Genome Mining Associated with Analysis of Structure, Antioxidant Activity Reveals the Potential Production of Levan-Rich Exopolysaccharides by Food-Derived Bacillus velezensis VTX20
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Bacteria Producing Exopolysaccharide
2.2. Exopolysaccharide Production and Quantification
2.3. Genome Sequencing, De Novo Assembly, and Annotation
2.4. Genome Comparisons
2.5. Water Solubility Index and Water-Holding Capacity
2.6. Monosaccharide and NMR Analysis of EPS
2.7. Anti-Oxidant Activity
3. Results
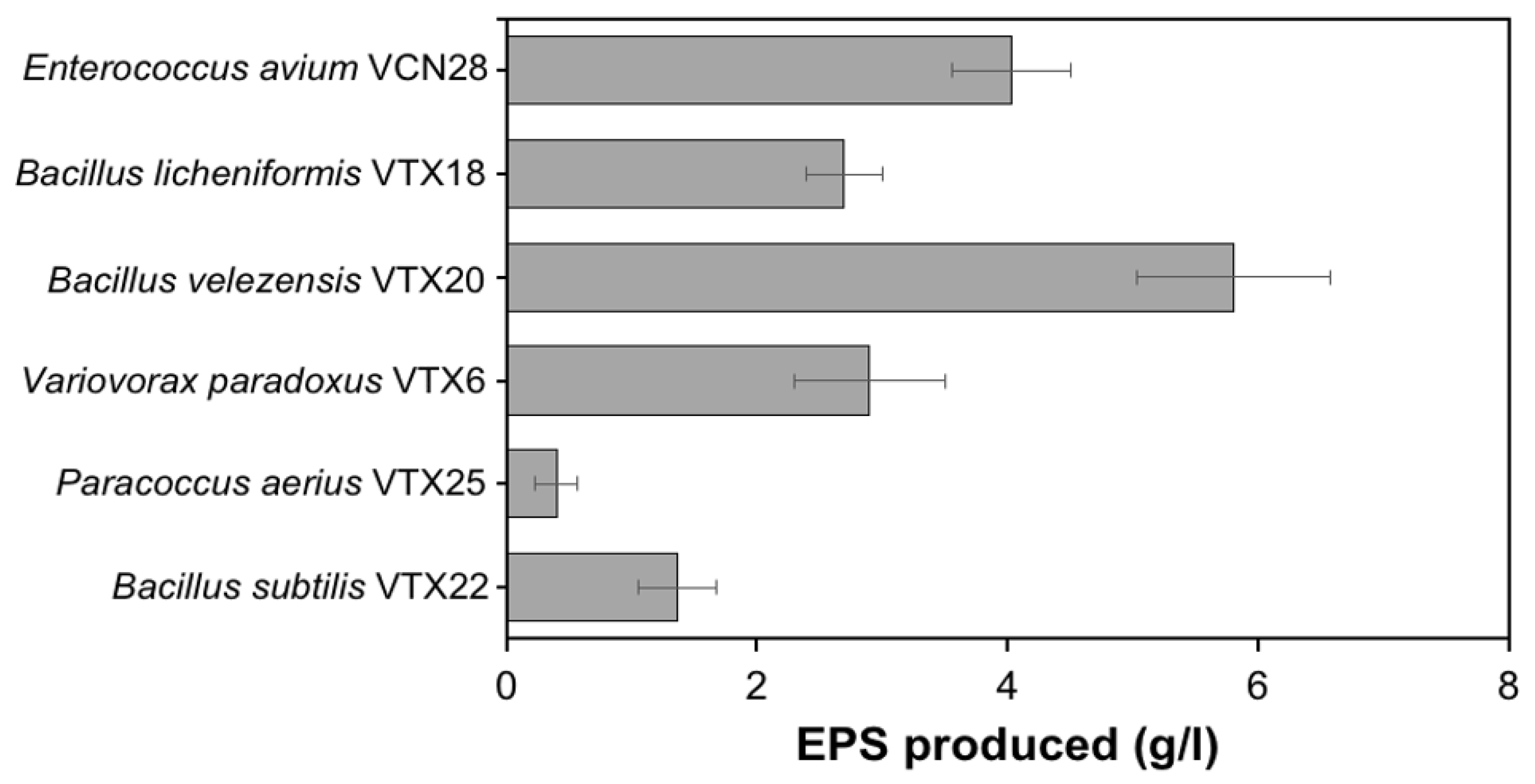
3.1. Isolation and Identification of High EPS-Producing Strain
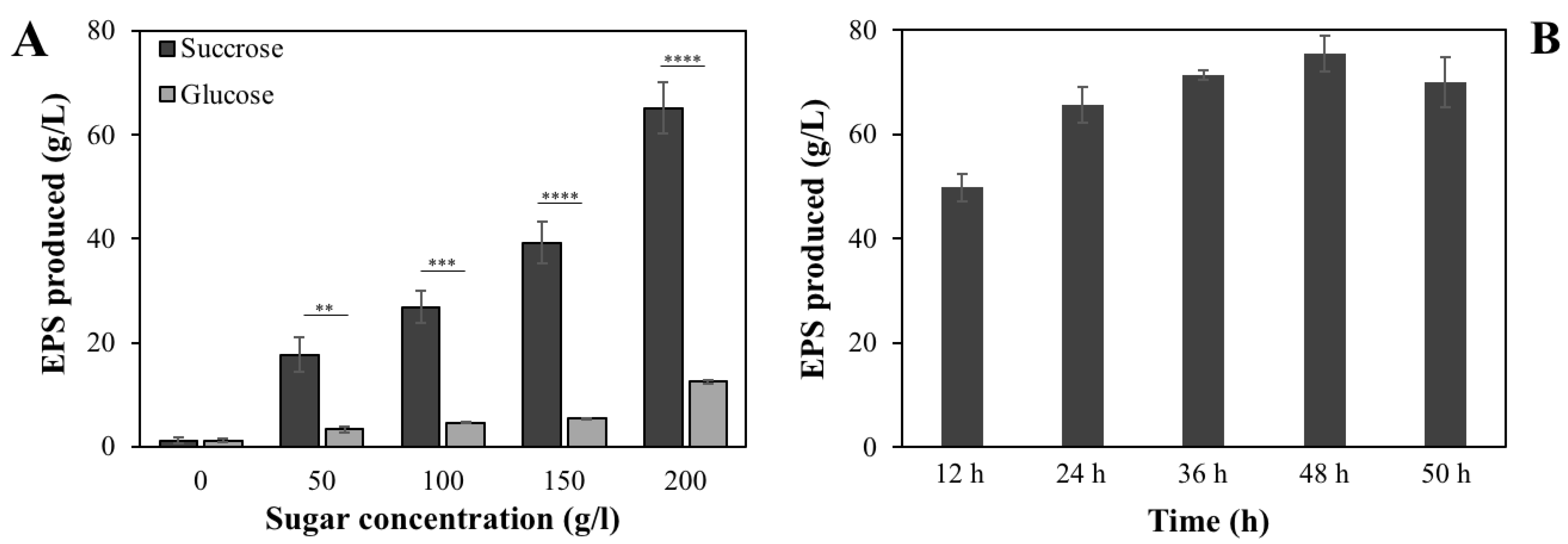
3.2. Effects of Different Sugars on EPS Production of B. velezensis VTX20
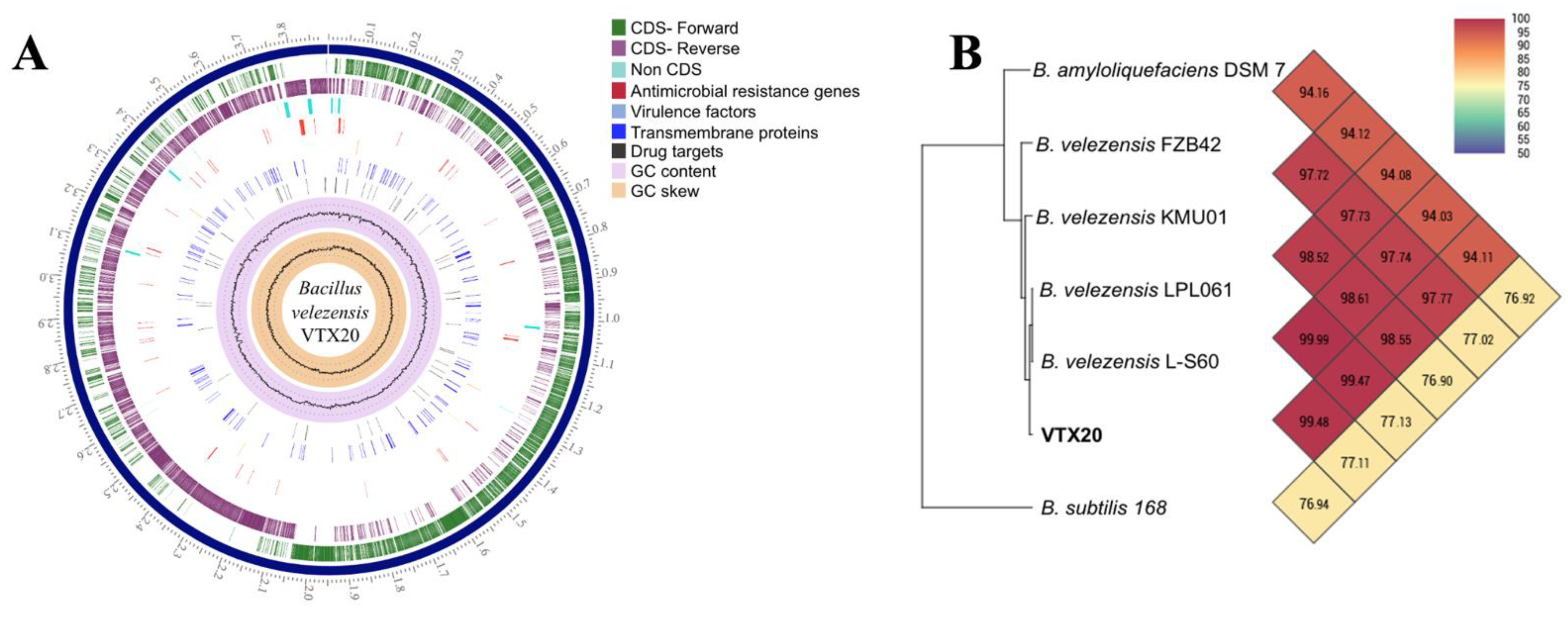
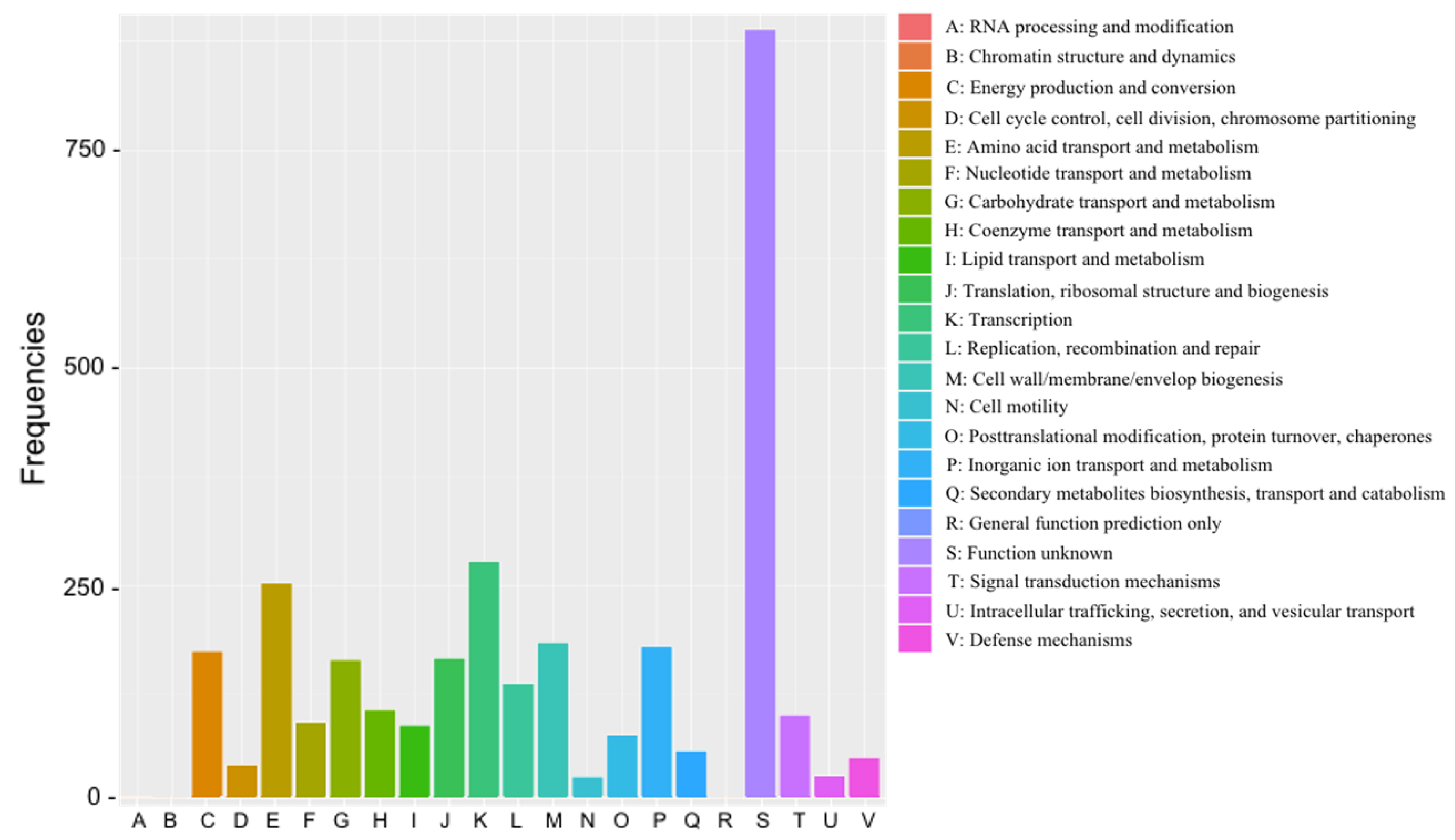
3.3. Genome Feature of EPS-Producing B. velezensis VTX20
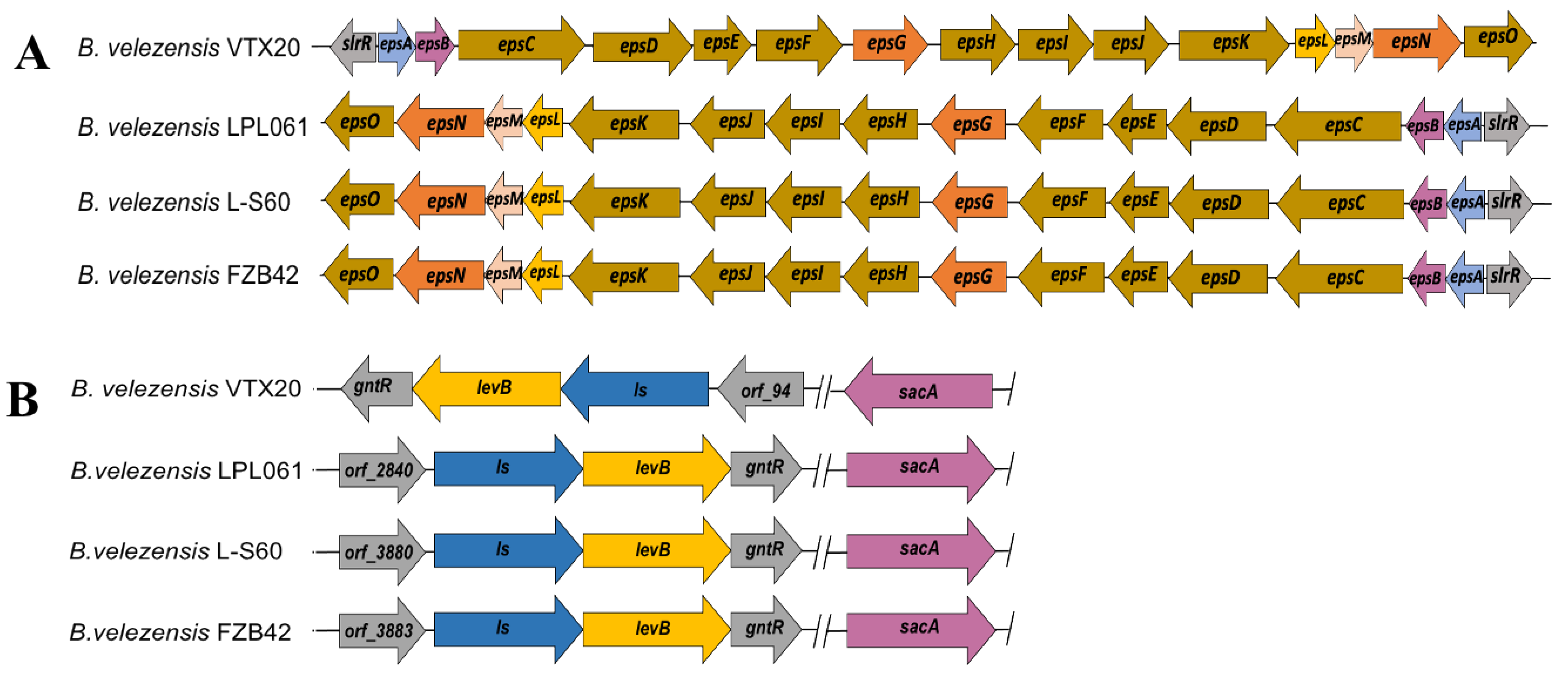
3.4. In Silico Analysis of EPS Biosynthetic Pathways
3.5. Characterization of EPSs Produced by B. velezensis VTX20
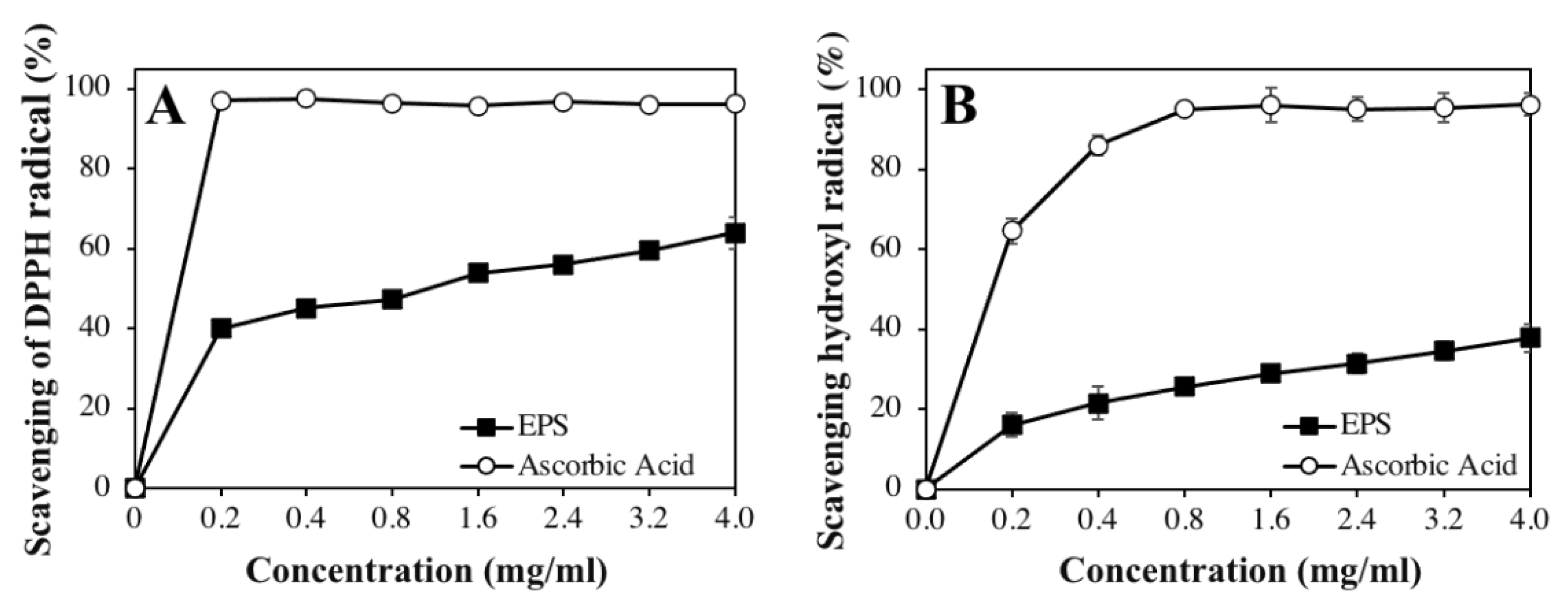
3.6. In Vitro Antioxidant Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freitas, F.; Alves, V.D.; Pais, J.; Carvalheira, M.; Costa, N.; Oliveira, R.; Reis, M.A.M. Production of a new exopolysaccharide (EPS) by Pseudomonas oleovorans NRRL B-14682 grown on glycerol. Process Biochem. 2010, 45, 297–305. [Google Scholar] [CrossRef]
- Parikh, A.; Madamwar, D. Partial characterization of extracellular polysaccharides from cyanobacteria. Bioresour. Technol. 2006, 97, 1822–1827. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, M.C.S.; Vespermann, K.A.C.; Pelissari, F.M.; Molina, G. Current status of biotechnological production and applications of microbial exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2020, 60, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Rajoka, M.S.R.; Mehwish, H.M.; Hayat, H.F.; Hussain, N.; Sarwar, S.; Aslam, H.; Nadeem, A.; Shi, J. Characterization, the