Effects of Connective Tissue Growth Factor on the Cell Viability, Proliferation, Osteogenic Capacity and mRNA Expression of Stem Cell Spheroids
Abstract
:1. Introduction
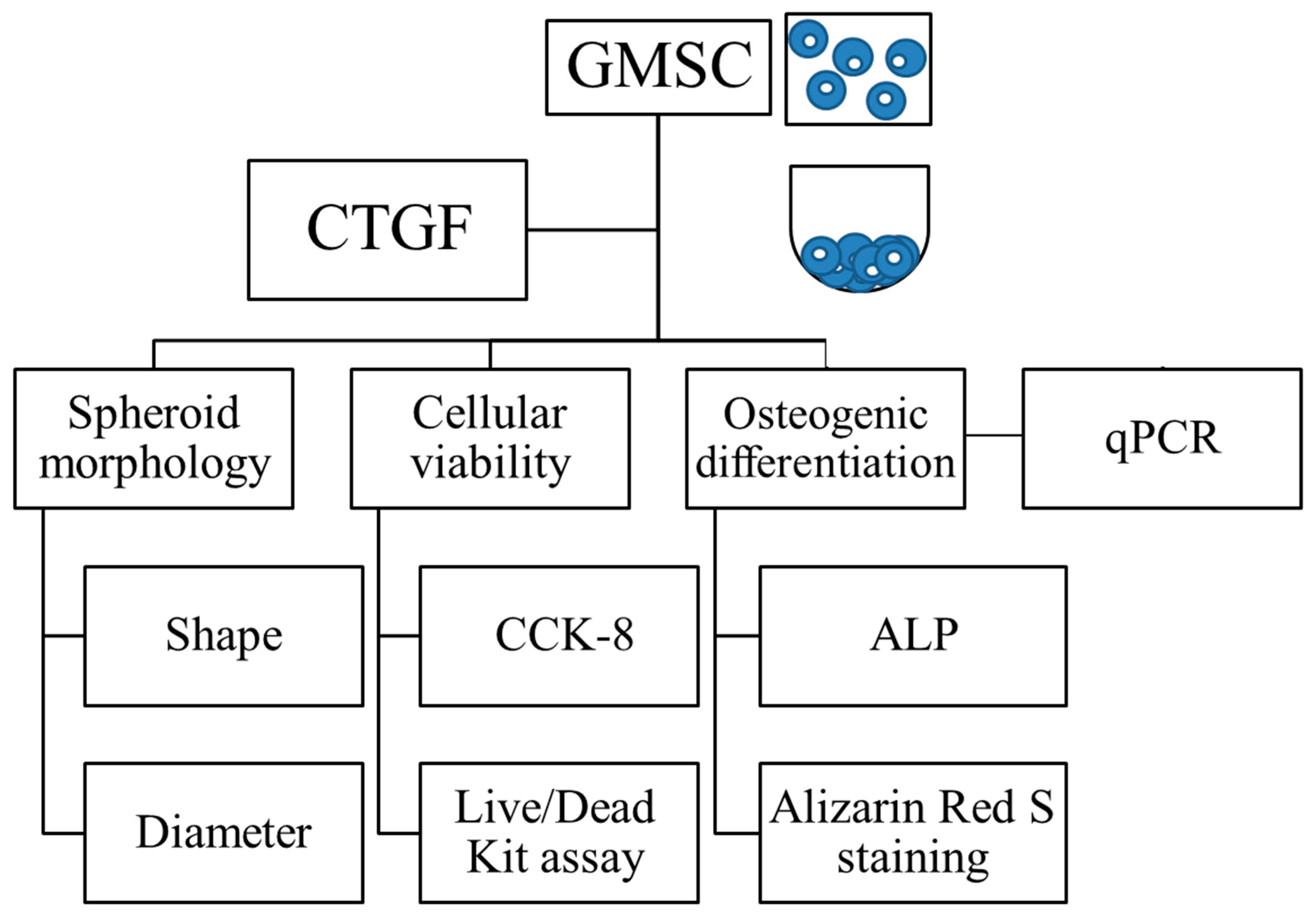
2. Materials and Methods
2.1. Cell Spheroids Composed of Human Gingiva-Derived Mesenchymal Stem Cells
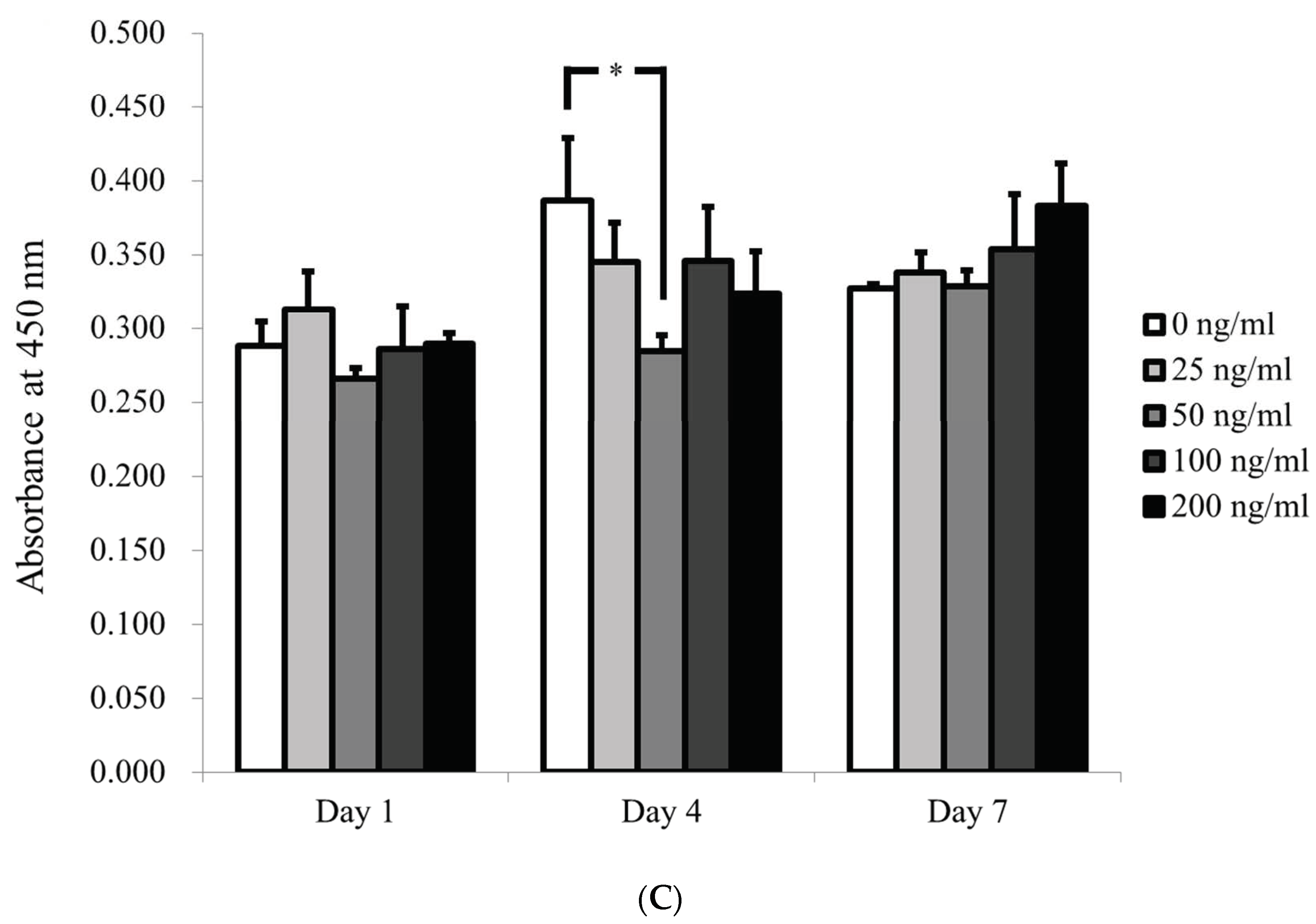
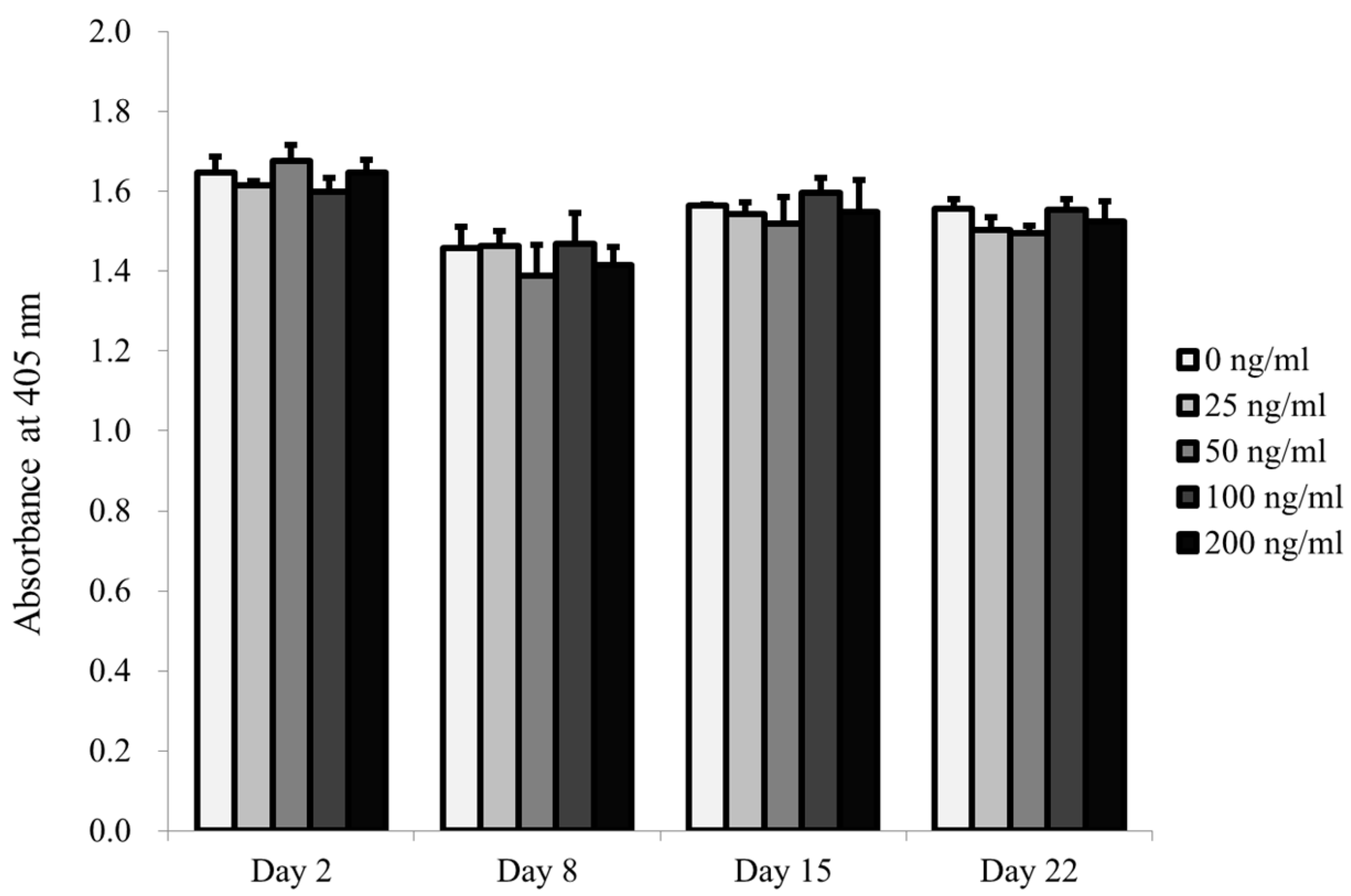
2.2. Determination of Cellular Viability
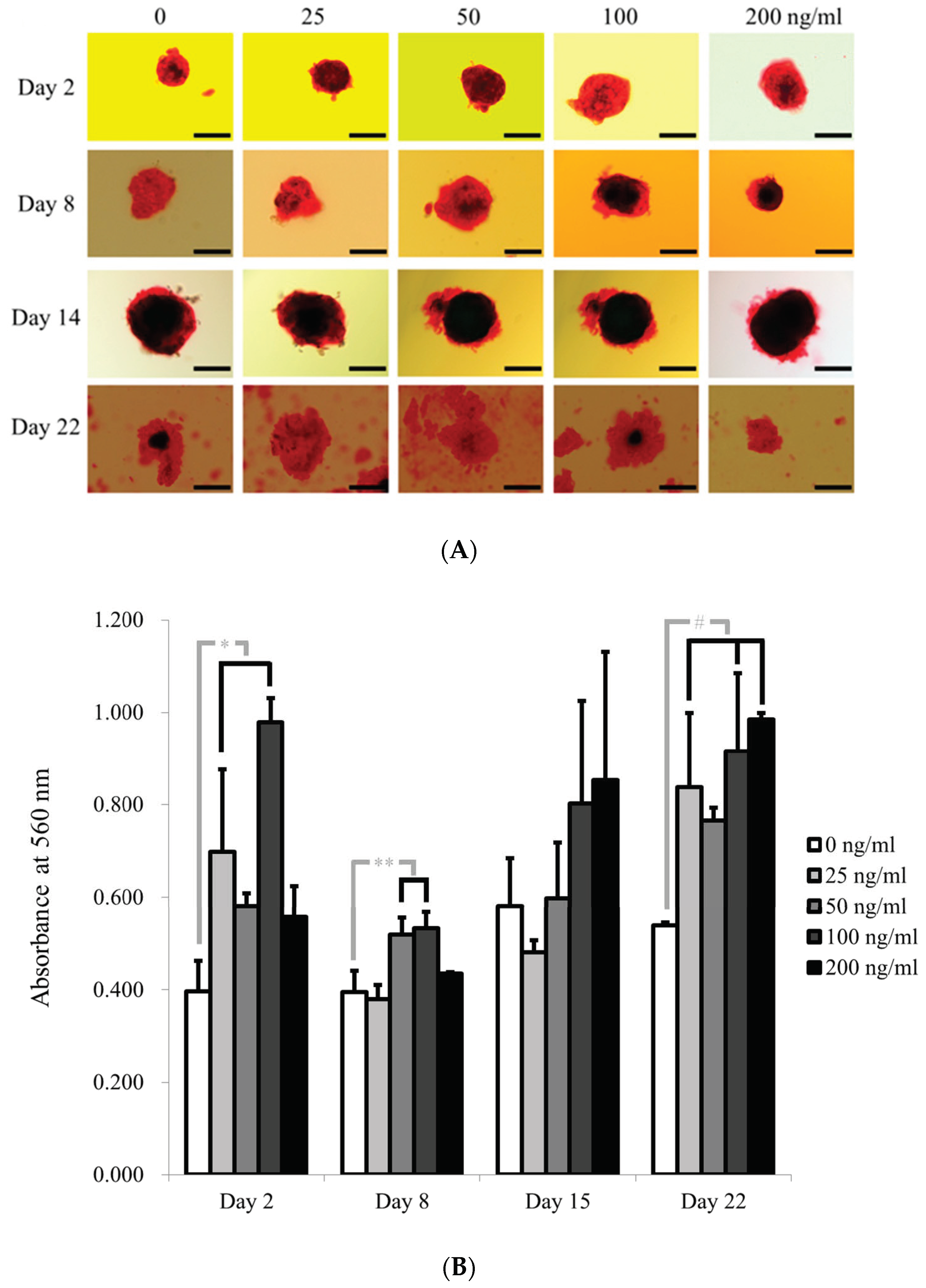
2.3. Evaluation of Alkaline Phosphatase Activity and Alizarin Red S Staining in Osteogenic Media
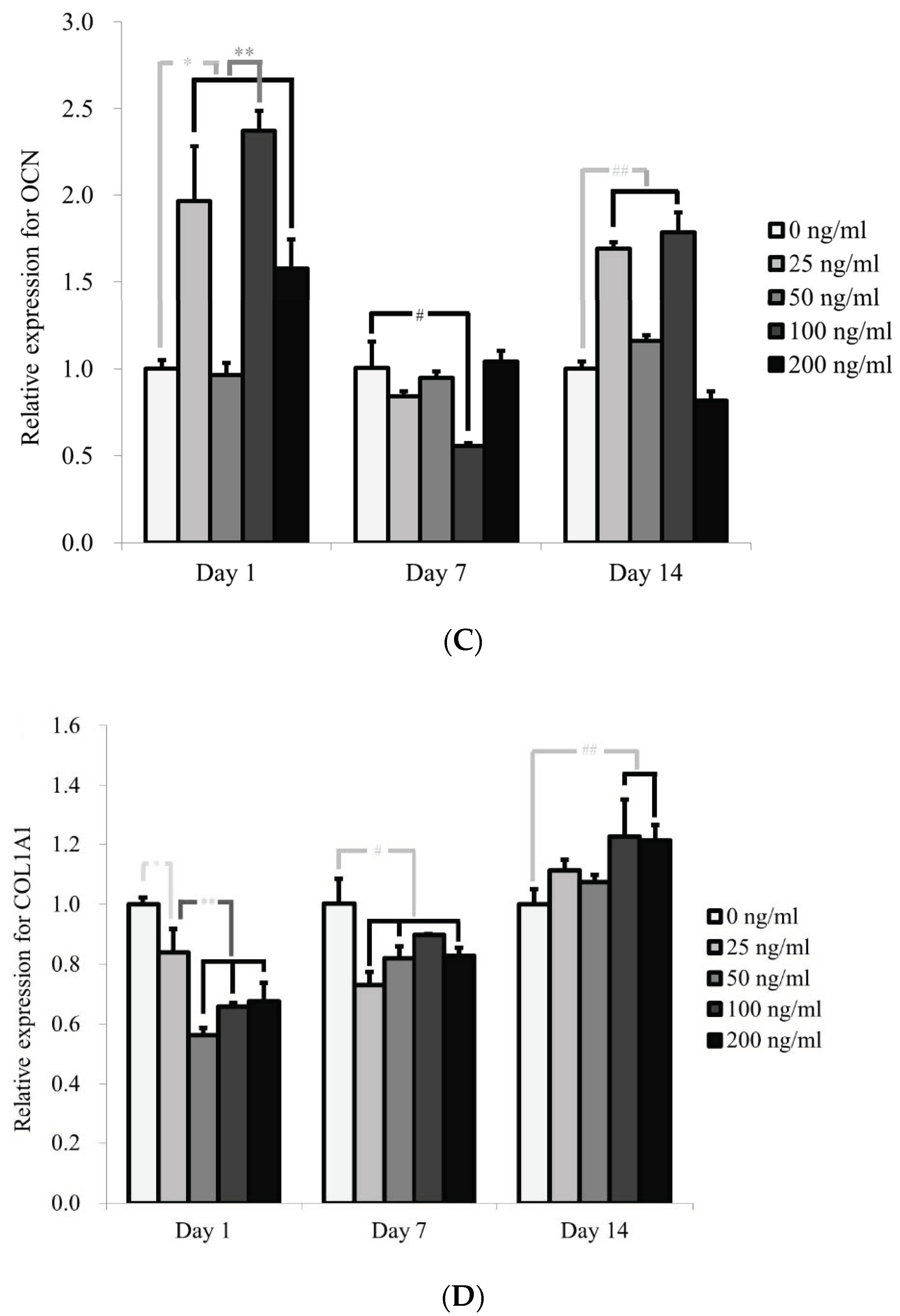
2.4. Total RNA Extraction and Quantification of Runt-Related Transcription Factor 2 (RUNX2), Bone Sialoprotein (BSP), Osteocalcin (OCN) and COL1A1 mRNA by Real-Time Quantitative Polymerase Chain Reaction (qPCR)
2.5. Statistical Analysis
3. Results
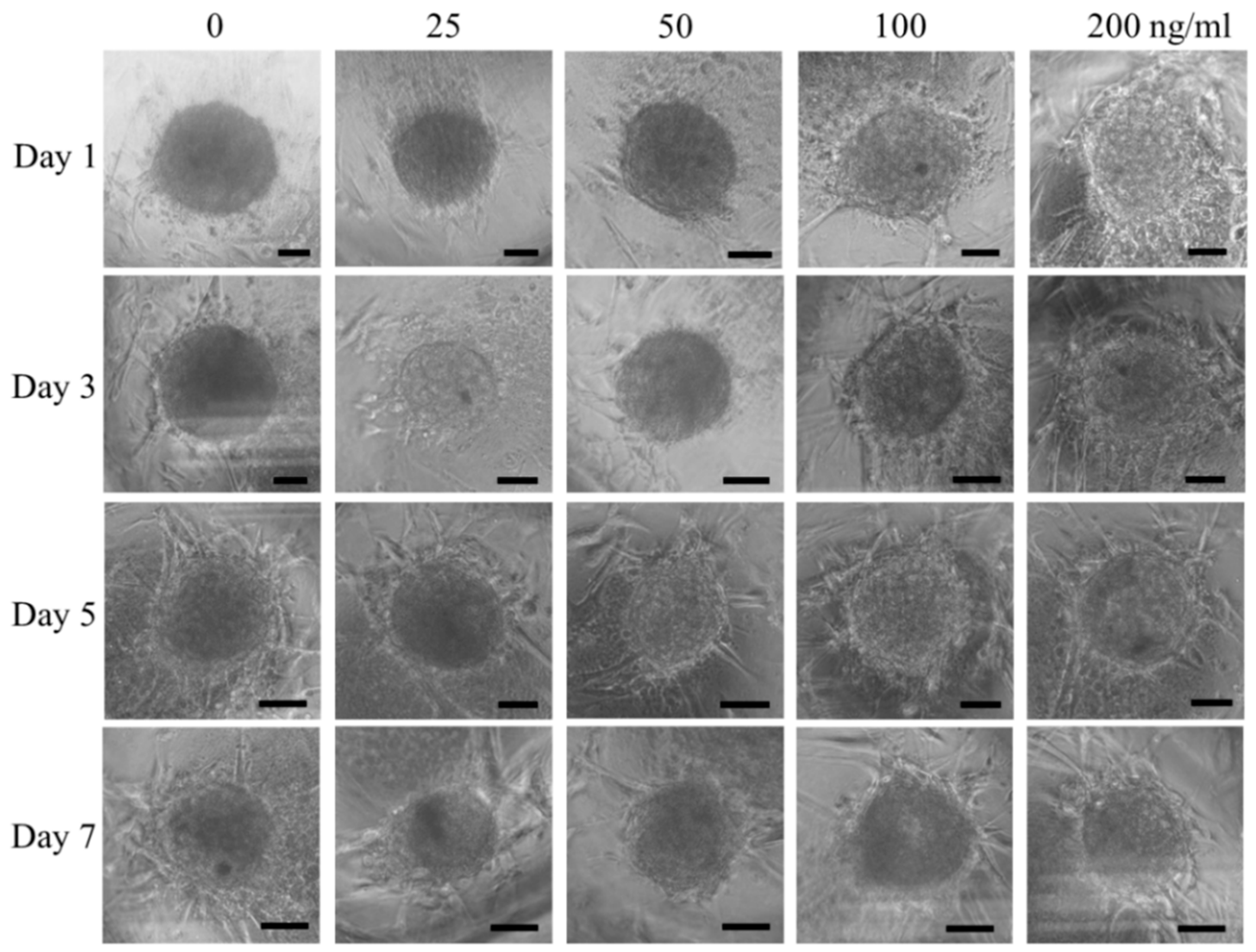
3.1. Cell Spheroids of Human Gingiva-Derived Mesenchymal Stem Cells
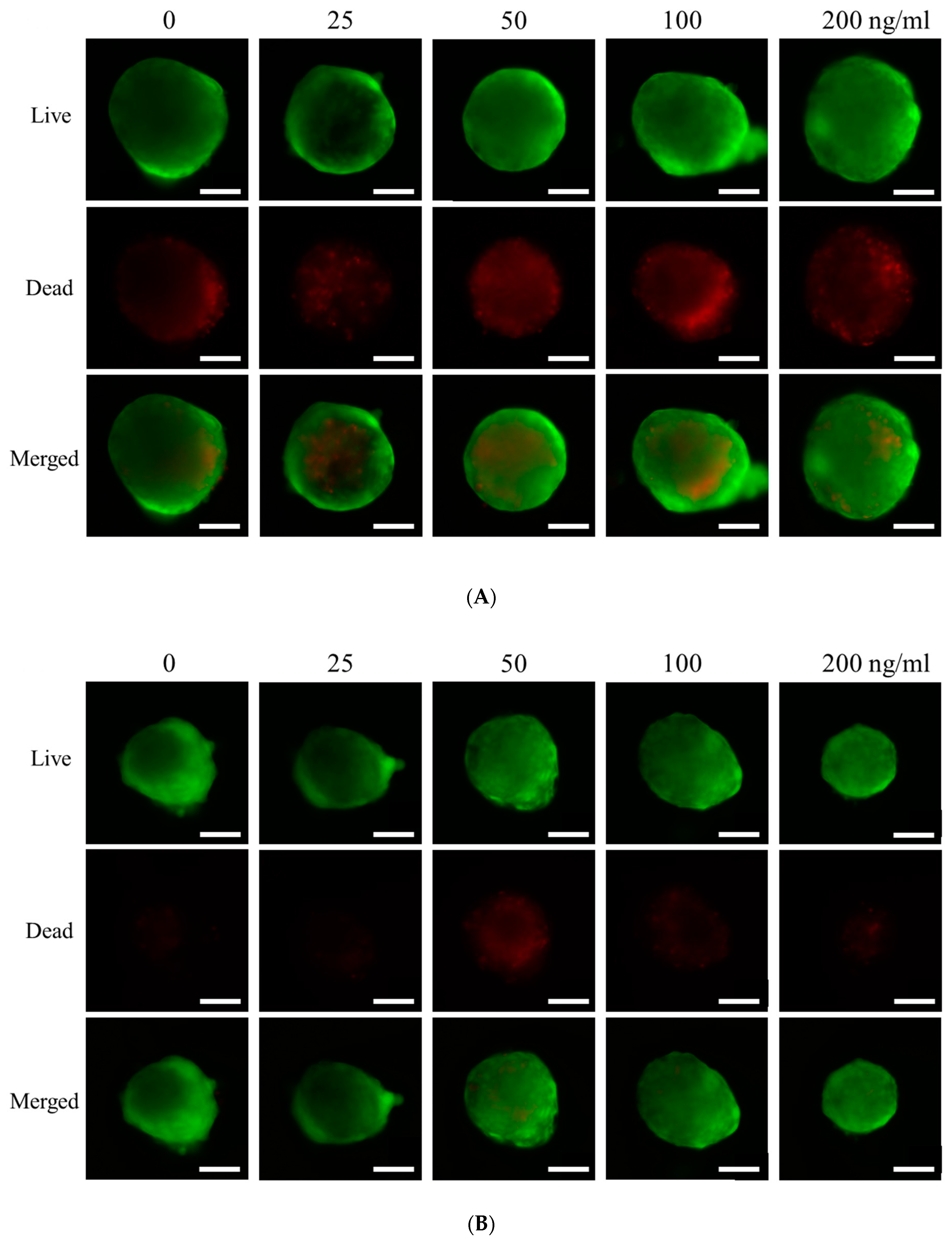
3.2. Qualitative Determination of Cell Viability
3.3. Evaluation of Alkaline Phosphatase Activity and Alizarin Red S Staining
3.4. Evaluation of RUNX2, BSP, OCN, and COL1A1 by qPCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, H.; Min, S.K.; Song, Y.; Park, Y.H.; Park, J.B. Bone morphogenetic protein-7 upregulates genes associated with osteoblast differentiation, including collagen I, Sp7 and IBSP in gingiva-derived stem cells. Exp. Ther. Med. 2019, 18, 2867–2876. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.I.; Ko, Y.; Park, J.B. Evaluation of the secretion and release of vascular endothelial growth factor from two-dimensional culture and three-dimensional cell spheroids formed with stem cells and osteoprecursor cells. Adv. Clin. Exp. Med. 2018, 27, 971–977. [Google Scholar] [CrossRef]
