1. Introduction
The concept of biomaterials, which is widely used nowadays, could include everything from metal to ceramic, plastic, and any other material that is well tolerated by the human body. In the field of fixed orthodontics, brackets are used very often during the treatment. These appliances are mostly made of metal alloys, polycarbonate, ceramic, zirconia [
1,
2], different kind of wires, and various bonding materials. Although the materials from which the brackets were made have significantly evolved, the metallic ones are still widely used due to their mechanical and chemical properties [
3]. Intense research over the years on these materials has led to a great diversity of ferroalloys used for brackets, wires, bands, and other orthodontic accessories, such as cobalt–chromium alloy (Cr–Co), stainless steel, and titanium alloys, all of which have nickel (Ni) as one of the main components [
4].
Ceramic brackets began to be used in the late 1980s, and nowadays, they are commonly used for orthodontic fixed therapy [
5]. They were designed in an attempt to satisfy the esthetic requirements of the patients with fixed orthodontic appliances. These translucent brackets are made from aluminum oxide (alumina) particles and are available in the monocrystalline and polycrystalline form [
5]. Aluminum oxide is an inert crystalline powder, insoluble in water and odorless [
6].
Aluminum oxide has a chemical formula Al
2O
3. It is amphoteric in nature and has been used in various chemical, industrial, and commercial applications. It is considered an indirect additive used in food contact substances by the FDA [
6]. The crystal structure of aluminum oxide is illustrative of the principles involved with ceramics having a substantial ionic bonding character. The crystal structure [
7] consists of a nearly hcp arrangement of the larger oxygen anions (O
2−), with the smaller aluminum cations (Al
3+) located in two-thirds of the octahedral interstitial sites in the hcp structure. The layers in the structure provide the maximum separation of the Al
3+ ions. These octahedral sites have sixfold coordination, i.e., each aluminum ion is surrounded by six oxygen ions. The crystal structure is determined by the ratio of the radii of the aluminum and oxygen ions, and the requirement of an electrically neutral unit cell.
Both physical and chemical characteristics of aluminum oxide products vary depending on the processing method that leads to changes in the crystal structure. The variety formed at very high temperatures is quite inert chemically [
5,
6].
Concern about enamel damage occurs especially when ceramic brackets are used. The first generations of ceramic brackets resulted in a major alteration of the enamel. Thus far, they have undergone significant improvements. The purpose of debracketing is to remove brackets and adhesive material from the tooth, giving it the initial appearance and contour, and minimizing the loss of iatrogenic hard tissue during this maneuver [
8]. The manufacturers of the new generations of ceramic brackets assure that their use in orthodontic treatment is safe, due to the design of the base. Technological evolution allows researchers to quantitatively measure the surface of the enamel, which can be reduced after debracketing. The use of metal brackets registers the presence of the remaining adhesive material and leads to a loss of hard tooth substance of 20–50 μm after their removal [
6,
8].
The objective of this in vitro study was to compare the changes in the enamel surface following the removal of ceramic and metal brackets, namely, the appearance of new cracks or the deepening of the existing ones. Quantitative analysis was performed using dispersive X-ray spectroscopy in the base of the brackets, and the ARI
tooth score [
9] is retained.
2. Materials and Methods
For analysis, a number of 174 freshly extracted premolars for orthodontic purposes were collected. After extraction, teeth were kept at room temperature in 10% formalin solution for a period of 7 days, which was subsequently replaced by saline solution (NaCl 0.9%) and was changed daily for 10 more days. After storage, teeth were examined and inspected. Teeth that showed signs of enamel degradation, caries, tartar, or fillings on the vestibular surface, as well as fluorosis or hypoplasia, were excluded from the study. On the subsequent stage of the study, 147 premolars with intact vestibular surfaces were analyzed. Teeth from the study group underwent periodontal ligament removal using a Gracey™ 5–6 curette and then were polished with Depural Neo™ Spofa Dental, Jičín, Czech Republic, and stored back in the saline solution.