antioxidant and antimicrobial activity of exopolysaccharide Isolated from poultry origin Lactobacilli. Probiotics Antimicrob. Proteins 2019, 11, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.T.; Chu, F.J.; Chou, C.C.; Yu, R.C. Antiproliferative and anticytotoxic effects of cell fractions and exopolysaccharides from Lactobacillus casei 01. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2011, 721, 157–162. [Google Scholar] [CrossRef]
- Kavita, K.; Singh, V.K.; Mishra, A.; Jha, B. Characterisation and anti-biofilm activity of extracellular polymeric substances from Oceanobacillus iheyensis. Carbohydr. Polym. 2014, 101, 29–35. [Google Scholar] [CrossRef]
- Ismail, B.; Nampoothiri, K.M. Production, purification and structural characterization of an exopolysaccharide produced by a probiotic Lactobacillus plantarum MTCC 9510. Arch. Microbiol. 2010, 192, 1049–1057. [Google Scholar] [CrossRef]
- Jolly, L.; Vincent, S.J.; Duboc, P.; Neeser, J.R. Exploiting expolysaccharides from lactic acid bacteria. Antonie Van Leeuwenhoek 2002, 82, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Welman, A.D.; Maddox, I.S. Exopolysaccharides from lactic acid bacteria: Perspectives and challenges. Trends Biotechnol. 2003, 21, 269–274. [Google Scholar] [CrossRef]
- Sayem, S.M.A.; Manzo, E.; Ciavatta, L.; Tramice, A.; Cordone, A.; Zanfardino, A.; De Felice, M.; Varcamonti, M. Anti-biofilm activity of an exopolysaccharide from a sponge-associated strain of Bacillus licheniformis. Microb. Cell Factories 2011, 10, 74. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-T.; Yuan, Q.; Shan, L.E.T.; Lin, M.-A.; Cheng, D.-Q.; Li, C.-Y. Antitumor activity of bacterial exopolysaccharides from the endophyte Bacillus amyloliquefaciens sp. isolated from Ophiopogon japonicus. Oncol. Lett. 2013, 5, 1787–1792. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Ye, H.-F. Characterization and flocculating properties of an extracellular biopolymer produced from a Bacillus subtilis DYU1 isolate. Process Biochem. 2007, 42, 1114–1123. [Google Scholar] [CrossRef]