- Lee, H.; Park, J.B. Evaluation of the effects of dimethylsulphoxide on morphology, cellular viability, mRNA, and protein expression of stem cells culture in growth media. Biomed. Rep. 2017, 7, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Son, J.; Tae, J.Y.; Min, S.K.; Ko, Y.; Park, J.B. Fibroblast growth factor-4 maintains cellular viability while enhancing osteogenic differentiation of stem cell spheroids in part by regulating RUNX2 and BGLAP expression. Exp. Therap. Med. 2020, 20, 2013–2020. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Hou, Y.; Song, P.; Zhou, M.; Nie, M.; Liu, X. Modulation of proliferation and differentiation of gingiva-derived mesenchymal stem cells by concentrated growth factors: Potential implications in tissue engineering for dental regeneration and repair. Int. J. Mol. Med. 2019, 44, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Mundy, C.; Gannon, M.; Popoff, S.N. Connective tissue growth factor (CTGF/CCN2) negatively regulates BMP-2 induced osteoblast differentiation and signaling. J. Cell. Physiol. 2014, 229, 672–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanaan, R.A.; Aldwaik, M.; Al-Hanbali, O.A. The role of connective tissue growth factor in skeletal growth and development. Med. Sci. Monit. 2006, 12, Ra277–Ra281. [Google Scholar]
- Safadi, F.F.; Xu, J.; Smock, S.L.; Kanaan, R.A.; Selim, A.H.; Odgren, P.R.; Marks, S.C., Jr.; Owen, T.A.; Popoff, S.N. Expression of connective tissue growth factor in bone: Its role in osteoblast proliferation and differentiation in vitro and bone formation in vivo. J. Cell Physiol. 2003, 196, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Yoo, J.H.; Lee, J.H.; Lee, Y.; Bae, M.K.; Kim, Y.D.; Kim, H.J. Connective tissue growth factor (CTGF) regulates the fusion of osteoclast precursors by inhibiting Bcl6 in periodontitis. Int. J. Med. Sci. 2020, 17, 647–656. [Google Scholar] [CrossRef] [Green Version]
