Quantitative Monitoring of Dynamic Blood Flows Using Coflowing Laminar Streams in a Sensorless Approach
Abstract
1. Introduction
2. Materials and Methods
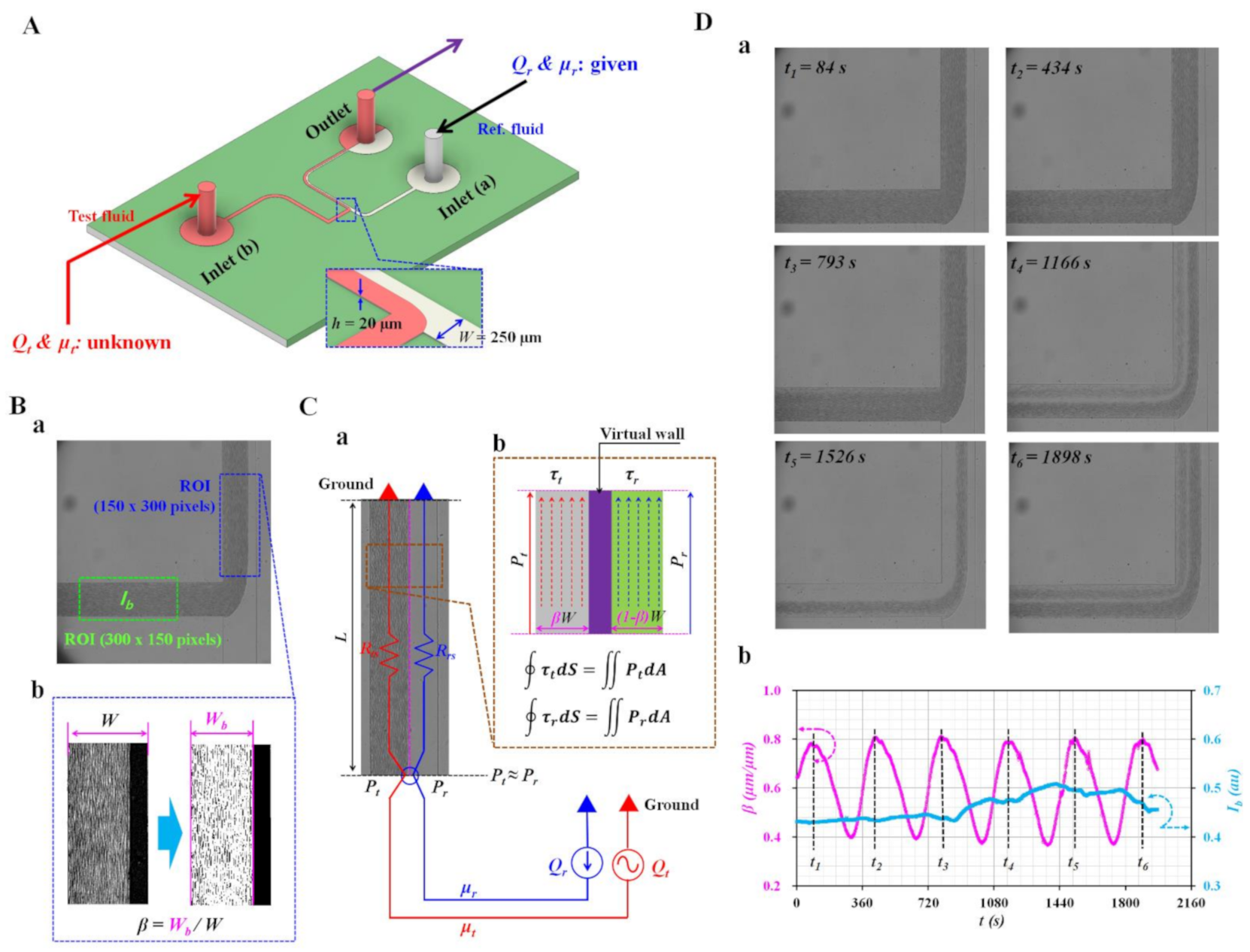
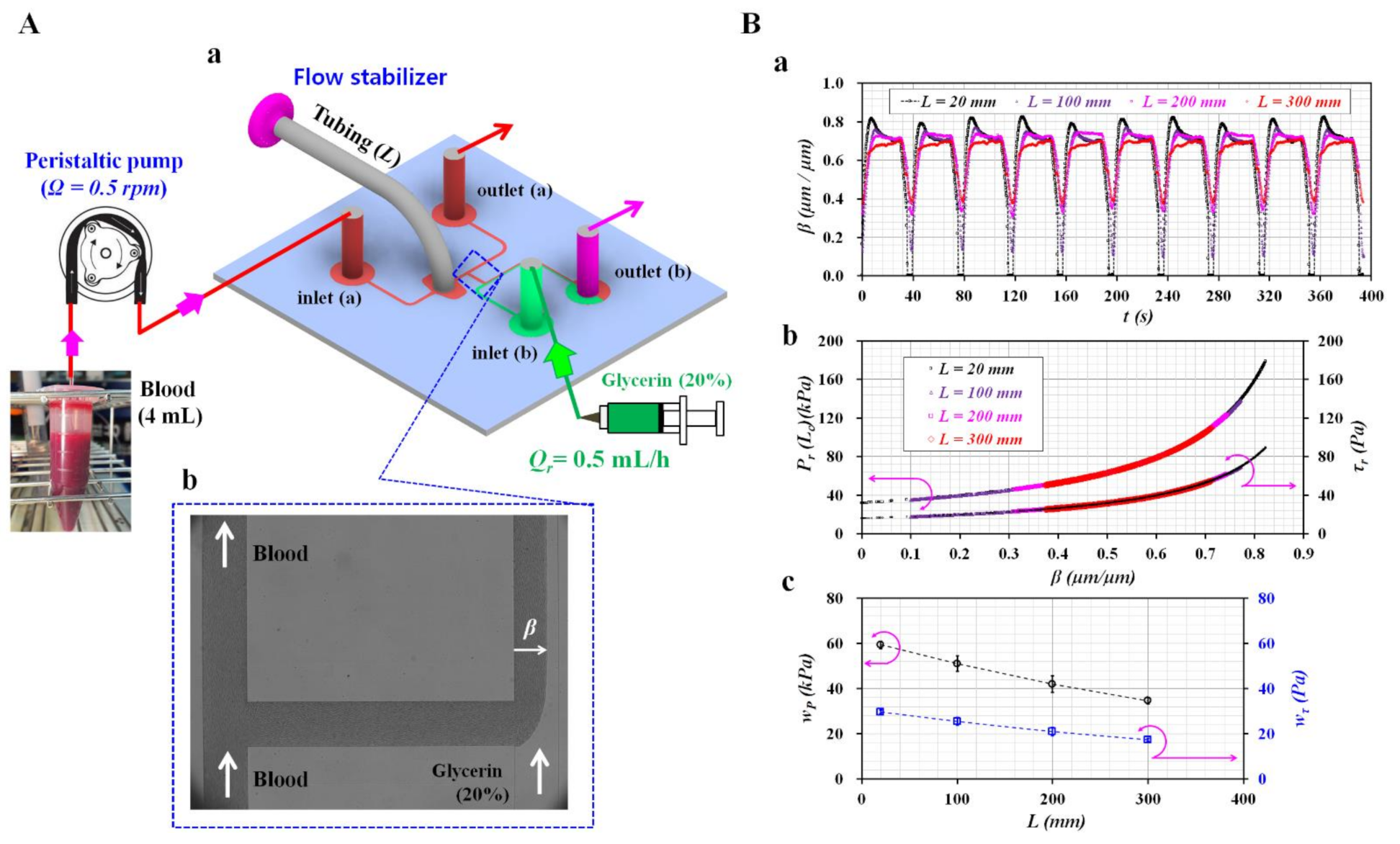
2.1. Microfluidic Device and Experimental Setup
2.2. Quantification of Image Intensity and Interfacial Location in Coflowing Channel
2.3. Mathematical Representation of Pressure and Shear Stress of Each Stream in Coflowing Channel
2.4. Quantification of Interface (β) and Intensity (Ib) as Preliminary Study
2.5. Suspended Blood Preparation Procedure
3. Results and Discussion
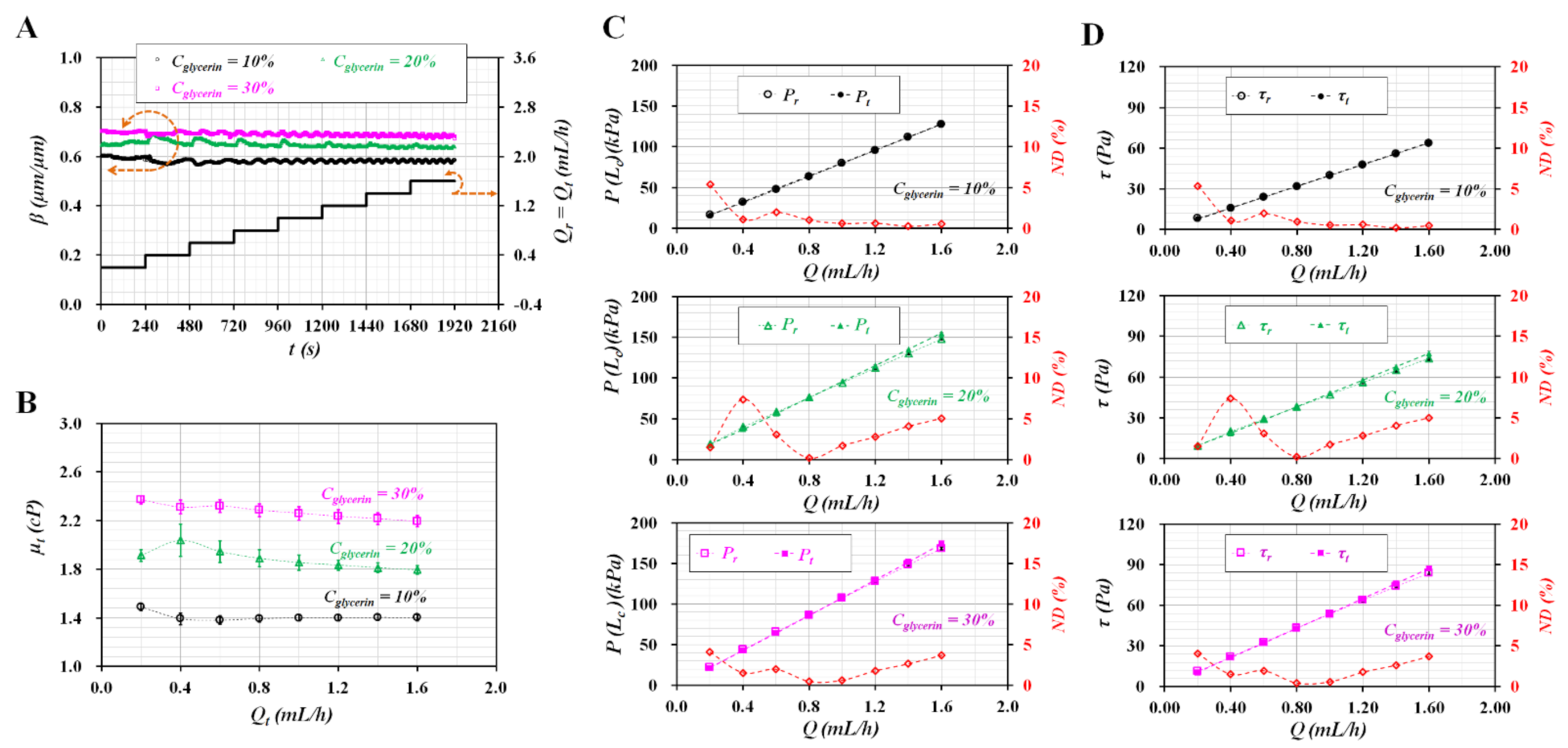
3.1. CFD Simulation for Validation of Analytical Expressions
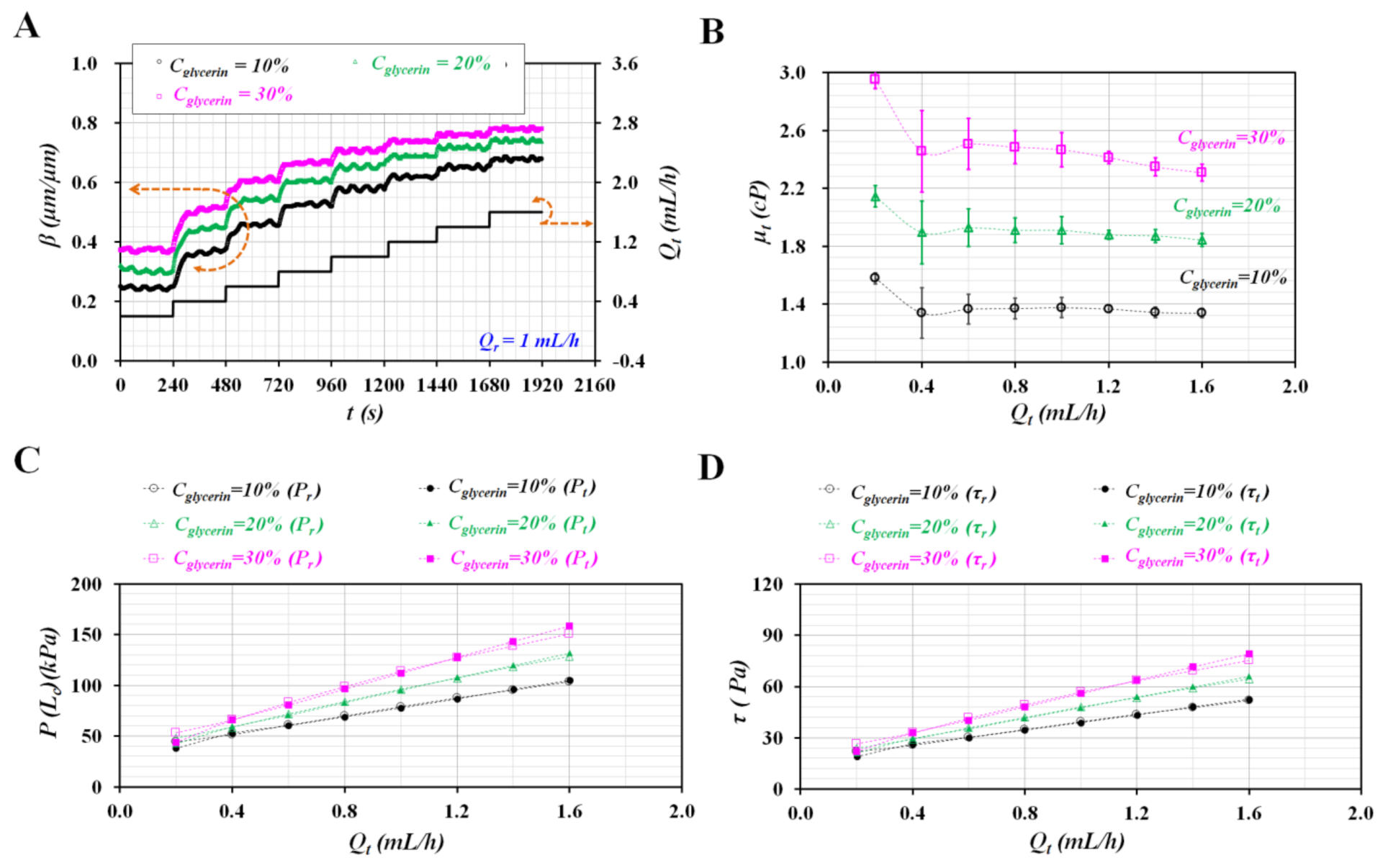
3.2. Various Flow Rate Patterns of Pure Liquid Controlled with Syringe Pump
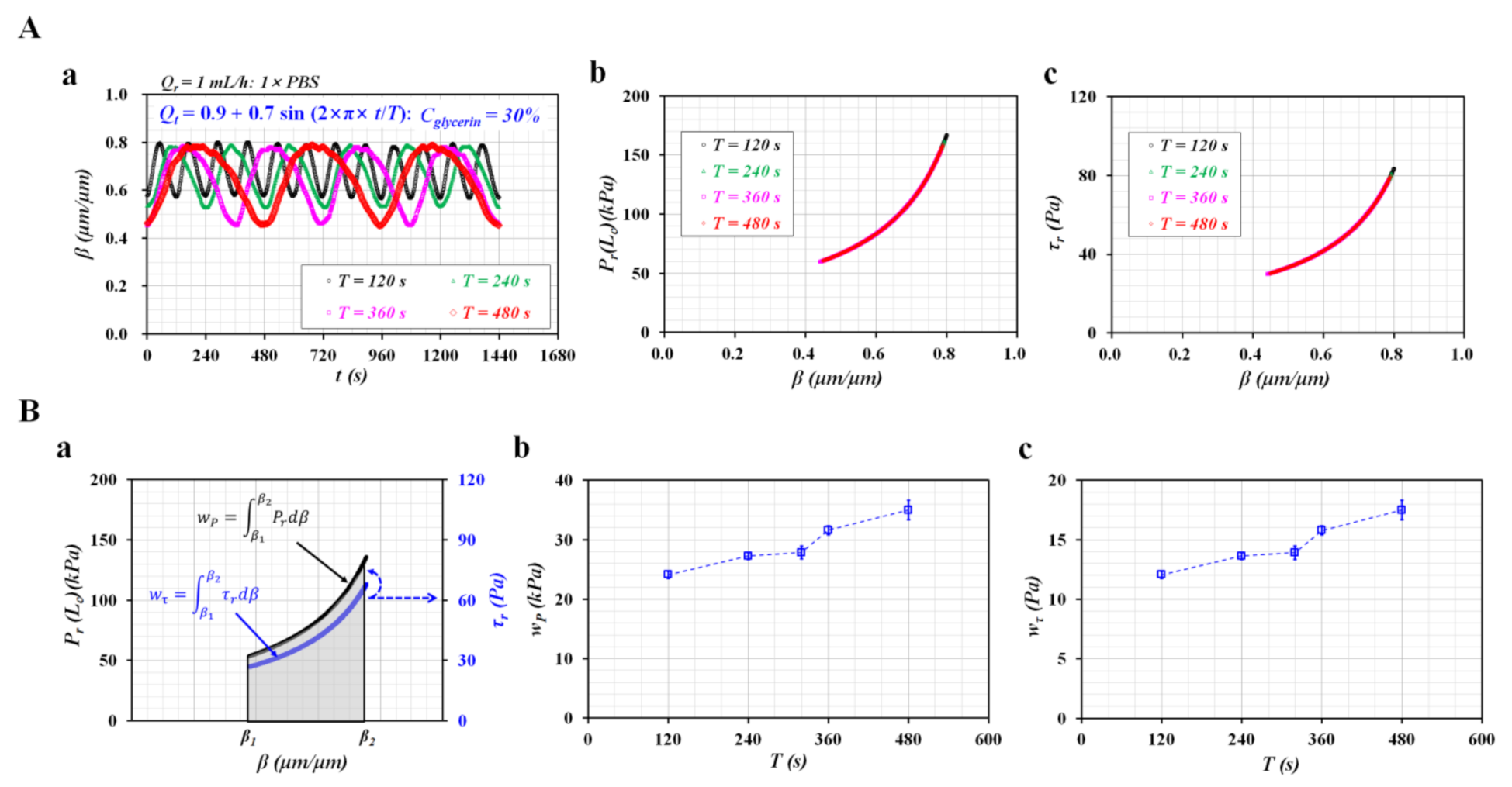
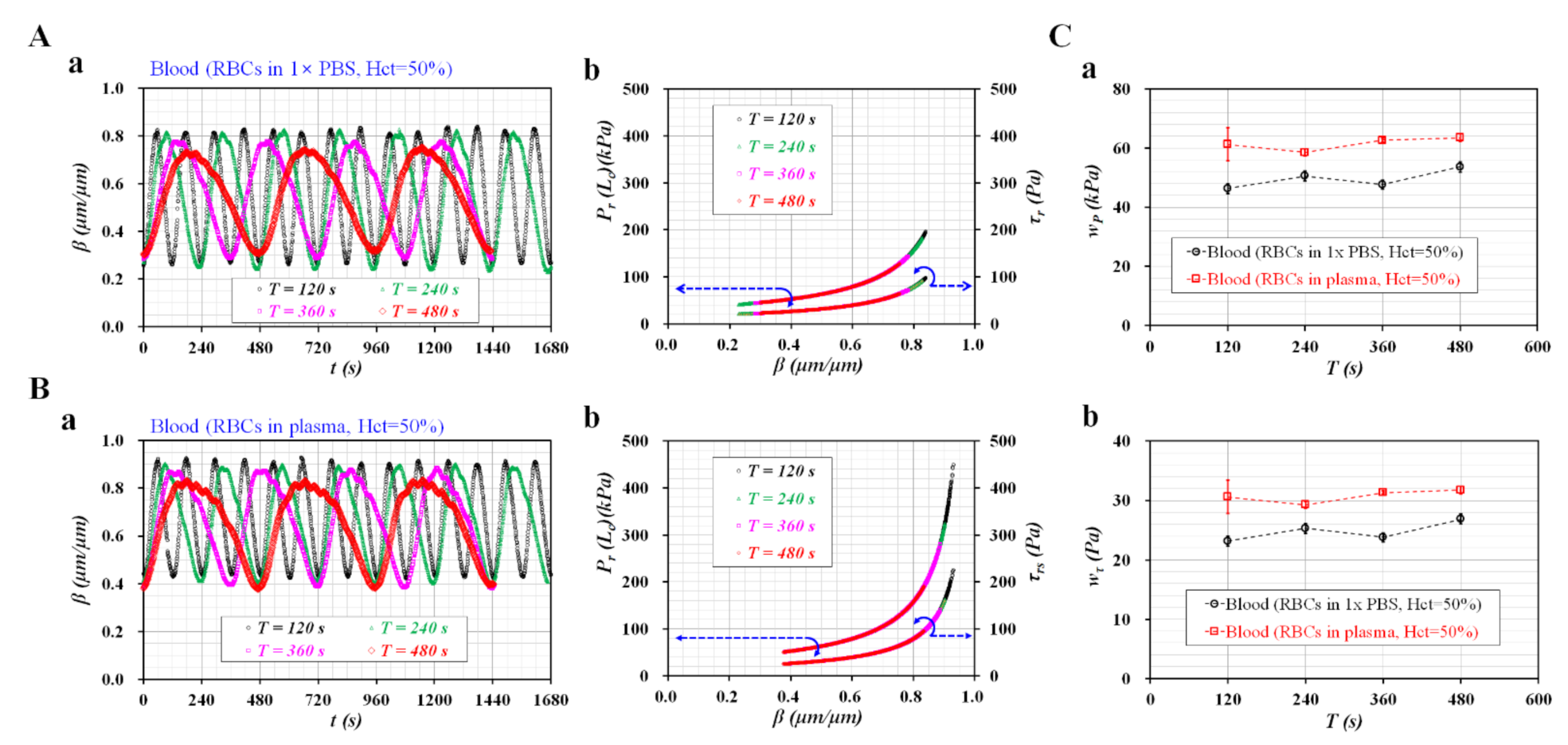
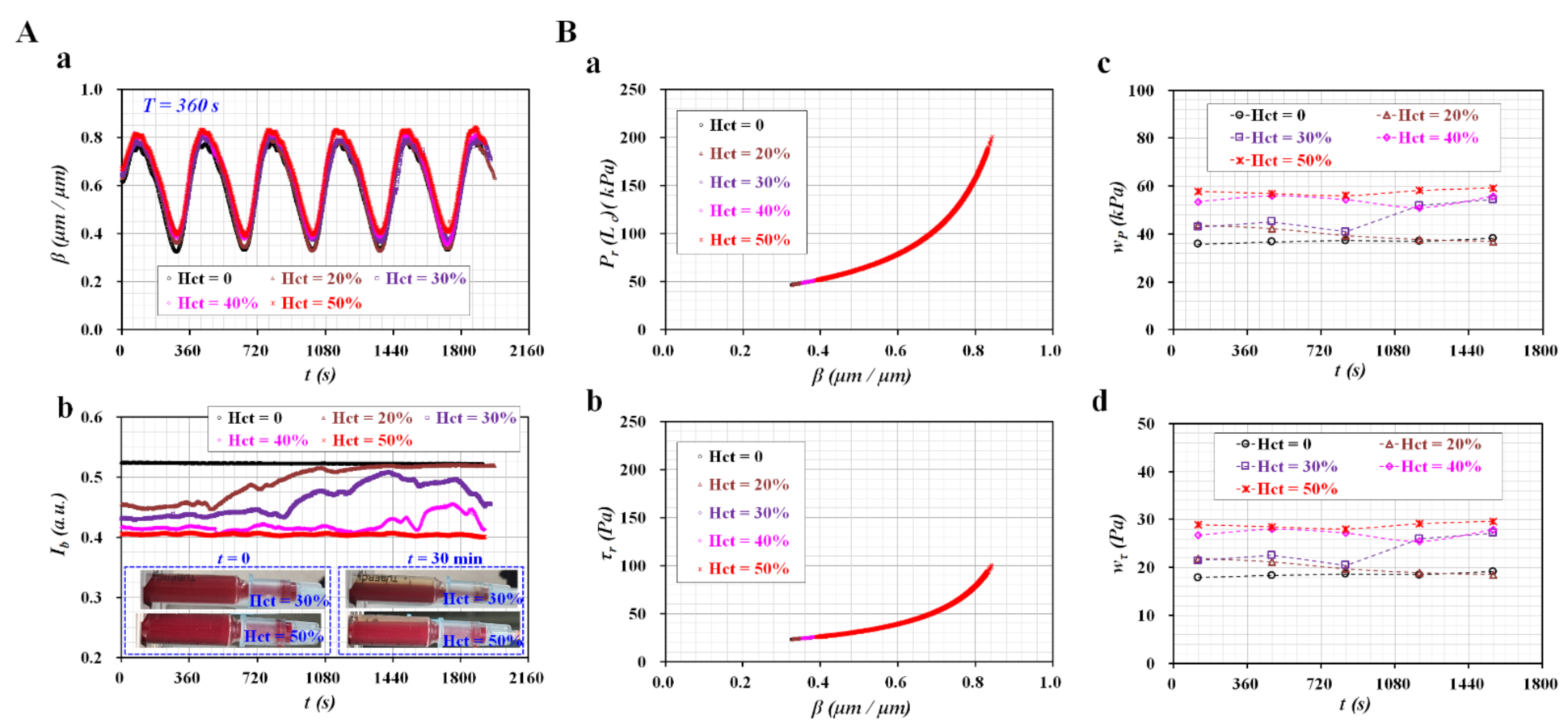
3.3. Sinusoidal Flow Rates of Blood Controlled with Syringe Pump
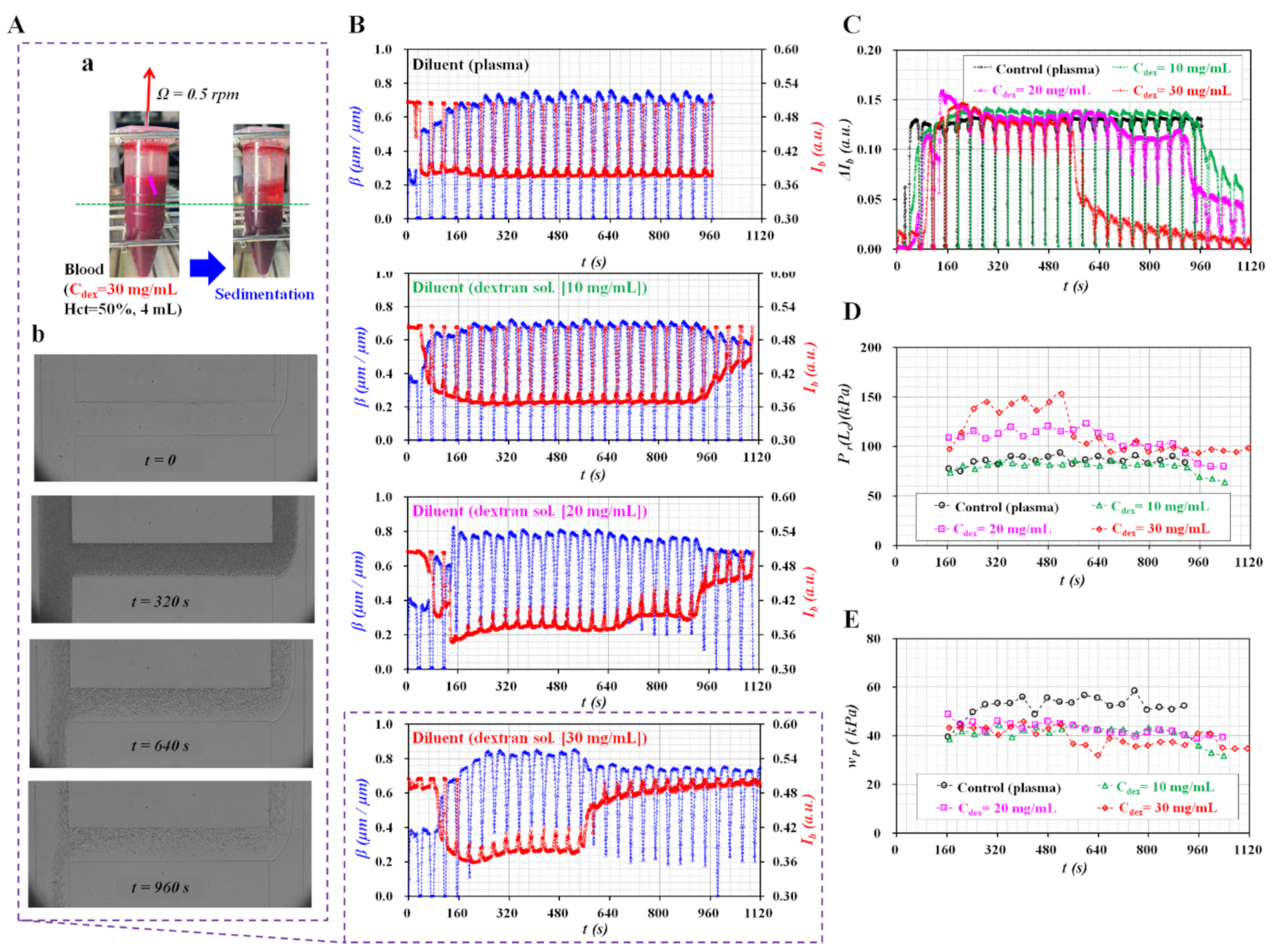
3.4. Blood Flows Controlled with Peristaltic Pump
4. Conclusions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Popel, A.S.; Johnson, P.C. Microcirculation and hemorheology. Annu. Rev. Fluid Mech. 2005, 37, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Tomaiuolo, G.; Carciati, A.; Caserta, S.; Guido, S. Blood linear viscoelasticity by small amplitude oscillatory flow. Rheol. Acta 2016, 55, 485–495. [Google Scholar] [CrossRef]
- Ahn, C.B.; Kang, Y.J.; Kim, M.G.; Yang, S.; Lim, C.H.; Son, H.S.; Kim, J.S.; Lee, S.Y.; Son, K.H.; Sun, K. The effect of pulsatile versus nonpulsatile blood flow on viscoelasticity and red blood cell aggregation in extracorporeal circulation. Korean J. Thorac. Cardiovasc. Surg. 2016, 49, 145–150. [Google Scholar] [CrossRef]