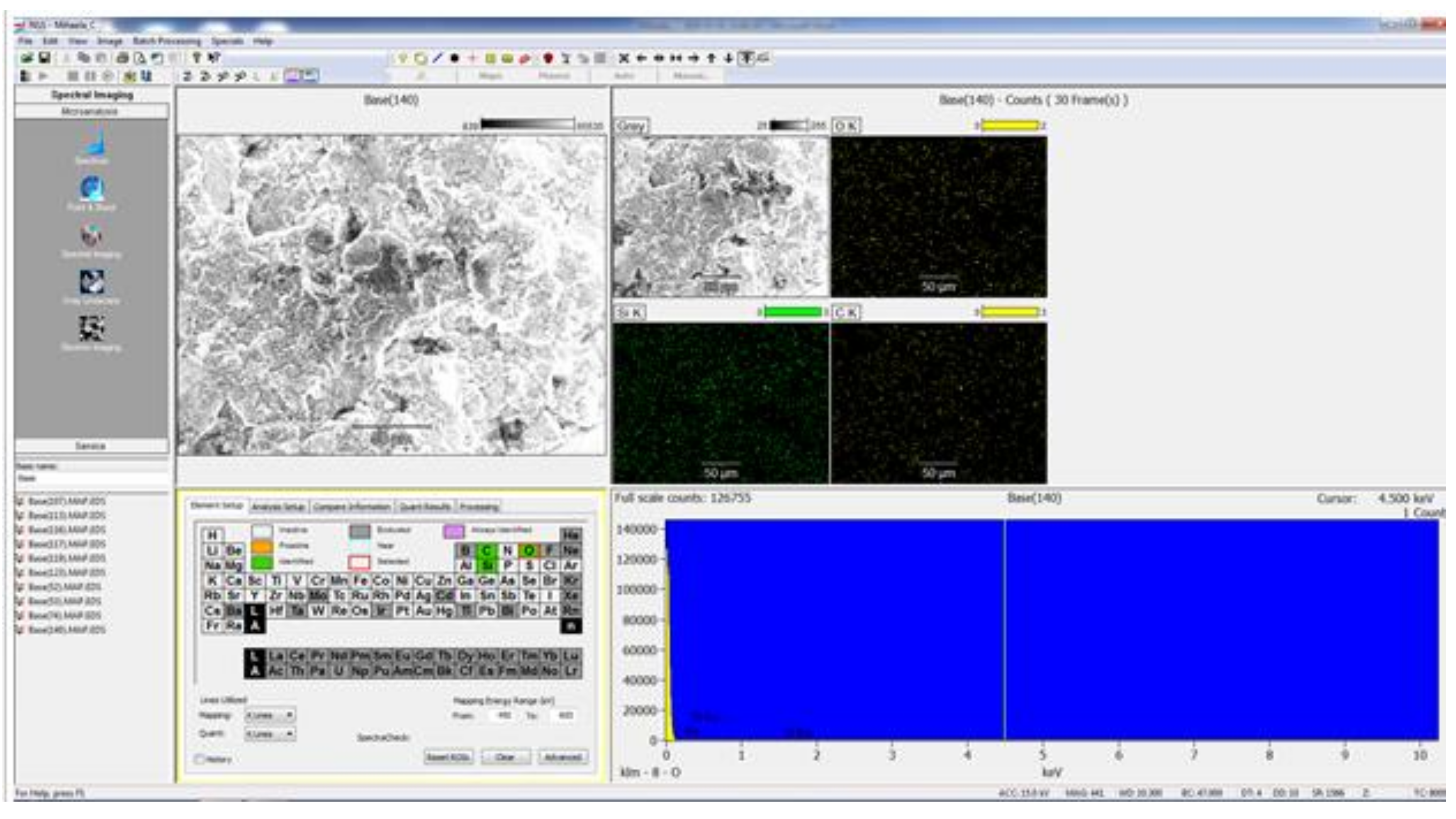
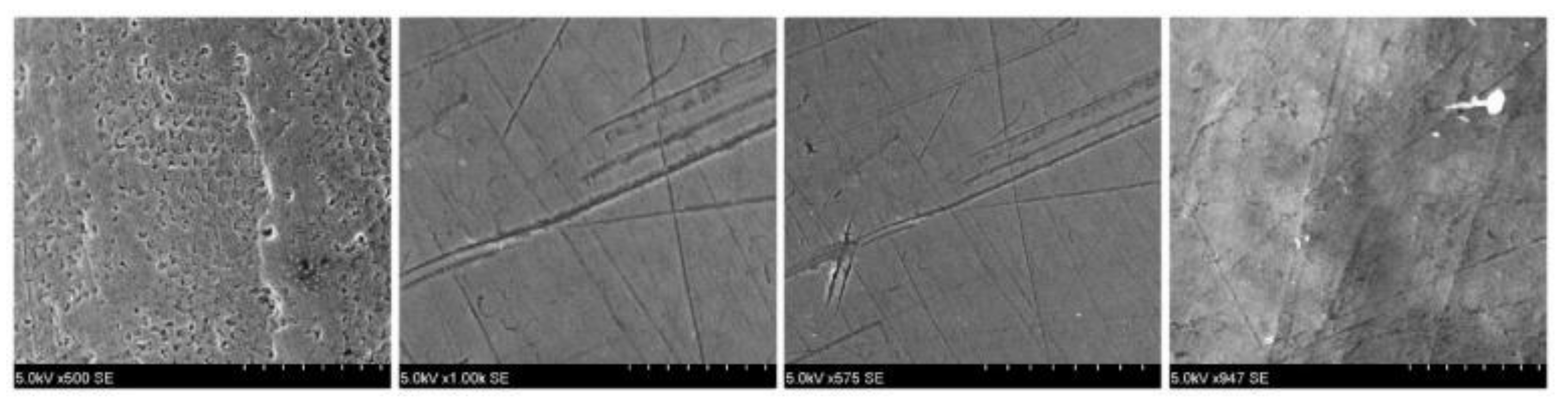
Later in the study, an S-3400N electron microscope, Hitachi, Chiyoda, Tōkyō, Japan, was used to analyze the enamel surface pretreatment (
Figure 1). Data were recorded and used as a benchmark later in the study for comparison with the images obtained post treatment.