- Smerdel-Ramoya, A.; Zanotti, S.; Stadmeyer, L.; Durant, D.; Canalis, E. Skeletal overexpression of connective tissue growth factor impairs bone formation and causes osteopenia. Endocrinology 2008, 149, 4374–4381. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Min, S.K.; Park, J.B. Effects of demographic factors on adipogenic and chondrogenic differentiation in bone marrow-derived stem cells. Exp. Ther. Med. 2019, 17, 3548–3554. [Google Scholar] [CrossRef] [Green Version]
- Ryu, N.E.; Lee, S.H.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.H.; Lee, J.E.; Yun, J.H.; Kim, I.; Ko, Y.; Park, J.B. Isolation and characterization of human mesenchymal stem cells from gingival connective tissue. J. Period. Res. 2015, 50, 461–467. [Google Scholar] [CrossRef]
- Lee, S.I.; Yeo, S.I.; Kim, B.B.; Ko, Y.; Park, J.B. Formation of size-controllable spheroids using gingiva-derived stem cells and concave microwells: Morphology and viability tests. Biomed. Rep. 2016, 4, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Tae, J.Y.; Ko, Y.; Park, J.B. Evaluation of fibroblast growth factor-2 on the proliferation of osteogenic potential and protein expression of stem cell spheroids composed of stem cells derived from bone marrow. Exp. Ther. Med. 2019, 18, 326–331. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.; Na, C.B.; Park, J.B. The effects of simvastatin on cellular viability, stemness and osteogenic differentiation using 3-dimensional cultures of stem cells and osteoblast-like cells. Adv. Clin. Exp. Med. Off. Organ Wroclaw Med. Univ. 2019, 28, 699–706. [Google Scholar] [CrossRef]