- Pop, G.A.; Chang, Z.-Y.; Slager, C.J.; Kooij, B.-J.; Deel, E.D.V.; Moraru, L.; Quak, J.; Meijer, G.C.; Duncker, D.J. Catheter-based impedance measurements in the right atrium for continuously monitoring hematocrit and estimating blood viscosity changes; an in vivo feasibility study in swine. Biosens. Bioelectron. 2004, 19, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.; Ishikawa, T.; Imai, Y.; Takeda, M.; Wada, S.; Yamaguchi, T. Radial dispersion of red blood cells in blood flowing through glass capillaries: The role of hematocrit and geometry. J. Biomech. 2008, 41, 2188–2197. [Google Scholar] [CrossRef] [PubMed]
- Shevkoplyas, S.S.; Yoshida, T.; Gifford, S.C.; Bitensky, M.W. Direct measurement of the impact of impaired erythrocyte deformability on microvascular network perfusion in a microfluidic device. Lab. Chip 2006, 6, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Chien, S. Red cell deformability and its relavance to blood flow. Ann. Rev. Physiol. 1987, 49, 177–192. [Google Scholar] [CrossRef]
- Ermolinskiy, P.; Lugovtsov, A.; Yaya, F.; Lee, K.; Kaestner, L.; Wagner, C.; Priezzhev, A. Eect of red blood cell aging in vivo on their aggregation properties in vitro: Measurements with laser tweezers. Appl. Sci. 2020, 10, 7581. [Google Scholar] [CrossRef]
- Wen, J.; Wan, N.; Bao, H.; Li, J. Quantitative measurement and evaluation of red blood cell aggregation in normal blood based on a modified hanai equation. Sensors 2019, 19, 1095. [Google Scholar] [CrossRef]
- Cho, Y.-I.; Cho, D.J. Hemorheology and Microvascular Disorders. Korean Cir. J. 2011, 41, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, B.; Dittrich, P.S. Microfluidics to mimic blood flow in health and disease. Annu. Rev. Fluid Mech. 2018, 50, 483–504. [Google Scholar] [CrossRef]
- Whittaker, S.R.F.; Winton, F.R. The apparent viscosity of blood flowing in the isolated hindlimb of the dog and its variation with corpuscular concentration. J. Physiol. 1933, 78, 339–369. [Google Scholar] [CrossRef] [PubMed]
- Lipowsky, H.H. Microvascular Rheology and Hemodynamics. Microcirculation 2005, 12, 5–15. [Google Scholar] [CrossRef]