The samples did not require prior preparation because the microscope has a vacuum function that allows the observation of nonconductive specimens at a working distance of 9 mm, without the need to apply any conductive layer. The acceleration voltage of the microscope was operated at 15 kV. The only intervention on the enamel surface was to draw an indicative perimeter of the future base of the bracket for an easier investigation of the surface. For this purpose, we used a round disk of 0.10 mm diameter.
After the preliminary analysis using SEM, the study group was divided into 2 subgroups as follows:
- -
Study sub-group 1 consisted of 72 premolars, in which we applied metal Gemini™ brackets (3M Unitec™, USA) with the following chemical composition: Ni 83.98%, Si 6.46%, Fe 5.90%, and Cr 3.52%;
- -
Study subgroup 2 consisted of 75 premolars, in which we applied ceramic brackets Forestadent™, Germany (Al2O3).
The enamel’s surface was dried for 10 s using the air spray, and 37% phosphoric acid (Unitek Etching Gel System from 3M Unitek) was applied for 30 s. The acid was removed by a jet of water applied for 10 s, followed by a jet of air from the unit’s spray for 10 s.To promote adhesion, a primer Transbond XT™ from 3M Unitek was used; the primer was applied with a micro brush by circular movements on the previously prepared surface then it was photo-polymerized for 20 s by using a high-power led lamp Elipar S10™, 3M ESPE, with the tip of the lamp curing tube placed 1 mm away from the vestibular surface of the tooth. The bracket’s base was loaded with the adhesive paste Transbond ™ from 3M Unitek, then placed very similar to and following the positioning bonding rules described by Armstrong et al. [
10] in the center of the vestibular surface. After correct positioning, brackets were pressed to 300 g using an orthodontic dynamometer. Excess composite resin was removed prior to light curing with a dental probe. The polymerization was performed mesially and distally from the bracket for 10 s on each side. After 24 h, the brackets were removed using straight take-off pliers and acting directly on the brackets’ bases. The adhesive material was removed with a high-speed multi-laminated tungsten