- Min, S.K.; Kim, M.; Park, J.B. Bone morphogenetic protein 2-enhanced osteogenic differentiation of stem cell spheres by regulation of Runx2 expression. Exp. Ther. Med. 2020, 20, 79. [Google Scholar] [CrossRef] [PubMed]
- Tae, J.-Y.; Park, Y.-H.; Ko, Y.; Park, J.-B. The Effects of Bone Morphogenetic Protein-4 on Cellular Viability, Osteogenic Potential, and Global Gene Expression on Gingiva-Derived Stem Cell Spheroids. Coatings 2020, 10, 1055. [Google Scholar] [CrossRef]
- Lee, H.-J.; Min, S.-K.; Park, Y.-H.; Park, J.-B. Application of Bone Morphogenetic Protein 7 Enhanced the Osteogenic Differentiation and Mineralization of Bone Marrow-Derived Stem Cells Cultured on Deproteinized Bovine Bone. Coatings 2021, 11, 642. [Google Scholar] [CrossRef]
- Kang, S.H.; Park, J.B.; Kim, I.; Lee, W.; Kim, H. Assessment of stem cell viability in the initial healing period in rabbits with a cranial bone defect according to the type and form of scaffold. J. Period. Implant Sci. 2019, 49, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Son, J.; Yi, G.; Koo, H.; Park, J.B. Cellular viability and osteogenic differentiation potential of human gingiva-derived stem cells in 2D culture following treatment with anionic, cationic, and neutral liposomes containing doxorubicin. Exp. Ther. Med. 2018, 16, 4457–4462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrobel, E.; Leszczynska, J.; Brzoska, E. The Characteristics Of Human Bone-Derived Cells (HBDCS) during osteogenesis in vitro. Cellul. Mol. Biol. Lett. 2016, 21, 26. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.E.; Kim, T.G.; Lee, Y.H.; Yi, H.K. Phelligridin D maintains the function of periodontal ligament cells through autophagy in glucose-induced oxidative stress. J. Period. Implant Sci. 2020, 50, 291–302. [Google Scholar] [CrossRef]