- Tomaiuolo, G.; Barra, M.; Preziosi, V.; Cassinese, A.; Rotoli, B.; Guido, S. Microfluidics analysis of red blood cell membrane viscoelasticity. Lab. Chip 2011, 11, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Campo-Deano, L.; Dullens, R.P.A.; Aarts, D.G.A.L.; Pinho, F.T.; Oliveira, M.S.N. Viscoelasticity of blood and viscoelastic blood analogues for use in polydymethylsiloxane in vitro models of the circulatory system. Biomicrofluidics 2013, 7, 034102. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Lee, S.-J. Blood viscoelasticity measurement using steady and transient flow controls of blood in a microfluidic analogue of Wheastone-bridge channel. Biomicrofluidics 2013, 7, 054122. [Google Scholar] [CrossRef]
- Kim, M.; Kim, A.; Kim, S.; Yang, S. Improvement of electrical blood hematocrit measurements under various plasma conditions using a novel hematocrit estimation parameter. Biosens. Bioelectron. 2012, 35, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yang, S. Improvement of the accuracy of continuous hematocrit measurement under various blood flow conditions. Appl. Phys. Lett. 2014, 104, 153508. [Google Scholar] [CrossRef]
- Lee, H.Y.; Barber, C.; Rogers, J.A.; Minerick, A.R. Electrochemical hematocrit determination in a direct current microfluidic device. Electrophoresis 2015, 36, 978–985. [Google Scholar] [CrossRef]
- Berry, S.B.; Fernandes, S.C.; Rajaratnam, A.; De Chiara, N.S.; Mace, C.R. Measurement of the hematocrit using paper-based microfluidic devices. Lab. Chip 2016, 16, 3689–3694. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, Y.S.; Zhbanov, A.; Yang, S. A physiometer for simultaneous measurement of whole blood viscosity and its determinants: Hematocrit and red blood cell deformability. Analyst 2019, 144, 3144–3157. [Google Scholar] [CrossRef]
- Jalal, U.M.; Kim, S.C.; Shim, J.S. Histogram analysis for smartphone-based rapid hematocrit determination. Biomed. Opt. Express 2017, 8, 3317–3328. [Google Scholar] [CrossRef] [PubMed]
