google Scholar] [CrossRef]
- Alexander, P.; Charlesby, A.; Ross, M. The degradation of solid polymethylmethacrylate by ionizing radiation. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1954, 223, 392–404. [Google Scholar]
- Hoecker, F.E.; Watkins, I. Radiation polymerization dosimetry. Int. J. Appl. Radiat. Isotopes. 1958, 3, 31–35. [Google Scholar] [CrossRef]
- Boni, A. A polyacrylamide gamma dosimeter. Radiat. Res. 1961, 14, 374–380. [Google Scholar] [CrossRef]
- Audet, C.; Schreiner, L. (Eds.) Radiation dosimetry by NMR relaxation time measurements of irradiated polymer solutions. In Proceedings of the International Society for Magnetic Resonance in Medicine, San Francisco, CA, USA, 10–16 August 1991. [Google Scholar]
- Gore, J.; Kang, Y. Measurement of radiation dose distributions by nuclear magnetic resonance (NMR) imaging. Phys. Med. Biol. 1984, 29, 1189. [Google Scholar] [CrossRef] [PubMed]
- Fricke, H.; Morse, S. The chemical action of roentgen rays on dilute ferrosulphate solutions as a measure of dose. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1927, 18, 430–432. [Google Scholar]
- Schreiner, L. (Ed.) Review of Fricke gel Dosimeters; IOP Publishing: Bristol, UK, 2004; Volume 3, p. 003. [Google Scholar]
- Kennan, R.; Maryanski, M.; Zhong, J.; Gore, J. Hydrodynamic effects and cross relaxation in cross linked polymer gels. Proc. Int. Soc. Magn. Reson. Med. (New York) 1992, 1, 11. [Google Scholar]
- Spevacek, J.; Dvorak, P.; Cechak, T.; Novotny, J., Jr.; Marek, M. The use of gel dosimetry of Fricke type in three-dimensional dosimetry; DRO: Demanovska Dolina, Slovak Republic, 2001; Volume 1, pp. 129–132. [Google Scholar]
- Appleby, A.; Christman, E.; Leghrouz, A. Imaging of spatial radiation dose distribution in agarose gels using magnetic resonance. Med. Phys. 1987, 14, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; DeGuzman, A.; Nguyen, D.; Gore, J. Dose-response curves for Fricke-infused agarose gels as obtained by nuclear magnetic resonance. Phys. Med. Biol. 1990, 35, 1611. [Google Scholar] [CrossRef]
- Olsson, L.E.; Westrin, B.A.; Fransson, A.; Nordell, B. Diffusion of ferric ions in agarose dosimeter gels. Phys. Med. Biol. 1992, 37, 2243. [Google Scholar] [CrossRef]
- Baldock, C.; Harris, P.; Piercy, A.; Healy, B. Experimental determination of the diffusion coefficient in two-dimensions in ferrous sulphate gels using the finite element method. Australas. Phys. Eng. Sci. Med. 2001, 24, 19. [Google Scholar] [CrossRef]
- Kron, T.; Jonas, D.; Pope, J.M. Fast T1 imaging of dual gel samples for diffusion measurements in NMR dosimetry gels. Magn. Reson. Imaging 1997, 15, 211–221. [Google Scholar] [CrossRef]
- Maryanski, M.; Gore, J.; Schulz, R. 3-D radiation dosimetry by MRI: Solvent proton relaxation enhancement by radiation-controlled polymerisation and cross-linking in gels. Proc. Int. Soc. Magn. Reson. Med. (New York) 1992. [Google Scholar]
- Maryanski, M.; Schulz, R.; Ibbott, G.; Gatenby, J.; Xie, J.; Horton, D.; Gore, J.C. Magnetic resonance imaging of radiation dose distributions using a polymer-gel dosimeter. Phys. Med. Biol. 1994, 39, 1437. [Google Scholar] [CrossRef]