- Tsai, M.T.; Li, W.J.; Tuan, R.S.; Chang, W.H. Modulation of osteogenesis in human mesenchymal stem cells by specific pulsed electromagnetic field stimulation. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2009, 27, 1169–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomar, G.B.; Srivastava, R.K.; Gupta, N.; Barhanpurkar, A.P.; Pote, S.T.; Jhaveri, H.M.; Mishra, G.C.; Wani, M.R. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem. Biophys. Res. Commun. 2010, 393, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Farsani, T.; Motevaseli, E.; Neyazi, N.; Khorramizadeh, M.R.; Zafarvahedian, E.; Ghahremani, M.H. Effect of Passage Number and Culture Time on the Expression and Activity of Insulin-Degrading Enzyme in Caco-2 Cells. Ir. Biomed. J. 2018, 22, 70–75. [Google Scholar] [CrossRef]
- van Setten, G.; Berglin, L.; Blalock, T.D.; Schultz, G. Detection of connective tissue growth factor in subretinal Fluid following retinal detachment: Possible contribution to subretinal scar formation, preliminary results. Ophth. Res. 2005, 37, 289–292. [Google Scholar] [CrossRef]
- Paradis, V.; Dargere, D.; Bonvoust, F.; Vidaud, M.; Segarini, P.; Bedossa, P. Effects and regulation of connective tissue growth factor on hepatic stellate cells. Lab. Investig. J. Tech. Methods Pathol. 2002, 82, 767–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Setten, G.B.; Blalock, T.D.; Grotendorst, G.; Schultz, G.S. Detection of connective tissue growth factor (CTGF) in human tear fluid: Preliminary results. Acta Ophthalmol. Scand. 2003, 81, 51–53. [Google Scholar] [CrossRef]
- Payr, S.; Rosado-Balmayor, E.; Tiefenboeck, T.; Schuseil, T.; Unger, M.; Seeliger, C.; van Griensven, M. Direct comparison of 3D and 2D cultivation reveals higher osteogenic capacity of elderly osteoblasts in 3D. J. Orthop. Surg. Res. 2021, 16, 13. [Google Scholar] [CrossRef]