carbide burr. The cleaning process was continued until the adhesive material was no longer visible to the visual inspection and the enamel became smooth, without roughness detectable on examination with the dental probe.
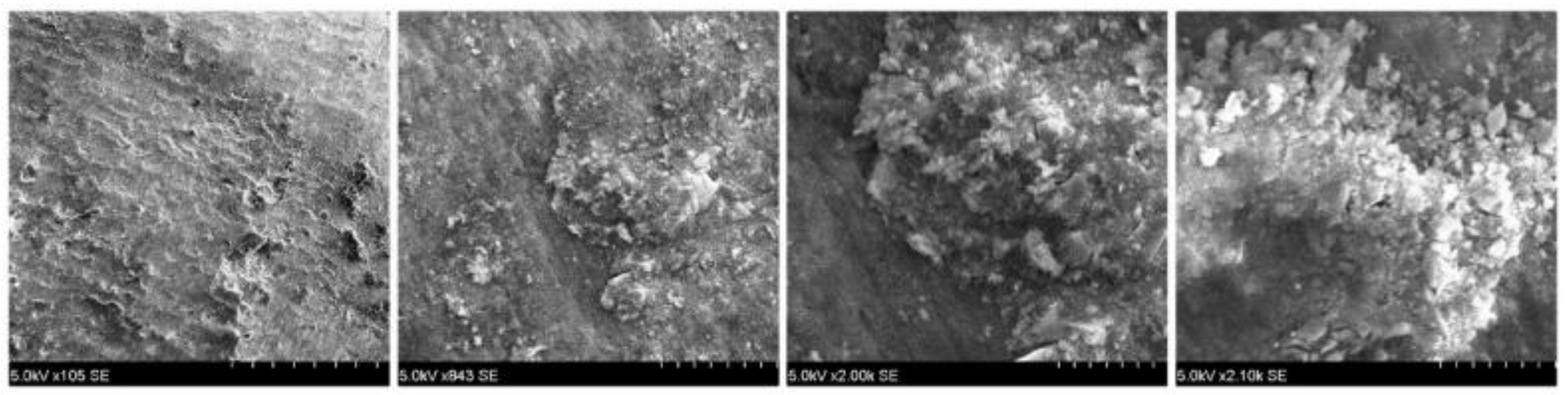
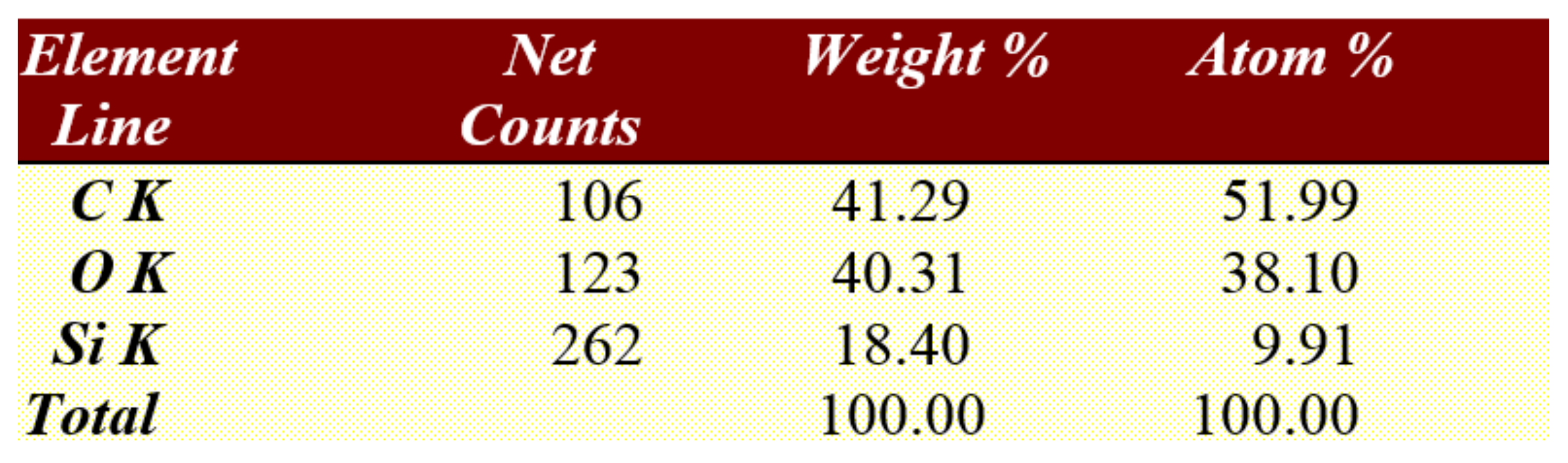
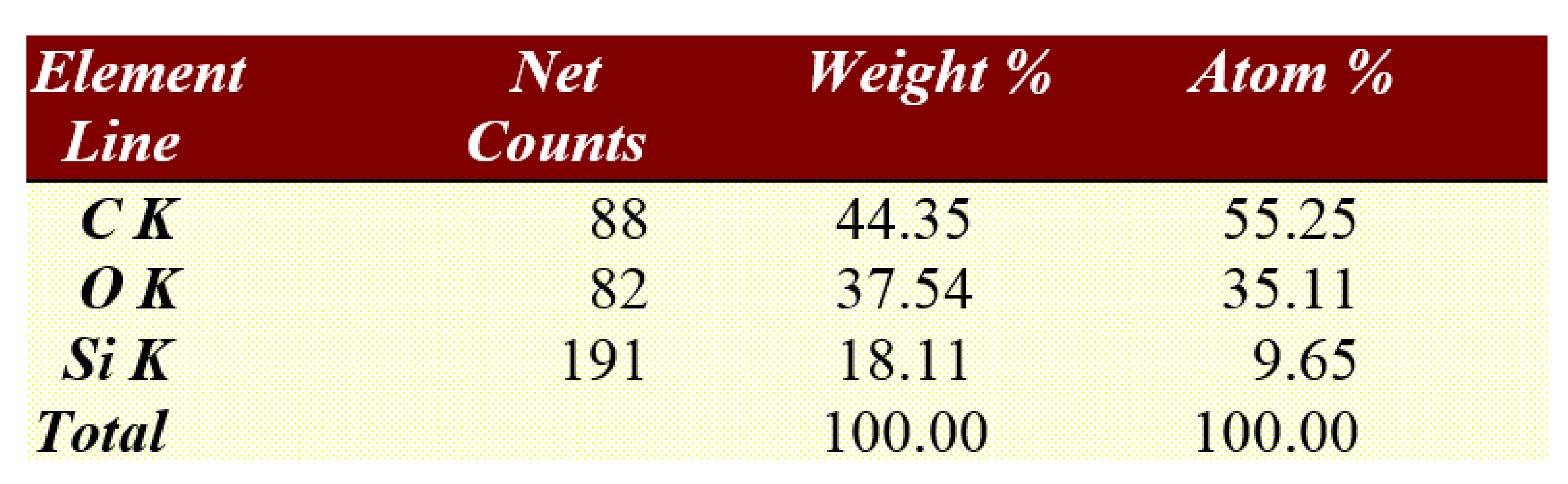
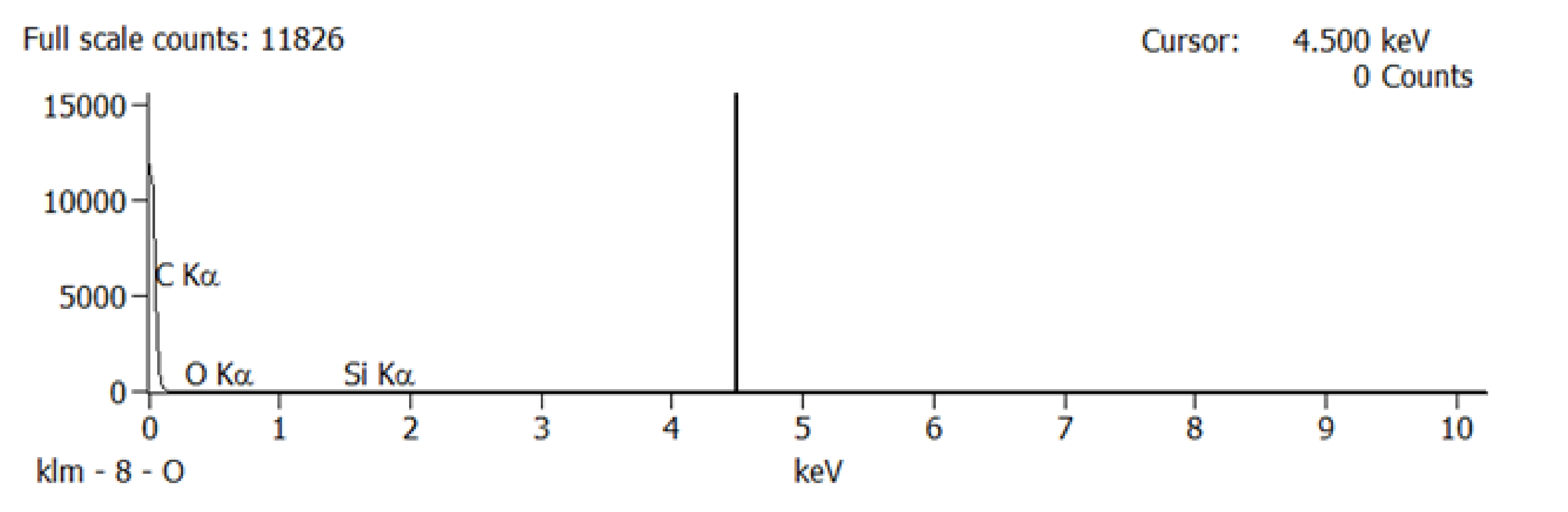
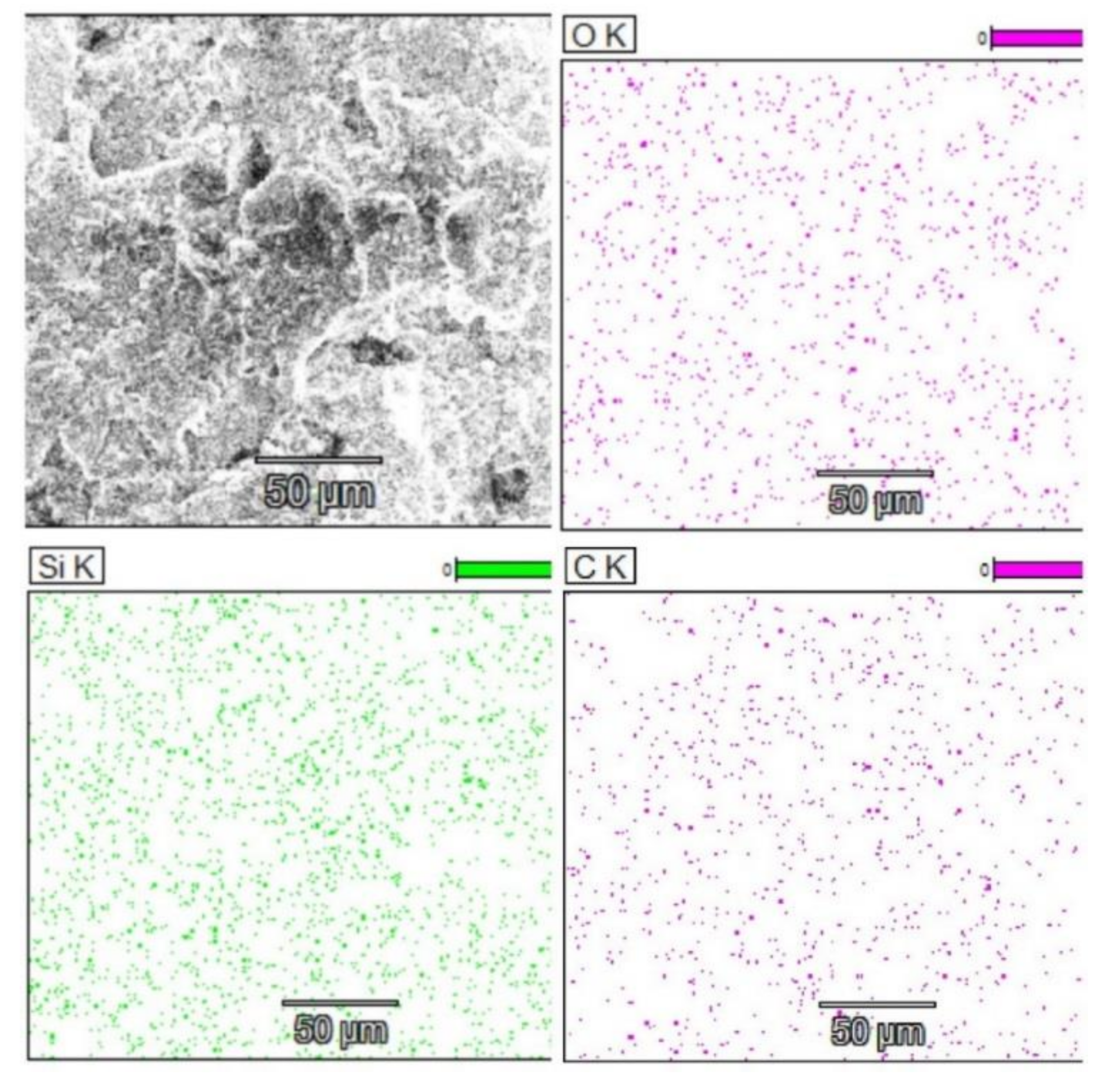
After the brackets were removed, the teeth were stored in saline solution at room temperature until the second analysis at SEM, when the samples were analyzed at the same parameters as before treatment, and possible irregularities and cracks in the enamel surface were monitored. In the next step of the study a quantitative analysis, the energy-dispersive X-ray spectroscopy (EDX) was also performed on the entire surface of the brackets’ soles to detect any traces of enamel. For analysis, we used APEX™, EDAX, Unterschleissheim Germany software for Windows™ (
Figure 2) that helped us identify the chemical elements and provided information on the quantitative composition of the sample.