- Maryanski, M.; Gore, J.; Schulz, R. Three-Dimensional Detection, Dosimetry and Imaging of an Energy Field by Formation of a Polymer in a Gel. U.S. Patent 5,321,357, 14 June 1994. [Google Scholar]
- Baldock, C.; Burford, R.; Billingham, N.; Wagner, G.; Patval, S.; Badawi, R.D.; Keevil, S.F. Experimental procedure for the manufacture and calibration of polyacrylamide gel (PAG) for magnetic resonance imaging (MRI) radiation dosimetry. Phys. Med. Biol. 1998, 43, 695. [Google Scholar] [CrossRef]
- Pappas, E.; Maris, T.; Angelopoulos, A.; Paparigopoulou, M.; Sakelliou, L.; Sandilos, P.; Voyiatzi, S.; Vlachos, L. A new polymer gel for magnetic resonance imaging (MRI) radiation dosimetry. Phys. Med. Biol. 1999, 44, 2677. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.M.; Keil, D.C.; Does, M.D.; Gore, J.C. Polymer gels for magnetic resonance imaging of radiation dose distributions at normal room atmosphere. Phys. Med. Biol. 2001, 46, 3105. [Google Scholar] [CrossRef]
- De Deene, Y.; Venning, A.; Hurley, C.; Healy, B.; Baldock, C. Dose–response stability and integrity of the dose distribution of various polymer gel dosimeters. Phys. Med. Biol. 2002, 47, 2459. [Google Scholar] [CrossRef]
- Senden, R.; De Jean, P.; McAuley, K.; Schreiner, L. Polymer gel dosimeters with reduced toxicity: A preliminary investigation of the NMR and optical dose–response using different monomers. Phys. Med. Biol. 2006, 51, 3301. [Google Scholar] [CrossRef]
- De Deene, Y.; Hurley, C.; Venning, A.; Vergote, K.; Mather, M.; Healy, B.J.; Baldock, C. A basic study of some normoxic polymer gel dosimeters. Phys. Med. Biol. 2002, 47, 3441. [Google Scholar] [CrossRef]
- Fernandes, J.P.; Pastorello, B.F.; de Araújo, D.B.; Baffa, O. (Eds.) Formaldehyde Increases MAGIC Gel Dosimeter Melting Point and Sensitivity; IOP Publishing: Bristol, UK, 2009; Volume 53, p. N53. [Google Scholar]
- Gustavsson, H.; Karlsson, A.; Bäck, S.A.J.; Olsson, L.E.; Haraldsson, P.; Engström, P.; Nyström, H. MAGIC-type polymer gel for three-dimensional dosimetry: Intensity-modulated radiation therapy verification. Med. Phys. 2003, 30, 1264–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, T.; Schwarcke, M.; Garrido, C.; Baffa, O.; Nicolucci, P. (Eds.) Dosimetric Properties of MAGIC-f Polymer Gel Assessed to Radiotherapy Clinical Beams; IOP Publishing: Bristol, UK, 2010; Volume 250, p. 012012. [Google Scholar]
- Pavoni, J.; Baffa, O. An evaluation of dosimetric characteristics of MAGIC gel modified by adding formaldehyde (MAGIC-f). Radiat. Meas. 2012, 47, 1074–1082. [Google Scholar] [CrossRef]
- Pavoni, J.; Pike, T.; Snow, J.; DeWerd, L.; Baffa, O. Tomotherapy dose distribution verification using MAGIC-f polymer gel dosimetry. Med. Phys. 2012, 39, 2877–2884. [Google Scholar] [CrossRef] [PubMed]
- Heufelder, J.; Stiefel, S.; Pfaender, M.; Lüdemann, L.; Grebe, G.; Heese, J. Use of BANG® polymer gel for dose measurements in a 68 MeV proton beam. Med. Phys. 2003, 30, 1235–1240. [Google Scholar] [CrossRef]