- Mu, D.; Zhou, Y.; Wu, X.; Montalban-Lopez, M.; Wang, L.; Li, X.; Zheng, Z. Secretion of Bacillus amyloliquefaciens levansucrase from Bacillus subtilis and Its application in the enzymatic synthesis of levan. ACS Food Sci. Technol. 2021, 1, 249–259. [Google Scholar] [CrossRef]
- Chai, Y.; Beauregard, P.B.; Vlamakis, H.; Losick, R.; Kolter, R. Galactose metabolism plays a crucial role in biofilm formation by Bacillus subtilis. mBio 2012, 3, e00184. [Google Scholar] [CrossRef] [Green Version]
- Srikanth, R.; Reddy, C.H.S.S.S.; Siddartha, G.; Ramaiah, M.J.; Uppuluri, K.B. Review on production, characterization and applications of microbial levan. Carbohydr. Polym. 2015, 120, 102–114. [Google Scholar] [CrossRef]
- Shih, I.-L.; Yu, Y.-T.; Shieh, C.-J.; Hsieh, C.-Y. Selective production and characterization of levan by Bacillus subtilis (Natto) Takahashi. J. Agric. Food Chem. 2005, 53, 8211–8215. [Google Scholar] [CrossRef]
- Jakob, F.; Gebrande, C.; Bichler, R.M.; Vogel, R.F. Insights into the pH-dependent, extracellular sucrose utilization and concomitant levan formation by Gluconobacter albidus TMW 2.1191. Antonie Van Leeuwenhoek 2020, 113, 863–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahech, I.; Belghith, K.S.; Hamden, K.; Feki, A.; Belghith, H.; Mejdoub, H. Antidiabetic activity of levan polysaccharide in alloxan-induced diabetic rats. Int. J. Biol. Macromol. 2011, 49, 742–746. [Google Scholar] [CrossRef]
- Esawy, M.A.; Ahmed, E.F.; Helmy, W.A.; Mansour, N.M.; El-Senousy, W.M.; El-Safty, M.M. Production of levansucrase from novel honey Bacillus subtilis isolates capable of producing antiviral levans. Carbohydr. Polym. 2011, 86, 823–830. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Z.; Tao, X.; Wei, H. Characterization and sulfated modification of an exopolysaccharide from Lactobacillus plantarum ZDY2013 and its biological activities. Carbohydr. Polym. 2016, 153, 25–33. [Google Scholar] [CrossRef]
