Effect of Sunflower, Almond, and Rapeseed Oils as Additives on Thermal Properties of a Machinery Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
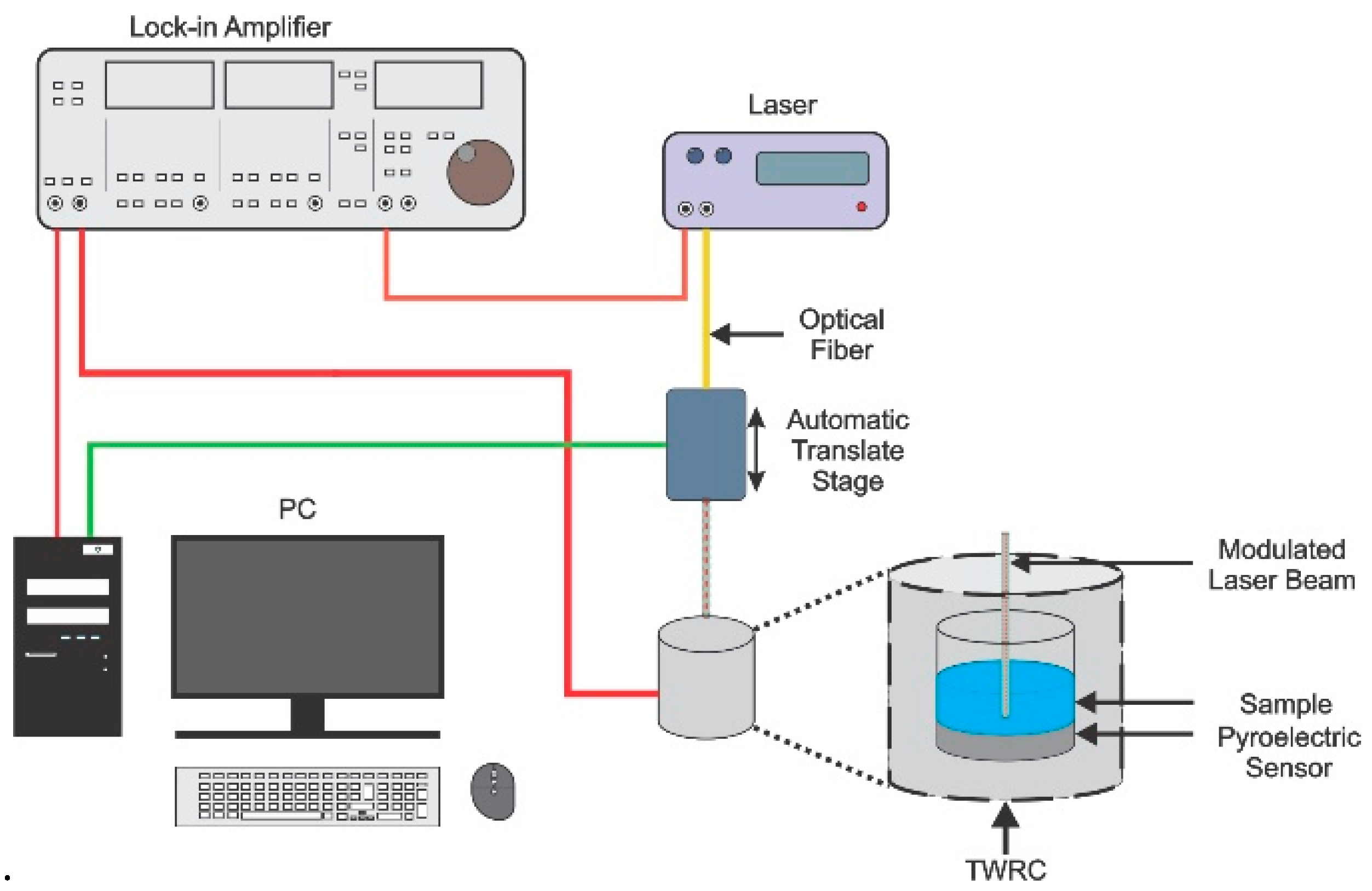
2.2. Thermal Effusivity
2.3. Thermal Diffusivity
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erhan, S.Z.; Sharma, B.K.; Perez, J.M. Oxidation and low temperature stability of vegetable oil-based lubricants. Ind. Crops Prod. 2006, 24, 292–299. [Google Scholar] [CrossRef]
- Malinowska, M. The effects of the addition of vegetable oil on the viscosity of used marine engine oil marinol RG 1240. New. Trends. Prod. Eng. 2018, 1, 479–485. [Google Scholar] [CrossRef][Green Version]
- Owuna, F.J. Stability of vegetable based oils used in the formulation of ecofriendly lubricants—A review. Egypt. J. Pet. 2020, 29, 251–256. [Google Scholar] [CrossRef]
- Jeevan, T.P.; Jayaram, S.R. Performance evaluation of jatropha and pongamia oil based environmentally friendly cutting fluids for turning AA 6061. Adv. Tribol. 2018, 2018, 2425619. [Google Scholar] [CrossRef]
- Dante, R.C. 2—Tribology of friction materials. In Handbook of Friction Materials and their Applications; Dante, R.C., Ed.; Woodhead Publishing: Boston, MA, USA, 2016; pp. 7–28. [Google Scholar] [CrossRef]
- Timilsina, G.R.; Shrestha, A. How much hope should we have for biofuels? Energy 2011, 36, 2055–2069. [Google Scholar] [CrossRef]
- Gallardo-Hernández, E.A.; Lara-Hernández, G.; Nieto-Camacho, F.; Domínguez-Pacheco, A.; Cruz-Orea, A.; Hernández-Aguilar, C.; Contreras-Gallegos, E.; Torres, M.V.; Flores-Cuautle, J.J.A. Thermal and tribological properties of jatropha oil as additive in commercial oil. Int. J. Thermophys. 2017, 38, 54. [Google Scholar] [CrossRef]
- Lara-Hernandez, G.; Benavides-Parra, J.C.; Cruz-Orea, A.; Contreras-Gallegos, E.; Hernández-Aguilar, C.; Flores-Cuautle, J.J.A. Thermal characterization of castor oil as additive in lubricant oil using photothermal techniques. Superf. Y. Vacio. 2018, 31, 6–9. [Google Scholar] [CrossRef]
- Lara-Hernandez, G.; Hernández-Aguilar, C.; Cruz-Orea, A.; Arias-Duque, N.P.; Wilches-Torres, M.A.; Flores-Cuautle, J.J.A. Wheat germ, mamey seed, walnut, coconut, and linseed oil thermal characterization using photothermal techniques. Rev. Mex. Fis. 2020, 66, 5. [Google Scholar] [CrossRef]