- Gustavsson, H.; Bäck, S.Å.J.; Medin, J.; Grusell, E.; Olsson, L.E. Linear energy transfer dependence of a normoxic polymer gel dosimeter investigated using proton beam absorbed dose measurements. Phys. Med. Biol. 2004, 49, 3847. [Google Scholar] [CrossRef]
- Zeidan, O.; Sriprisan, S.; Lopatiuk-Tirpak, O.; Kupelian, P.; Meeks, S.; Hsi, W.; Li, Z.; Palta, J.R.; Maryanski, M.J. Dosimetric evaluation of a novel polymer gel dosimeter for proton therapy. Med. Phys. 2010, 37, 2145–2152. [Google Scholar] [CrossRef]
- Jirasek, A.; Duzenli, C. Relative effectiveness of polyacrylamide gel dosimeters applied to proton beams: Fourier transform Raman observations and track structure calculations. Med. Phys. 2002, 29, 569–577. [Google Scholar] [CrossRef]
- Lopatiuk-Tirpak, O.; Su, Z.; Li, Z.; Zeidan, O.; Meeks, S.; Maryanski, M. Direct response to proton beam linear energy transfer (LET) in a novel polymer gel dosimeter formulation. Technol. Cancer Res. Treat. 2012, 11, 441–445. [Google Scholar] [CrossRef]
- Gustavsson, H.; Bäck, S.Å.J.; Lepage, M.; Rintoul, L.; Baldock, C. Development and optimization of a 2-hydroxyethylacrylate MRI polymer gel dosimeter. Phys. Med. Biol. 2004, 49, 227. [Google Scholar] [CrossRef]
- Bäck, S.Å.J.; Medin, J.; Magnusson, P.; Olsson, P.; Grusell, E.; Olsson, L.E. Ferrous sulphate gel dosimetry and MRI for proton beam dose measurements. Phys. Med. Biol. 1999, 44, 1983. [Google Scholar] [CrossRef] [PubMed]
- Pianoschi, T.; Alva, M.; Santanna, M.; Baffa, O.; Nicolucci, P. MAGIC-f Gel Dosimetry for Clinical Electron Beam; IOP Publishing: Bristol, UK, 2010; Volume 250, p. 012037. [Google Scholar]
- Oldham, M.; Siewerdsen, J.H.; Shetty, A.; Jaffray, D.A. High resolution gel-dosimetry by optical-CT and MR scanning. Med. Phys. 2001, 28, 1436–1445. [Google Scholar] [CrossRef] [Green Version]
- Hilts, M.; Audet, C.; Duzenli, C.; Jirasek, A. Polymer gel dosimetry using x-ray computed tomography: A feasibility study4. Phys. Med. Biol. 2000, 45, 2559. [Google Scholar] [CrossRef] [PubMed]
- De Deene, Y.; De Wagter, C.; Van Duyse, B.; Derycke, S.; De Neve, W.; Achten, E. Three-dimensional dosimetry using polymer gel and magnetic resonance imaging applied to the verification of conformal radiation therapy in head-and-neck cancer. Radiother. Oncol. 1998, 48, 283–291. [Google Scholar] [CrossRef]
- Maryanski, M.J.; Gore, J.C.; Kennan, R.P.; Schulz, R.J. NMR relaxation enhancement in gels polymerized and cross-linked by ionizing radiation: A new approach to 3D dosimetry by MRI. Magn. Reson. Imaging 1993, 11, 253–258. [Google Scholar] [CrossRef]
- Mather, M.L.; De Deene, Y.; Whittaker, A.K.; Simon, G.P.; Rutgers, R.; Baldock, C. Investigation of ultrasonic properties of PAG and MAGIC polymer gel dosimeters. Phys. Med. Biol. 2002, 47, 4397. [Google Scholar] [CrossRef]
- Mather, M.L.; Whittaker, A.K.; Baldock, C. Ultrasound evaluation of polymer gel dosimeters. Phys. Med. Biol. 2002, 47, 1449. [Google Scholar] [CrossRef]