- Liu, J.; Luo, J.; Ye, H.; Sun, Y.; Lu, Z.; Zeng, X. Production, characterization and antioxidant activities in vitro of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr. Polym. 2009, 78, 275–281. [Google Scholar] [CrossRef]
- Moghannem, S.A.M.; Farag, M.M.S.; Shehab, A.M.; Azab, M.S. Exopolysaccharide production from Bacillus velezensis KY471306 using statistical experimental design. Braz. J. Microbiol. 2018, 49, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, L.; Zhao, F.; Wang, J.; Zhao, B.; Zhou, Z.; Han, Y. Cloning and expression of levansucrase gene of Bacillus velezensis BM-2 and enzymatic synthesis of levan. Processes 2021, 9, 317. [Google Scholar] [CrossRef]
- Yang, H.; Deng, J.; Yuan, Y.; Fan, D.; Zhang, Y.; Zhang, R.; Han, B. Two novel exopolysaccharides from Bacillus amyloliquefaciens C-1: Antioxidation and effect on oxidative stress. Curr. Microbiol. 2015, 70, 298–306. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P.; de los Reyes-Gavilán, C.G. Invited review: Methods for the screening, isolation, and characterization of exopolysaccharides produced by lactic acid bacteria. J. Dairy Sci. 2005, 88, 843–856. [Google Scholar] [CrossRef] [Green Version]
- Bikric, S.; Aslim, B.; Dincer, İ.; Yuksekdag, Z.; Ulusoy, S.; Yavuz, S. Characterization of exopolysaccharides (EPSs) obtained from Ligilactobacillus salivarius strains and investigation at the prebiotic potential as an alternative to plant prebiotics at poultry. Probiotics Antimicrob. Proteins 2021. [Google Scholar] [CrossRef]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Galperin, M.Y.; Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Expanded microbial genome coverage and improved protein family annotation in the COG database. Nucleic Acids Res. 2015, 43, D261–D269. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Ouk Kim, Y.; Park, S.C.; Chun, J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2016, 66, 1100–1103. [Google Scholar] [CrossRef]