- Boas, L.V.; Faustino, V.; Lima, R.; Miranda, J.M.; Minas, G.; Fernandes, C.S.V.; Catarino, S.O. Assessment of the deformability and velocity of healthy and artificially impaired red blood cells in narrow polydimethylsiloxane (PDMS) microchannels. Micromachines 2018, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.F.; Mancuso, J.E.; Zivkovic, A.M.; Smilowitz, J.T.; Ristenpart, W.D. Red blood cells from individuals with abdominal obesity or metabolic abnormalities exhibit less deformability upon entering a constriction. PLoS ONE 2016, 11, e0156070. [Google Scholar] [CrossRef]
- Liu, L.; Huang, S.; Xu, X.; Han, J. Study of individual erythrocyte deformability susceptibility to INFeD and ethanol using a microfluidic chip. Sci. Rep. 2016, 6, 22929. [Google Scholar] [CrossRef] [PubMed]
- Namgung, B.; Lee, T.; Tan, J.K.S.; Poh, D.K.H.; Park, S.; Chng, K.Z.; Agrawal, R.; Park, S.-Y.; Leo, H.L.; Kim, S. Vibration motor-integrated low-cost, miniaturized system for rapid quantification of red blood cell aggregation. Lab. Chip 2020, 20, 3930–3937. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, W.H.; Piety, N.Z.; Shevkoplyas, S.S. Influence of red blood cell aggregation on perfusion of an artificial microvascular network. Microcirculation 2017, 24, e12317. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Yeom, E.; Lee, S.-J. Microfluidic biosensor for monitoring temporal variations of hemorheological and hemodynamic properties using an extracorporeal rat bypass loop. Anal. Chem. 2013, 85, 10503–10511. [Google Scholar] [CrossRef]
- Yeom, E.; Kang, Y.J.; Lee, S.J. Hybrid system for ex-vivo hemorheological and hemodynamic analysis: A feasibility study. Sci. Rep. 2015, 5, 11064. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J. Periodic and simultaneous quantification of blood viscosity and red blood cell aggregation using a microfluidic platform under in-vitro closed-loop circulation. Biomicrofluidics 2018, 12, 024116. [Google Scholar] [CrossRef]
- Arjmandi, N.; Liu, C.; Roy, W.V.; Lagae, L.; Borghs, G. Method for flow measurement in microfluidic channels based on electrical impedance spectroscopy. Microfluid. Nanofluid. 2012, 12, 17–23. [Google Scholar] [CrossRef][Green Version]
- Stern, L.; Bakal, A.; Tzur, M.; Veinguer, M.; Mazurski, N.; Cohen, N.; Levy, U. Doppler-based flow rate sensing in microfluidic channels. Sensors 2014, 14, 16799–16807. [Google Scholar] [CrossRef] [PubMed]