- Lv, H.; Yang, H.; Wang, Y. Effects of miR-103 by negatively regulating SATB2 on proliferation and osteogenic differentiation of human bone marrow mesenchymal stem cells. PLoS ONE 2020, 15, e0232695. [Google Scholar] [CrossRef] [PubMed]
- Malaval, L.; Wade-Guéye, N.M.; Boudiffa, M.; Fei, J.; Zirngibl, R.; Chen, F.; Laroche, N.; Roux, J.P.; Burt-Pichat, B.; Duboeuf, F.; et al. Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. J. Exp. Med. 2008, 205, 1145–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Song, Y.; Park, Y.H.; Uddin, M.S.; Park, J.B. Evaluation of the Effects of Cuminum cyminum on Cellular Viability, Osteogenic Differentiation and Mineralization of Human Bone Marrow-Derived Stem Cells. Med. Kaunas Lith. 2021, 57, 38. [Google Scholar] [CrossRef]
- Murphy, C.M.; Duffy, G.P.; Schindeler, A.; O’Brien F, J. Effect of collagen-glycosaminoglycan scaffold pore size on matrix mineralization and cellular behavior in different cell types. J. Biomed. Mater. Res. Part A 2016, 104, 291–304. [Google Scholar] [CrossRef]
- Köllmer, M.; Buhrman, J.S.; Zhang, Y.; Gemeinhart, R.A. Markers Are Shared Between Adipogenic and Osteogenic Differentiated Mesenchymal Stem Cells. J. Dev. Biol. Tissue Eng. 2013, 5, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Zerdoum, A.B.; Duncan, R.L.; Jia, X. Dynamic vibration cooperates with connective tissue growth factor to modulate stem cell behaviors. Tissue Eng. Part A 2014, 20, 1922–1934. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Min, S.K.; Park, Y.-H.; Park, J.-B. The Role of Insulin-Like Growth Factor-2 on the Cellular Viability and Differentiation to the Osteogenic Lineage and Mineralization of Stem Cells Cultured on Deproteinized Bovine Bone Mineral. Appl. Sci. 2020, 10, 5471. [Google Scholar] [CrossRef]