- Ustra, M.K.; Silva, J.R.F.; Ansolin, M.; Balen, M.; Cantelli, K.; Alkimim, I.P.; Mazutti, M.A.; Voll, F.A.P.; Cabral, V.F.; Cardozo-Filho, L.; et al. Effect of temperature and composition on density, viscosity and thermal conductivity of fatty acid methyl esters from soybean, castor and Jatropha curcas oils. J. Chem. Thermodyn. 2013, 58, 460–466. [Google Scholar] [CrossRef]
- Lara-Hernández, G.; Flores-Cuautle, J.J.A.; Hernandez-Aguilar, C.; Suaste-Gómez, E.; Cruz-Orea, A. Thermal properties of jojoba oil between 20 °C and 45 °C. Int. J. Thermophys. 2017, 38, 115. [Google Scholar] [CrossRef]
- Yang, L.; Mao, M.; Huang, J.; Ji, W. Enhancing the thermal conductivity of SAE 50 engine oil by adding zinc oxide nano-powder: An experimental study. Powder Technol. 2019, 356, 335–341. [Google Scholar] [CrossRef]
- Castro, M.P.P.; Andrade, A.A.; Franco, R.W.A.; Miranda, P.C.M.L.; Sthel, M.; Vargas, H.; Constantino, R.; Baesso, M.L. Thermal properties measurements in biodiesel oils using photothermal techniques. Chem. Phys. Lett. 2005, 411, 18–22. [Google Scholar] [CrossRef]
- Bisht, R.P.S.; Sivasankaran, G.A.; Bhatia, V.K. Additive properties of jojoba oil for lubricating oil formulations. Wear 1993, 161, 193–197. [Google Scholar] [CrossRef]
- Choi, U.S.; Ahn, B.G.; Kwon, O.K.; Chun, Y.J. Tribological behavior of some antiwear additives in vegetable oils. Tribol. Int. 1997, 30, 677–683. [Google Scholar] [CrossRef]
- Glushkov, D.; Nyashina, G.; Medvedev, V.; Vershinina, K. Relative environmental, economic, and energy performance indicators of fuel compositions with biomass. Appl. Sci. 2020, 10, 2092. [Google Scholar] [CrossRef]
- Niki, E.; Abe, K. CHAPTER 1 Vitamin E: Structure, Properties and Functions. In Vitamin E: Chemistry and Nutritional Benefits, 1st ed.; The Royal Society of Chemistry: London, UK, 2019; pp. 1–11. [Google Scholar] [CrossRef]
- Melhaoui, R.; Fauconnier, M.-L.; Sindic, M.; Addi, M.; Mihamou, A.; Serghini-Caid, H.; Elamrani, A. Tocopherol analysis of almond oils produced in eastern Morocco. In Proceedings of the 23rd National Symposium for Applied Biological Sciences, Brussels, Belgium, 8 February 2018; pp. 75–77. [Google Scholar] [CrossRef]
- Putt, E.D. Chapter 1: Early history of sunflower. In Sunflower Technology and Production, 2nd ed.; Albert, A.S., Ed.; AMA/CSSA/SSSA: Madison, WI, USA, 1997; pp. 1–19. [Google Scholar] [CrossRef]
- Tabio-García, D.; Díaz-Domínguez, Y.; Rondón-Macias, M.; Fernández-Santana, E.; Piloto-Rodríguez, R. Extracción de aceites de origen vegetal. Available online: https://www.researchgate.net/publication/317007345_Extraccion_de_aceites_de_origen_vegetal (accessed on 8 August 2021).