- Lucchetta, D.E.; Vita, F.; Francescangeli, D.; Francescangeli, O.; Simoni, F. Optical measurement of flow rate in a microfluidic channel. Microfluid. Nanofluid. 2016, 20, 9. [Google Scholar] [CrossRef]
- Cheri, M.S.; Latifi, H.; Sadeghi, J.; Moghaddam, M.S.; Shahraki, H.; Hajghassem, H. Real-time measurement of flow rate in microfluidic devices using a cantilever-based optofluidic sensor. Anayst 2014, 139, 431–438. [Google Scholar]
- Mohd, O.; Sotoudegan, M.S.; Ligler, F.S.; Walker, G.M. A simple cantilever system for measurement of flow rates in paper microfluidic devices. Eng. Res. Express 2019, 1, 025019. [Google Scholar] [CrossRef]
- Carroll, N.J.; Jensen, K.H.; Parsa, S.; Holbrook, N.M.; Weitz, D.A. Measurement of flow velocity and inference of liquid viscosity in a microfluidic channel by fluorescence photobleaching. Langmuir 2014, 30, 4868–4874. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Sadabadi, H.; Hejazi, S.H.; Daneshmand, M.; Sanati-Nezhad, A. Noncontact and nonintrusive microwave-microfluidic flow sensor for energy and biomedical engineering. Sci. Rep. 2018, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.-J.; Gan, R.; Huang, L. A microfluidic flow meter with micromachined thermal sensing elements. Rev. Sci. Instrum. 2020, 91, 105006. [Google Scholar] [CrossRef]
- Delaneya, C.; McCluskey, P.; Coleman, S.; Whyte, J.; Kent, N.; Diamond, D. Precision control of flow rate in microfluidic channels using photoresponsive soft polymer actuators. Lab. Chip 2017, 17, 2013–2021. [Google Scholar] [CrossRef]
- Pitts, K.L.; Mehri, R.; Mavriplis, C.; Fenech, M. Micro-particle image velocimetry measurement of blood flow: Validation and analysis of data pre-processing and processing methods. Meas. Sci. Technol. 2012, 23, 105302. [Google Scholar] [CrossRef]
- Kang, Y.J. Microfluidic-Based Biosensor for Blood Viscosity and Erythrocyte Sedimentation Rate Using Disposable Fluid Delivery System. Micromachines 2020, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Ha, Y.-R.; Lee, S.-J. Microfluidic-based measurement of erythrocyte sedimentation rate for biophysical assessment of blood in an in vivo malaria-infected mouse. Biomicrofluidics 2014, 8, 044114. [Google Scholar] [CrossRef]