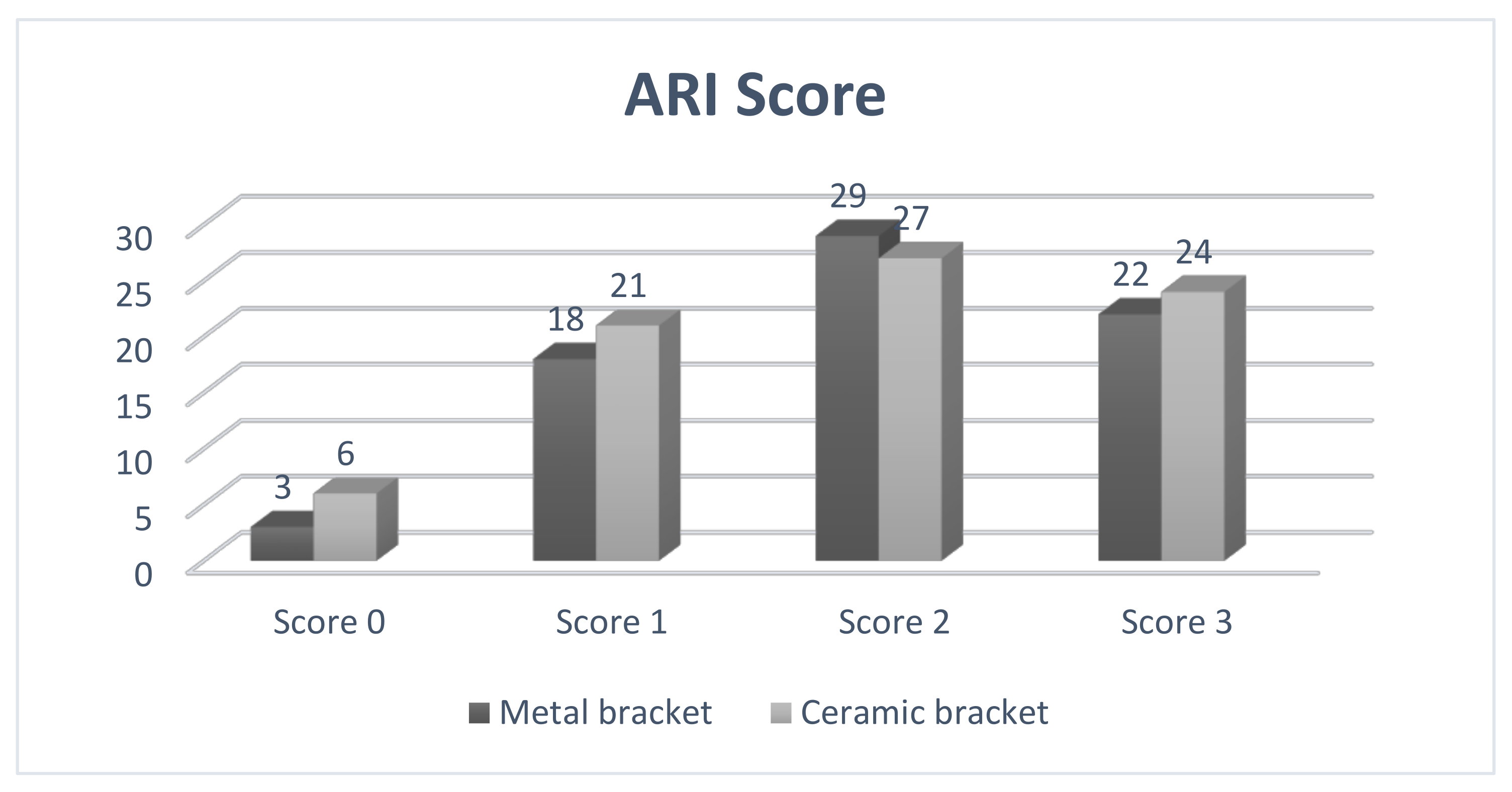
ARI Score
After the second SEM analysis was performed, the adhesive remnant index (ARI) was evaluated. ARI score records the amount of residual adhesive located on the tooth or bracket sole. For analysis, we chose to evaluate the amount of adhesive present on the enamel surface after removing the brackets using the values that were proposed by Artün and Bergland in 1984 [
11].
Score 0—without adhesive on the tooth surface;
Score 1—less than 50% adhesive present on the tooth surface;
Score 2—more than 50% adhesive present on the tooth surface;
Score 3—all the adhesive present on the tooth surface.