- Rashid, U.; Anwar, F.; Moser, B.R.; Ashraf, S. Production of sunflower oil methyl esters by optimized alkali-catalyzed methanolysis. Biomass Bioenergy 2008, 32, 1202–1205. [Google Scholar] [CrossRef]
- Atapour, M.; Kariminia, H.-R. Characterization and transesterification of Iranian bitter almond oil for biodiesel production. Appl. Energy 2011, 88, 2377–2381. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Pardal, A.; Martínez, G. Rape oil transesterification over heterogeneous catalysts. Fuel Process. Technol. 2010, 91, 1530–1536. [Google Scholar] [CrossRef]
- Alcalde, M.T.; del Pozo, A. Aceite de Argán. Offarm 2010, 29, 92–93. [Google Scholar]
- Kuliev, R.S.; Kuliev, F.A.; Mutalibova, A.A.; Kulieva, S.R. Improving the antioxidant and anticorrosion properties of vegetable oils. Chem. Technol. Fuels Oils 2006, 42, 55–59. [Google Scholar] [CrossRef]
- Mujeeda, B.; Prasad, N.; Siddaramaiah. Effect of antioxidant on thermal stability of vegetable oils by using ultrasonic studies. Int. Food. Res. J. 2016, 23, 528–536. [Google Scholar]
- Frandas, A.; Bicanic, D. Thermal properties of fruit juices as a function of concentration and temperature determined using the photopyroelectric (PPE) method. J. Sci. Food Agric. 1999, 79, 1361–1366. [Google Scholar] [CrossRef]
- Mexicana de Lubricantes. Available online: http://www.mexicanadelubricantes.com.mx/web/prodficha.php?id=276577 (accessed on 19 July 2021).
- Lara-Hernandez, G.; Cruz-Orea, A.; Suaste-Gomez, E.; Flores-Cuautle, J.J.A. Comparative performance of PLZT and PVDF pyroelectric sensors used to the thermal characterization of liquid samples. Adv. Mater. Sci. Eng. 2013, 2013, 5. [Google Scholar] [CrossRef]
- Balderas-López, J.A.; Mandelis, A. Photopyroelectric spectroscopy of pure fluids and liquid mixtures: Foundations and state-of-the-art applications. Int. J. Thermophys. 2020, 41, 78. [Google Scholar] [CrossRef]
- Balderas-Lopez, J.; Monsivais-Alvarado, T.; Galvez-Coyt, G.; Muñoz-Diosdado, A.; Diaz-Reyes, J. Thermal characterization of vegetable oils by means of photoacoustic techniques. Rev. Mex. Fis. 2013, 59, 5. [Google Scholar]
- Carbajal-Valdez, R.; Jiménez-Pérez, J.L.; Cruz-Orea, A.; Correa-Pacheco, Z.N.; Alvarado-Noguez, M.L.; Romero-Ibarra, I.C.; Mendoza-Alvarez, J.G. Thermal properties of centrifuged oils measured by alternative photothermal techniques. Thermochim. Acta 2017, 657, 66–71. [Google Scholar] [CrossRef]
- Balderas-Lopez, J.A. Measurements of the termal effusivity of transparent liquids by means of a photopyroelectric technique. Rev. Mex. Fis. 2003, 49, 5. [Google Scholar]
- Samyn, P.; Schoukens, G.; Vonck, L.; Stanssens, D.; Van den Abbeele, H. Quality of brazilian vegetable oils evaluated by (modulated) differential scanning calorimetry. J. Therm. Anal. Calorim. 2012, 110, 1353–1365. [Google Scholar] [CrossRef]