- Srivastava, N.; Burns, M.A. Microfluidic pressure sensing using trapped air compression. Lab. Chip 2007, 7, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Ai, M.; Ma, J.; Li, Z.; Xue, S. An easy method for pressure measurement in microchannels using trapped air compression in a one-end-sealed capillary. Micromachines 2020, 11, 914. [Google Scholar] [CrossRef]
- Chen, Y.; Chan, H.N.; Michael, S.A.; Shen, Y.; Chen, Y.; Tian, Q.; Huang, L.; Wu, H. A microfluidic circulatory system integrated with capillary-assisted pressure sensors. Lab. Chip 2017, 17, 653–662. [Google Scholar] [CrossRef]
- Tsai, C.-H.D.; Kaneko, M. On-chip pressure sensor using single-layer concentric chambers. Biomicrofluidics 2016, 10, 024116. [Google Scholar] [CrossRef]
- Jung, T.; Yang, S. Highly stable liquid metal-based pressure sensor integrated with a microfludic channel. Sensors 2015, 15, 11823–11835. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J. Microfluidic-based measurement of RBC aggregation and the ESR using a driving syringe system. Anal. Methods 2018, 10, 1805–1816. [Google Scholar] [CrossRef]
- Lee, J.; Tripathi, A. Intrinsic Viscosity of Polymers and Biopolymers Measured by Microchip. Anal. Chem. 2005, 77, 7137–7147. [Google Scholar] [CrossRef]
- Lan, W.J.; Li, S.W.; Xu, J.H.; Luo, G.S. Rapid measurement of fluid viscosity using co-flowing in a co-axial microfluidic device. Microfluid. Nanofluid. 2010, 8, 687–693. [Google Scholar] [CrossRef]
- Vanapalli, S.A.; Ende, D.V.D.; Duits, M.H.G.; Mugele, F. Scaling of interface displacement in a microfluidic comparator. Appl. Phys. Lett. 2007, 90, 114109. [Google Scholar] [CrossRef]