- Anderson, R.A.; Conway, H.F.; Peplinski, A.J. Gelatinization of corn grits by roll cooking, extrusion cooking and steaming. Starch Stärke 1970, 22, 130–135. [Google Scholar] [CrossRef]
- Feng, F.; Zhou, Q.; Yang, Y.; Zhao, F.; Du, R.; Han, Y.; Xiao, H.; Zhou, Z. Characterization of highly branched dextran produced by Leuconostoc citreum B-2 from pineapple fermented product. Int. J. Biol. Macromol. 2018, 113, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Yang, X.; Zhao, Y.; Ruan, Y.; Yang, Y.; Wang, Z. Separation and quantification of component monosaccharides of the tea polysaccharides from Gynostemma pentaphyllum by HPLC with indirect UV detection. Food Chem. 2009, 112, 742–746. [Google Scholar] [CrossRef]
- Kadaikunnan, S.; Rejiniemon, T.; Khaled, J.M.; Alharbi, N.S.; Mothana, R. In-vitro antibacterial, antifungal, antioxidant and functional properties of Bacillus amyloliquefaciens. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Yu, J.; Zhao, S.; Wu, S.; He, P.; Jia, X.; Liu, Y.; Mao, D. Effect of carbon source on production, characterization and bioactivity of exopolysaccharide produced by Phellinus vaninii Ljup. An. Acad. Bras. Ciências 2017, 89, 2033–2041. [Google Scholar] [CrossRef] [Green Version]
- Güvener, Z.T.; McCarter, L.L. Multiple regulators control capsular polysaccharide production in Vibrio parahaemolyticus. J. Bacteriol. 2003, 185, 5431–5441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marvasi, M.; Visscher, P.T.; Casillas Martinez, L. Exopolymeric substances (EPS) from Bacillus subtilis: Polymers and genes encoding their synthesis. FEMS Microbiol. Lett. 2010, 313, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Velázquez-Hernández, M.L.; Baizabal-Aguirre, V.M.; Bravo-Patiño, A.; Cajero-Juárez, M.; Chávez-Moctezuma, M.P.; Valdez-Alarcón, J.J. Microbial fructosyltransferases and the role of fructans. J. Appl. Microbiol. 2009, 106, 1763–1778. [Google Scholar] [CrossRef]