- Baldock, C.; Rintoul, L.; Keevil, S.; Pope, J.; George, G. Fourier transform Raman spectroscopy of polyacrylamide gels (PAGs) for radiation dosimetry. Phys. Med. Biol. 1998, 43, 3617. [Google Scholar] [CrossRef]
- Trapp, J.; Bäck, S.Å.J.; Lepage, M.; Michael, G.; Baldock, C. An experimental study of the dose response of polymer gel dosimeters imaged with x-ray computed tomography. Phys. Med. Biol. 2001, 46, 2939. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, H.; Mokhtari-Dizaji, M.; Arbabi, A.; Bakhshandeh, M. Determine the dose distribution using ultrasound parameters in MAGIC-f polymer gels. Dose-Response 2016, 14, 1559325815625647. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, Y.; Kubo, H. A variable echo-number method for estimating in MRI-based polymer gel dosimetry. Med. Phys. 2011, 38, 975–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibbott, G.S. (Ed.) Applications of Gel Dosimetry; IOP Publishing: Bristol, UK, 2004; Volume 3, p. 007. [Google Scholar]
- McJury, M.; Oldham, M.; Cosgrove, V.P.; Murphy, P.S.; Doran, S.; O Leach, M.; Webb, S. Radiation dosimetry using polymer gels: Methods and applications. Br. J. Radiol. 2000, 73, 919–929. [Google Scholar] [CrossRef]
- MacDougall, N.; Pitchford, W.; Smith, M. A systematic review of the precision and accuracy of dose measurements in photon radiotherapy using polymer and Fricke MRI gel dosimetry. Phys. Med. Biol. 2002, 47, R107. [Google Scholar] [CrossRef]
- Andrews, H.L.; Murphy, R.E.; LeBrun, E.J. Gel dosimeter for depth-dose measurements. Rev. Sci. Instrum. 1957, 28, 329–332. [Google Scholar] [CrossRef]
- Haraldsson, P.; Bäck, S.; Magnusson, P.; Olsson, L. Dose response characteristics and basic dose distribution data for a polymerization-based dosemeter gel evaluated using MR. Br. J. Radiol. 2000, 73, 58–65. [Google Scholar] [CrossRef]
- Oldham, M.; Baustert, I.; Lord, C.; Smith, T.A.D.; McJury, M.; Warrington, A.P.; O Leach, M.; Webb, S. An investigation into the dosimetry of a nine-field tomotherapy irradiation using BANG-gel dosimetry. Phys. Med. Biol. 1998, 43, 1113. [Google Scholar] [CrossRef] [PubMed]
- Baldock, C.; Hasler, C.; Keevil, S.; Greener, A.; Billingham, N.; Burford, R. A dosimetry phantom for external beam radiation therapy of the breast using radiation-sensitive polymer gels and MRI. Med. Phys. 1996, 23, 1490. [Google Scholar]
- Love, P.; Evans, P.; Leach, M.; Webb, S. Polymer gel measurement of dose homogeneity in the breast: Comparing MLC intensity modulation with standard wedged delivery. Phys. Med. Biol. 2003, 48, 1065. [Google Scholar] [CrossRef]
- Trapp, J.; Partridge, M.; Hansen, V.N.; Childs, P.; Bedford, J.; Warrington, A.P.; O Leach, M.; Webb, S. The use of gel dosimetry for verification of electron and photon treatment plans in carcinoma of the scalp. Phys. Med. Biol. 2004, 49, 1625. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.; Venning, A.; Baldock, C. The dose response of normoxic polymer gel dosimeters measured using X-ray CT. Br. J. Radiol. 2005, 78, 623–630. [Google Scholar] [CrossRef]