Statistical processing was performed using GraphPad Prism™ V6.01 software for Windows™. The D’Agostino and Pearson omnibus normality test was used to determine the normality of the data. The chosen significance threshold was alpha = 0.05, considering p significant when p <0.05.
4. Discussion
In this paper, the debracketing technique was used by positioning the pliers at the base of the bracket and the presence of enamel was not identified either at the level of the ceramic or metal brackets.
Artun and Bergland found that all brackets analyzed with EDX contain calcium, and by default enamel, in the form of a thin layer, fragments, or even in those where no trace of residual material is visible. The debracketing was performed in this experiment by positioning the pliers at the level of the wings [
11]. Further studies that analyzed the presence of calcium on ceramic, metal (SS), and self-ligating brackets confirm that the highest amount of calcium was recorded in ceramic (4%), followed by metal (1.44%), and finally, self-ligating (1.42%). In this study, the debracketing was performed by positioning the pliers at the base of the bracket [
12,
13].
Furthermore, in another study comparing the two methods of debracketing metal brackets, i.e., wings and soles, it was found that the ARI
bracket score is similar (WM = 66.7%, BM = 68.7%), which assumes that the rupture occurred at either the adhesive–adhesive or adhesive–enamel interface [
14]. The two debracketing techniques were expected to result in different ARI scores. However, the ARI score and the presence of calcium were similar in the two methods, despite the difference in force applied, which was previously measured [
15]. The explanation for this dichotomy is that the crack is probably initiated at the level of the adhesive layer and propagated at the bracket–adhesive or adhesive–enamel interface. It should also be taken into account the fact that metal brackets cast with light-curable material have an initially lower adhesion strength at the bracket–adhesive interface than the enamel–adhesive interface because the light-cured resin under the sole of the bracket has reflected light. This is supported by a short-term study that showed that the rupture occurs at the bracket–adhesive interface [
16]. Further recent studies [
14,
15] demonstrated that after a longer period of time, 24 months, the composite is completely polymerized with a strong adhesion force. Another aspect to consider is that the enamel under the sole of the bracket may be affected by decalcification resulting from food debris and poor hygiene that damages the enamel–adhesive bond. The authors claimed that regardless of the method of debracketing chosen, the rupture occurred at the adhesive–enamel interface [
15,
16]. Türkkahraman et al. [
17] reported that the biggest part of brackets’ failures occurred within the adhesive layer.
An analysis of photoelastic stress showed that the force applied to the outer wings of the bracket transfers less stress to the enamel, compared to the force applied to the base of the bracket and the adhesion area, which creates a region of stress concentration in the enamel, thus leading to a separation at the adhesive–enamel interface. Nevertheless, Brosh et al. [
14] did not find any significant difference between the two possibilities of applying the forceps (on the wings/at the base of the bracket) in terms of calcium score.
In this situation, a question arises: is there a correlation between the ARI score and the presence of calcium on the sole of the bracket?
One study claims that a thin layer of enamel is removed when the bracket is cut out [
18]. This is due to acid etching and the fact that the adhesive material penetrates the micropores produced by this process. Most likely, the partially demineralized enamel around the pores detaches more easily from the tooth and remains adhered to the adhesive material during debracketing. The study was performed in vivo with a treatment duration of 24 months +/− 3 months. The bracket–adhesive interface is considered to be the most favorable for debracketing, thus leaving most of the adhesive on the tooth surface, as happens at an ARI
tooth score of 2 and 3 [
19,
20,
21].
Because partial demineralization of the enamel may occur, some authors suggested the use of nano-hydroxyapatite in demineralized enamel before orthodontic bonding of brackets [
22]. Scribante et al. [
22], in their study, showed that a great visual remineralization effect can be noticed already after the second application cycle of biomimetic nano-hydroxyapatite. On the other hand, there are studies that demonstrated that the use of pre-orthodontic treatment fluoride lacquer or varnish develops lower bond strengths, which is likely to result in a higher frequency of premature detachment of the bracket [
23]. More recently, Bącela J et al. [
24], in their study regarding coating the orthodontic devices, as well as modifying their surface with additives, showed that this method improves their mechanical properties and can minimize bacterial adhesion on the surface. Appropriate coating techniques enable the reduction in corrosivity, wear and tear, and its delamination. The material used in the coating may accelerate orthodontic treatment. The analysis of additives such as TiO2, ZnO, Ag, and silver nanoparticles, as well as uncoated arches, can compare antimicrobial activity and environmental resistance. The layer thickness of coatings applied to the archwires is also relevant for the antibacterial effect.
After bracketing, it is recommended to preserve the integrity of the enamel layer; in the meantime, the persistence of the adhesive material on the tooth is an objective to be pursued. This goal was achieved in this study, as the ARI scores obtained were 2 and 3 for most of the ceramic and metal brackets.
4.1. Roughness
The degree of roughness depends on the tools used to remove the adhesive material.