- Niknezhad, S.V.; Najafpour-Darzi, G.; Morowvat, M.H.; Ghasemi, Y. Eexopolysaccharide production of Pantoea sp. BCCS 001 GH: Physical characterizations, emulsification, and antioxidant activities. Int. J. Biol. Macromol. 2018, 118, 1103–1111. [Google Scholar] [CrossRef]
- Han, J.; Xu, X.; Gao, C.; Liu, Z.; Wu, Z.; Elkins, C.A. Levan-producing Leuconostoc citreum strain BD1707 and its growth in tomato juice supplemented with sucrose. Appl. Environ. Microbiol. 2016, 82, 1383–1390. [Google Scholar] [CrossRef] [Green Version]
- Abid, Y.; Azabou, S.; Casillo, A.; Gharsallah, H.; Jemil, N.; Lanzetta, R.; Attia, H.; Corsaro, M.M. Isolation and structural characterization of levan produced by probiotic Bacillus tequilensis-GM from Tunisian fermented goat milk. Int. J. Biol. Macromol. 2019, 133, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Haddar, A.; Hamed, M.; Bouallegue, A.; Bastos, R.; Coelho, E.; Coimbra, M.A. Structural elucidation and interfacial properties of a levan isolated from Bacillus mojavensis. Food Chem. 2021, 343, 128456. [Google Scholar] [CrossRef]
- Vijitra, L.-I.; Worachot, S. Characterization and bioactivities of a novel exopolysaccharide produced from lactose by Bacillus tequilensis PS21 isolated from Thai Milk Kefir. Microbiol. Biotechnol. Lett. 2018, 46, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.S.; Amer, S.K.; Selim, M.S.; Rifaat, H.M. Characterization and applications of exopolysaccharide produced by marine Bacillus altitudinis MSH2014 from Ras Mohamed, Sinai, Egypt. Egypt. J. Basic Appl. Sci. 2018, 5, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Choi, I.S.; Ko, S.H.; Lee, M.E.; Kim, H.M.; Yang, J.E.; Jeong, S.G.; Lee, K.H.; Chang, J.Y.; Kim, J.C.; Park, H.W. Production, characterization, and antioxidant activities of an exopolysaccharide extracted from spent media wastewater after Leuconostoc mesenteroides WiKim32 fermentation. ACS Omega 2021, 6, 8171–8178. [Google Scholar] [CrossRef]