- Kim, G.; Jeong, S.; Kang, Y.J. Ultrasound standing wave-based cell-to-liquid separation for measuring viscosity and aggregation of blood sample. Sensors 2020, 20, 2284. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Ryu, J.; Lee, S.-J. Label-free viscosity measurement of complex fluids using reversal flow switching manipulation in a microfluidic channel. Biomicrofluidics 2013, 7, 044106. [Google Scholar] [CrossRef] [PubMed]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man. Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Solomon, D.E.; Vanapalli, S.A. Multiplexed microfluidic viscometer for high-throughput complex fluid rheology. Microfluid. Nanofluid. 2014, 16, 677–690. [Google Scholar] [CrossRef]
- Hintermüller, M.A.; Offenzeller, C.; Jakoby, B. A microfluidic viscometer with capcative readout using screen-printed electrodes. IEEE Sensors J. 2021, 21, 2565–2572. [Google Scholar] [CrossRef]
- Kang, Y.J.; Ha, Y.-R.; Lee, S.-J. High-throughput and label-free blood-on-a-chip for malaria diagnosis. Anal. Chem. 2016, 88, 2912–2922. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Yang, S. Fluidic low pass filter for hydrodynamic flow stabilization in microfluidic environments. Lab. Chip 2012, 12, 1881–1889. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Yan, J.; Zhu, T.; Guo, S.; Li, S.; Li, T. Standing air bubble-based micro-hydraulic capacitors for flow stabilization in syringe pump-driven systems. Micromachines 2020, 11, 396. [Google Scholar] [CrossRef]
- Inman, W.; Domansky, K.; Serdy, J.; Owens, B.; Trumper, D.; Griffith, L.G. Design, modeling and fabrication of a constant flow pneumatic micropump. J. Micromech. Microeng. 2007, 17, 891–899. [Google Scholar] [CrossRef]
- Kim, J.; Kang, M.; Jensen, E.C.; Mathies, R.A. Lifting gate PDMS microvalves and pumps for microfluidic control. Anal. Chem. 2012, 84, 2067–2071. [Google Scholar] [CrossRef]
- Kim, B.H.; Kim, I.C.; Kang, Y.J.; Ryu, J.; Lee, S.J. Effect of phase shift on optimal operation of serial-connected valveless micropumps. Sens. Actuator A Phys. 2014, 209, 133–139. [Google Scholar] [CrossRef]
- Yang, B.; Lin, Q. A Compliance-Based Microflow Stabilizer. J. Microelectromech. Syst. 2009, 18, 539–546. [Google Scholar] [CrossRef]