- Liu, J.; Yu, F.; Sun, Y.; Jiang, B.; Zhang, W.; Yang, J.; Xu, G.T.; Liang, A.; Liu, S. Concise reviews: Characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells 2015, 33, 627–638. [Google Scholar] [CrossRef]
- Ramírez-Rámiz, A.; Brunet, L.L.; Lahor-Soler, E.; Miranda-Rius, J. On the Cellular and Molecular Mechanisms of Drug-Induced Gingival Overgrowth. Open Dent. J. 2017, 11, 420–435. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.B.; Tae, J.Y.; Ko, Y.; Park, J.B. Lovastatin increases the proliferation and osteoblastic differentiation of human gingiva-derived stem cells in three-dimensional cultures. Exp. Therap. Med. 2019, 18, 3425–3430. [Google Scholar] [CrossRef] [Green Version]
- Mitrano, T.I.; Grob, M.S.; Carrión, F.; Nova-Lamperti, E.; Luz, P.A.; Fierro, F.S.; Quintero, A.; Chaparro, A.; Sanz, A. Culture and characterization of mesenchymal stem cells from human gingival tissue. J. Periodontol. 2010, 81, 917–925. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.Y.; Lee, J.S.; Cha, J.K.; Kim, S.K.; Kim, C.S. Periodontal healing using a collagen matrix with periodontal ligament progenitor cells in a dehiscence defect model in beagle dogs. J. Period. Implant Sci. 2019, 49, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Tewari, S.; Narula, S.C.; Sharma, R.K.; Tanwar, N. Platelet-rich fibrin along with a modified minimally invasive surgical technique for the treatment of intrabony defects: A randomized clinical trial. J. Period. Implant Sci. 2019, 49, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Nancarrow-Lei, R.; Mafi, P.; Mafi, R.; Khan, W. A Systemic Review of Adult Mesenchymal Stem Cell Sources and their Multilineage Differentiation Potential Relevant to Musculoskeletal Tissue Repair and Regeneration. Curr. Stem Cell Res. Ther. 2017, 12, 601–610. [Google Scholar] [CrossRef]