- Zahra, Y.-N.; Zahra, P.-V. A study on the specifications of cold pressed colza oil. Recent Patents on Food, Nutr. Agric. 2015, 7, 47–52. [Google Scholar] [CrossRef]



| Sample | Slope | Intercept | R2 |
|---|---|---|---|
| Sunflower | −1.08 | 7.25 | 0.32 |
| Almond | −4.54 | 8.02 | 0.93 |
| Rapeseed | 3 | 7.12 | 1 |
| Vegetable Oil to Motor Oil | Effusivity | Diffusivity | Conductivity (Calculated) | ||
|---|---|---|---|---|---|
| VO% | MO% | ||||
| Motor Oil | - | 100 | 4.80 ± 0.04 | 8.88 ± 0.04 | 1.43 ± 0.01 |
| Sunflower | 5 | 95 | 5.20 ± 0.04 | 7.14 ± 0.01 | 1.39 ± 0.01 |
| 10 | 90 | 4.63 ± 0.01 | 7.32 ± 0.02 | 1.25 ± 0.01 | |
| 15 | 85 | 4.77 ± 0.02 | 7.04 ± 0.02 | 1.26 ± 0.01 | |
| 20 | 80 | 4.81 ± 0.02 | 7.02 ± 0.02 | 1.27 ± 0.01 | |
| 100 | - | 5.75 ± 0.07 | 5.75 ± 0.05 | 1.38 ± 0.02 | |
| Almonds | 5 | 95 | 5.04 ± 0.08 | 7.81 ± 0.02 | 1.41 ± 0.02 |
| 10 | 90 | 5.05 ± 0.08 | 7.50 ± 0.02 | 1.38 ± 0.02 | |
| 15 | 85 | 5.00 ± 0.09 | 7.50 ± 0.01 | 1.37 ± 0.02 | |
| 20 | 80 | 4.86 ± 0.09 | 7.08 ± 0.02 | 1.29 ± 0.02 | |
| 100 | - | 5.72 ± 0.03 | 8.86 ± 0.02 | 1.70 ± 0.02 | |
| Rapeseed | 5 | 95 | 4.93 ± 0.10 | 7.27 ± 0.03 | 1.33 ± 0.02 |
| 10 | 90 | 4.95 ± 0.07 | 7.42 ± 0.02 | 1.35 ± 0.02 | |
| 15 | 85 | 4.92 ± 0.09 | 7.57 ± 0.01 | 1.35 ± 0.02 | |
| 20 | 80 | 4.55 ± 0.02 | 7.72 ± 0.03 | 1.26 ± 0.01 | |
| 100 | - | 5.58 ± 0.03 | 8.54 ± 0.02 | 1.63 ± 0.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores Cuautle, J.d.J.A.; Sandoval González, O.O.; González Morán, C.O.; Rodríguez Jarquin, J.P.; Trujillo Romero, C.J.; Lara Hernandez, G. Effect of Sunflower, Almond, and Rapeseed Oils as Additives on Thermal Properties of a Machinery Oil. Appl. Sci. 2021, 11, 7441. https://doi.org/10.3390/app11167441
Flores Cuautle JdJA, Sandoval González OO, González Morán CO, Rodríguez Jarquin JP, Trujillo Romero CJ, Lara Hernandez G. Effect of Sunflower, Almond, and Rapeseed Oils as Additives on Thermal Properties of a Machinery Oil. Applied Sciences. 2021; 11(16):7441. https://doi.org/10.3390/app11167441
Chicago/Turabian StyleFlores Cuautle, José de Jesús Agustín, Oscar Osvaldo Sandoval González, Carlos Omar González Morán, José Pastor Rodríguez Jarquin, Citlalli Jessica Trujillo Romero, and Gemima Lara Hernandez. 2021. "Effect of Sunflower, Almond, and Rapeseed Oils as Additives on Thermal Properties of a Machinery Oil" Applied Sciences 11, no. 16: 7441. https://doi.org/10.3390/app11167441
APA StyleFlores Cuautle, J. d. J. A., Sandoval González, O. O., González Morán, C. O., Rodríguez Jarquin, J. P., Trujillo Romero, C. J., & Lara Hernandez, G. (2021). Effect of Sunflower, Almond, and Rapeseed Oils as Additives on Thermal Properties of a Machinery Oil. Applied Sciences, 11(16), 7441. https://doi.org/10.3390/app11167441