- Veenstra, T.T.; Sharma, N.R.; Forster, F.K.; Gardeniers, J.G.E.; Elwenspoek, M.C.; van den Berg, A. The design of an in-plane compliance structure for microfluidical systems. Sens. Actuator B Chem. 2002, 81, 377–383. [Google Scholar] [CrossRef]
- Nilsson, A.M.K.; Alsholm, P.; Karlsson, A.; Andersson-Engels, S. T-matrix computations of light scattering by red blood cells. Appl. Opt. 1998, 37, 2735–2748. [Google Scholar]
- Mroczka, J.; Wysoczanski, D. Optical parameters and scattering properties of red blood cells. Opt. Appl. 2002, 32, 691–700. [Google Scholar]
- Myers, D.R.; Lam, W.A. Vascularized microfluidics and their untapped potential for discovery in diseases of the microvasculature. Annu. Rev. Biomed. Eng. 2021, 23, 407–432. [Google Scholar] [CrossRef]
- Hesh, C.A.; Qiu, Y.; Lam, W.A. Vascularized microfluidics and the blood–endothelium interface. Micromachines 2020, 11, 18. [Google Scholar] [CrossRef]
- Islam, M.M.; Beverung, S.; Steward, R., Jr. Bio-inspired microdevices that mimic the human vasculature. Micromachines 2017, 8, 299. [Google Scholar] [CrossRef]
- Yu, Z.T.F.; Yong, K.M.A.; Fu, J. Microfl uidic blood cell sorting: Now and beyond. Small 2013, 10, 1687–1703. [Google Scholar] [CrossRef]
- Toner, M.; Irimia, D. Blood-on-a-chip. Annu. Rev. Biomed. Eng. 2005, 7, 77–103. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.J. Quantitative Monitoring of Dynamic Blood Flows Using Coflowing Laminar Streams in a Sensorless Approach. Appl. Sci. 2021, 11, 7260. https://doi.org/10.3390/app11167260
Kang YJ. Quantitative Monitoring of Dynamic Blood Flows Using Coflowing Laminar Streams in a Sensorless Approach. Applied Sciences. 2021; 11(16):7260. https://doi.org/10.3390/app11167260
Chicago/Turabian StyleKang, Yang Jun. 2021. "Quantitative Monitoring of Dynamic Blood Flows Using Coflowing Laminar Streams in a Sensorless Approach" Applied Sciences 11, no. 16: 7260. https://doi.org/10.3390/app11167260
APA StyleKang, Y. J. (2021). Quantitative Monitoring of Dynamic Blood Flows Using Coflowing Laminar Streams in a Sensorless Approach. Applied Sciences, 11(16), 7260. https://doi.org/10.3390/app11167260