- Webb, S. The Physics of Conformal Radiotherapy: Advances in Technology (PBK); CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Schreiner, L.; Crooks, I.; Evans, M.; Keller, B.; Parker, W. Imaging of HDR brachytherapy dose distributions using NMR Fricke-gelatin dosimetry. Magn. Reson. Imaging 1994, 12, 901–907. [Google Scholar] [CrossRef]
- Olsen, D.; Hellesnes, J. Absorbed dose distribution measurements in brachytherapy using ferrous sulphate gel and magnetic resonance imaging. Br. J. Radiol. 1994, 67, 1121–1126. [Google Scholar] [CrossRef]
- Gelfi, C.; Righetti, P.G. Polymerization kinetics of polyacrylamide gels II. Effect of temperature. Electrophoresis 1981, 2, 220–228. [Google Scholar] [CrossRef]
- Maryanski, M.; Audet, C.; Gore, J. Effects of crosslinking and temperature on the dose response of a BANG polymer gel dosimeter. Phys. Med. Biol. 1997, 42, 303. [Google Scholar] [CrossRef]
- Omidian, H.; Hashemi, S.; Sammes, P.; Meldrum, I. Modified acrylic-based superabsorbent polymers. Effect of temperature and initiator concentration. Polymer 1998, 39, 3459–3466. [Google Scholar] [CrossRef]
- Gambarini, G.; Gomarasca, G.; Marchesini, R.; Pecci, A.; Pirola, L.; Tomatis, S. Three-dimensional determination of absorbed dose by spectrophotometric analysis of ferrous-sulphate agarose gel. Nucl. Instrum. Methods Phys. Res. Sect. A: Accel. Spectrometers Detect. Assoc. Equip. 1999, 422, 643–648. [Google Scholar] [CrossRef]
- Gambarini, G. Gel Dosimetry in Neutron Capture Therapy. In Proceedings of the DOSGEL 2001, 2nd International Conference on Radiotherapy Gel Dosimetry, Brisbane, Australia, 18–21 November 2001; p. 92. [Google Scholar]
- Gambarini, G.; Birattari, C.; Colombi, C.; Pirola, L.; Rosi, G. Fricke gel dosimetry in boron neutron capture therapy. Radiat. Prot. Dosim. 2002, 101, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, P.; Bousbouras, P.; Sandaltzopoulos, R.; Kaldoudi, E. Investigating the potential of polymer gel dosimetry for interventional radiology: First results. Phys. Med. Biol. 2008, 53, N127. [Google Scholar] [CrossRef] [PubMed]
- Maryanski, M.; Schulz, R.; Gore, G. Three dimensional dose distributions for 160 MeV protons using MRI of the tissue-equivalent BANG polymer-gel dosimeter. In Proceedings of the PTCOG Newsletter, MA, USA, 1994; Volume 14, pp. 10–11. [Google Scholar]
- Schubert, J. Radiation Chemistry—An Introduction: AJ Swallow; Longman Group, Ltd.: Pergamon, Turkey; London, UK, 1973; p. 275. [Google Scholar]
- Ramm, U.W.U.; Bocks, M.; Kraemer, M.; Bankamp, A.; Damrau, M.; Thilman, C.; Bottcher, H.D.; LaK, G.S. Three-dimensional BANG™gel dosimetry in conformal carbon. Phys. Med. Biol. 2000, 45, N95. [Google Scholar] [CrossRef] [PubMed]
- Pantelis, E.; Karlis, A.; Kozicki, M.; Papagiannis, P.; Sakelliou, L.; Rosiak, J. Polymer gel water equivalence and relative energy response with emphasis on low photon energy dosimetry in brachytherapy. Phys. Med. Biol. 2004, 49, 3495. [Google Scholar] [CrossRef]
- Vergote, K.; De Deene, Y.; Claus, F.; De Gersem, W.; Van Duyse, B.; Paelinck, L.; Achten, E.; De Neve, W.; De Wagter, C. Application of monomer/polymer gel dosimetry to study the effects of tissue inhomogeneities on intensity-modulated radiation therapy (IMRT) dose distributions. Radiother. Oncol. 2003, 67, 119–128. [Google Scholar] [CrossRef]