- Kodali, V.P.; Sen, R. Antioxidant and free radical scavenging activities of an exopolysaccharide from a probiotic bacterium. Biotechnol. J. 2008, 3, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Freitas, F.; Alves, V.D.; Reis, M.A. Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef]
- Pabst, M.J. Levan and levansucrase of Actinomyces viscosus. Infect. Immun. 1977, 15, 518–526. [Google Scholar] [CrossRef] [Green Version]
- Jensen, S.L.; Diemer, M.B.; Lundmark, M.; Larsen, F.H.; Blennow, A.; Mogensen, H.K.; Nielsen, T.H. Levanase from Bacillus subtilis hydrolyses β-2,6 fructosyl bonds in bacterial levans and in grass fructans. Int. J. Biol. Macromol. 2016, 85, 514–521. [Google Scholar] [CrossRef]
- Ullah, M.W.; Ul-Islam, M.; Khan, T.; Park, J.K. Chapter 31—Recent developments in the synthesis, properties, and applications of various microbial polysaccharides. In Handbook of Hydrocolloids, 3rd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 975–1015. [Google Scholar] [CrossRef]
- Pantelić, I.; Lukić, M.; Gojgić-Cvijović, G.; Jakovljević, D.; Nikolić, I.; Lunter, D.J.; Daniels, R.; Savić, S. Bacillus licheniformis levan as a functional biopolymer in topical drug dosage forms: From basic colloidal considerations to actual pharmaceutical application. Eur. J. Pharm. Sci. 2020, 142, 105109. [Google Scholar] [CrossRef]
- Li, W.; Ji, J.; Chen, X.; Jiang, M.; Rui, X.; Dong, M. Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2-1. Carbohydr. Polym. 2014, 102, 351–359. [Google Scholar] [CrossRef]
- Liu, C.F.; Tseng, K.C.; Chiang, S.S.; Lee, B.H.; Hsu, W.H.; Pan, T.M. Immunomodulatory and antioxidant potential of Lactobacillus exopolysaccharides. J. Sci. Food Agric. 2011, 91, 2284–2291. [Google Scholar] [CrossRef]
| EPS-Producing Bacteria | Monosaccharides | Antioxidant Activity | References |
|---|---|---|---|
| B. mojavensis MT012152 | Fructose | ND | [42] |