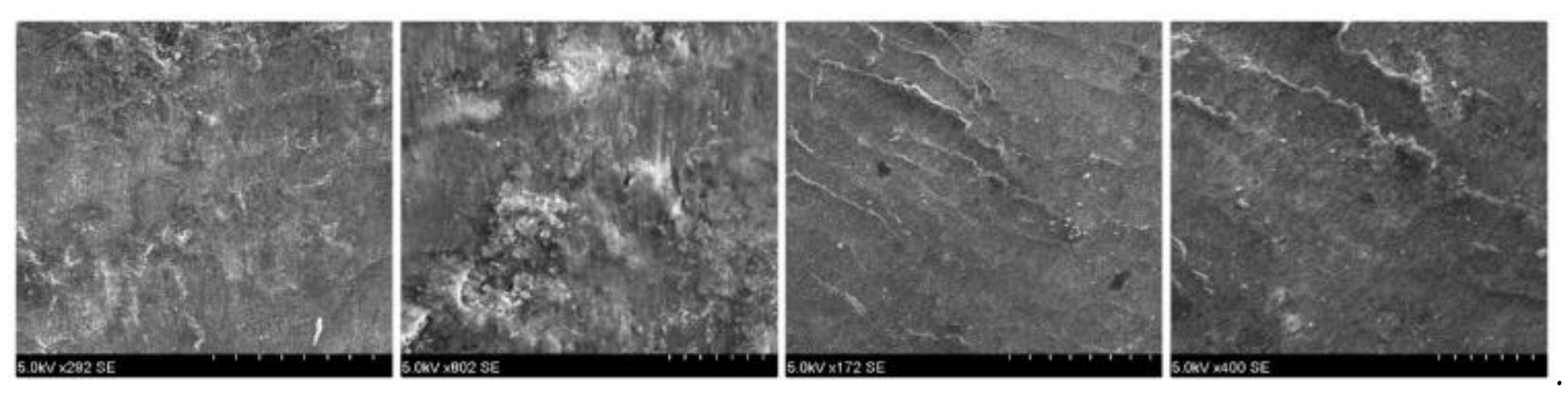
It has been shown that by assessing the roughness before and after debracketing, it is increased in all cases at the end of treatment [
25]. Goel et al. claim that using the Sof-Lex disc and the self-etching primer achieves a degree of roughness in the enamel surface similar to the pretreated one; this is followed by the combination of using the self-etching primer and the tungsten carbide burr [
19].
Further studies state that the best results were obtained with the help of the tungsten carbide burr operated at low speed. The study compared low-speed tungsten carbide burr, tungsten carbide burr, and ultrafine diamond burr, both at high speed, together with cooling and Er: YAG laser instrument, with a wavelength of 2940 nm [
20].
Another recent study reports that the most efficient is the high-speed multifilament tungsten carbide cutter, followed by the low-speed multifilament tungsten carbide cutter. The fiberglass cutter is inefficient, leaving a large amount of adhesive behind [
21].
4.2. Integrity of Brackets after Debonding
Over 60% of the ceramic brackets were broken during debonding, while the metal brackets remained intact. This is due to the increased adhesion force of the ceramic brackets. The adhesion force of metal brackets is lower than that of ceramic brackets with mechanical retention, which indicates a higher risk of enamel degradation while debracketing [
26].
The study conducted by Ciocan et al. stated that the increased adhesion strength of ceramic brackets may be due to the design of the bracket sole, which provides strong bonding and requires special debracketing procedures recommended by the manufacturer [
27].
Several studies indicated that the design of the base can influence the adhesion force and can play a role in the absorption of stress by the enamel and producing iatrogenic effects at this level [
28,
29].
When evaluating the results of these studies, it should be borne in mind that clinical debonding may differ from in vitro conditions. Debracketing forces can be applied slightly differently, while temperature, humidity, and other oral factors can affect the adhesion force and therefore damage to the enamel. Patients should be informed of the possible side effects of the removal of the brackets on enamel [
29]. The extracted teeth used during the studies may have undetectable cracks in the surface, due to forceps, amplifying the degradation of the enamel. However, the results of in vitro studies remain very relevant [
8].
It should be noted that in this study, the teeth were subjected to a pretreatment analysis, the debracketing technique consisted in applying the pliers at the base of the bracket, and the study was performed in vitro. These are the factors that contribute to the identification of the enamel on the sole of the bracket in the conditions of the examination of the samples collected from the patients. Other in vitro studies performed debracketing after 24 h [
16,
30] as we did in this scientific paper, and the difficulty with which the brackets were removed, both metal and ceramic, did not confirm the hypothesis of insufficient polymerization of the adhesive material.
5. Conclusions
Unfortunately, debracketing may lead to bracket fracture. Bracket fragments may detach (loose fragments) or remain attached to the enamel surface. Low-speed or high-speed grinding of the bracket fragments with no water coolant may bring forth permanent damage and necrosis of the dental pulps. Therefore, water cooling is absolutely necessary during the grinding/removal of ceramic bracket fragments. Analyzing the pretreatment and posttreatment results, no crack was observed in the enamel, only increased roughness in both types of brackets. The sole of the bracket did not show traces of calcium to confirm the loss of substance.
The persistence of the adhesive material on the tooth is a goal to be pursued, and the placement of the straight orthodontic plier at the base of the bracket makes this possible, thus preserving the integrity of the enamel. Although more brittle and prone to breakage, ceramic brackets did not favor the loss of enamel, similar to the metallic ones.