- Gum, F.; Scherer, J.; Bogner, L.; Solleder, M.; Rhein, B.; Bock, M. Preliminary study on the use of an inhomogeneous anthropomorphic Fricke gel phantom and 3D magnetic resonance dosimetry for verification of IMRT treatment plans. Phys. Med. Biol. 2002, 47, N67. [Google Scholar] [CrossRef]
- Ølberg, S.; Skretting, A.; Bruland, Ø.; Olsen, D.R. Dose distribution measurements by MRI of a phantom containing lung tissue equivalent compartments made of ferrous sulphate gel. Phys. Med. Biol. 2000, 45, 2761. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.; BenComo, J.; Ibbott, G. A 3 Dimensional Gel Dosimetry Lung Equivalent (WIP). In Proceedings of the AAPM Annual Meeting, San Diego, CA, USA, 10–14 August 2003. [Google Scholar]
- Schwarcke, M.; Marques, T.; Garrido, C.; Nicolucci, P.; Baffa, O. (Eds.) MAGIC-f Gel in Nuclear Medicine Dosimetry: Study in an External Beam of Iodine-131; IOP Publishing: Bristol, UK, 2010; Volume 250, p. 012082. [Google Scholar]
- da Silveira, M.A.; Pavoni, J.F.; Salmon, C.E.G.; Baffa, O. Tridimensional dosimetry for prostate IMRT treatments using MAGIC-f gel by MRI. Radiat. Meas. 2014, 71, 369–373. [Google Scholar] [CrossRef]
- Quevedo, A.; Luo, G.; Galhardo, E.; Price, M.; Nicolucci, P.; Gore, J.C.; Zu, Z. Polymer gel dosimetry by nuclear Overhauser enhancement (NOE) magnetic resonance imaging. Phys. Med. Biol. 2018, 63, 15NT03. [Google Scholar] [CrossRef]
- Alva, M.; Pianoschi, T.; Takeda, F.; Alves, T.; Haddad, C.; Nicolucci, P. SU-GG-I-90: Dose Distribution of Small Fields through MAGIC-F Gel Dosimetry and PENELOPE-Monte Carlo Simulation. Med. Phys. 2010, 37, 3122. [Google Scholar] [CrossRef]
- Alva, M.; Pianoschi, T.; Marques, T.; Santanna, M.M.; Baffa, O.; Nicolucci, P. Monte Carlo Simulation of MAGIC-f gel for Radiotherapy using PENELOPE. JPhCS 2010, 250, 012067. [Google Scholar]
- Khosravi, H.; Ghazikhanlousani, K.; Rahimi, A. Use of gold nanoparticles in MAGIC-f gels to 18 MeV photon enhancement. Nanomed. J. 2019, 6, 67–73. [Google Scholar]
- Watanabe, Y.; Perera, G.M.; Mooij, R.B. Image distortion in MRI-based polymer gel dosimetry of gamma knife stereotactic radiosurgery systems. Med. Phys. 2002, 29, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Hilts, M.; Jirasek, A.; Duzenli, C. Technical considerations for implementation of x-ray CT polymer gel dosimetry. Phys. Med. Biol. 2005, 50, 1727. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Venning, A.; De Deene, Y.; Vial, P.; Oliver, L.; Adamovics, J.; Baldock, C. Radiological properties of the PRESAGE and PAGAT polymer dosimeters. Appl. Radiat. Isot. 2008, 66, 1970–1974. [Google Scholar] [CrossRef] [PubMed]
- Haraldsson, P.; Karlsson, A.; Wieslander, E.; Gustavsson, H.; Bäck, S.Å.J. Dose response evaluation of a low-density normoxic polymer gel dosimeter using MRI. Phys. Med. Biol. 2006, 51, 919. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).