| B. amyloliquefaciens C-1 | Glucose, mannose, galactose, arabinose | Superoxide, hydroxyl radicals | [25] |
| B. tequilensis PS21 | Glucose, xylose, ribose, rhamnose, galactose | DPPH, ABTS | [43] |
| B. altitudinis MSH2014 | Glucose | DPPH | [44] |
| B. tequilensis GM | Fructose | ND | [41] |
| B. velezensis VTX20 | Fructose, glucose | DPPH, hydroxyl radicals | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, T.H.N.; Quach, N.T.; Nguyen, N.A.; Nguyen, H.T.; Ngo, C.C.; Nguyen, T.D.; Ho, P.-H.; Hoang, H.; Chu, H.H.; Phi, Q.-T. Genome Mining Associated with Analysis of Structure, Antioxidant Activity Reveals the Potential Production of Levan-Rich Exopolysaccharides by Food-Derived Bacillus velezensis VTX20. Appl. Sci. 2021, 11, 7055. https://doi.org/10.3390/app11157055
Vu THN, Quach NT, Nguyen NA, Nguyen HT, Ngo CC, Nguyen TD, Ho P-H, Hoang H, Chu HH, Phi Q-T. Genome Mining Associated with Analysis of Structure, Antioxidant Activity Reveals the Potential Production of Levan-Rich Exopolysaccharides by Food-Derived Bacillus velezensis VTX20. Applied Sciences. 2021; 11(15):7055. https://doi.org/10.3390/app11157055
Chicago/Turabian StyleVu, Thi Hanh Nguyen, Ngoc Tung Quach, Ngoc Anh Nguyen, Huyen Trang Nguyen, Cao Cuong Ngo, Tien Dat Nguyen, Phu-Ha Ho, Ha Hoang, Hoang Ha Chu, and Quyet-Tien Phi. 2021. "Genome Mining Associated with Analysis of Structure, Antioxidant Activity Reveals the Potential Production of Levan-Rich Exopolysaccharides by Food-Derived Bacillus velezensis VTX20" Applied Sciences 11, no. 15: 7055. https://doi.org/10.3390/app11157055
APA StyleVu, T. H. N., Quach, N. T., Nguyen, N. A., Nguyen, H. T., Ngo, C. C., Nguyen, T. D., Ho, P.-H., Hoang, H., Chu, H. H., & Phi, Q.-T. (2021). Genome Mining Associated with Analysis of Structure, Antioxidant Activity Reveals the Potential Production of Levan-Rich Exopolysaccharides by Food-Derived Bacillus velezensis VTX20. Applied Sciences, 11(15), 7055. https://doi.org/10.3390/app11157055