- Hwang, J.H.; Oh, S.; Kim, S. Improvement of the osteogenic potential of ErhBMP-2-/EGCG-coated biphasic calcium phosphate bone substitute: In vitro and in vivo activity. J. Period. Implant Sci. 2019, 49, 114–126. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnahash, A.Z.; Song, Y.-M.; Min, S.-K.; Lee, H.-J.; Kim, M.-J.; Park, Y.-H.; Park, J.-U.; Park, J.-B. Effects of Connective Tissue Growth Factor on the Cell Viability, Proliferation, Osteogenic Capacity and mRNA Expression of Stem Cell Spheroids. Appl. Sci. 2021, 11, 6572. https://doi.org/10.3390/app11146572
Alnahash AZ, Song Y-M, Min S-K, Lee H-J, Kim M-J, Park Y-H, Park J-U, Park J-B. Effects of Connective Tissue Growth Factor on the Cell Viability, Proliferation, Osteogenic Capacity and mRNA Expression of Stem Cell Spheroids. Applied Sciences. 2021; 11(14):6572. https://doi.org/10.3390/app11146572
Chicago/Turabian StyleAlnahash, Abdullah Zaki, Young-Min Song, Sae-Kyung Min, Hyun-Jin Lee, Min-Ji Kim, Yoon-Hee Park, Je-Uk Park, and Jun-Beom Park. 2021. "Effects of Connective Tissue Growth Factor on the Cell Viability, Proliferation, Osteogenic Capacity and mRNA Expression of Stem Cell Spheroids" Applied Sciences 11, no. 14: 6572. https://doi.org/10.3390/app11146572
APA StyleAlnahash, A. Z., Song, Y.-M., Min, S.-K., Lee, H.-J., Kim, M.-J., Park, Y.-H., Park, J.-U., & Park, J.-B. (2021). Effects of Connective Tissue Growth Factor on the Cell Viability, Proliferation, Osteogenic Capacity and mRNA Expression of Stem Cell Spheroids. Applied Sciences, 11(14), 6572. https://doi.org/10.3390/app11146572






