Effect of a Training Program on Hepatic Fat Content and Cardiometabolic Risk in Postmenopausal Women: The Randomized Controlled Trial
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement and Reporting Philosophy
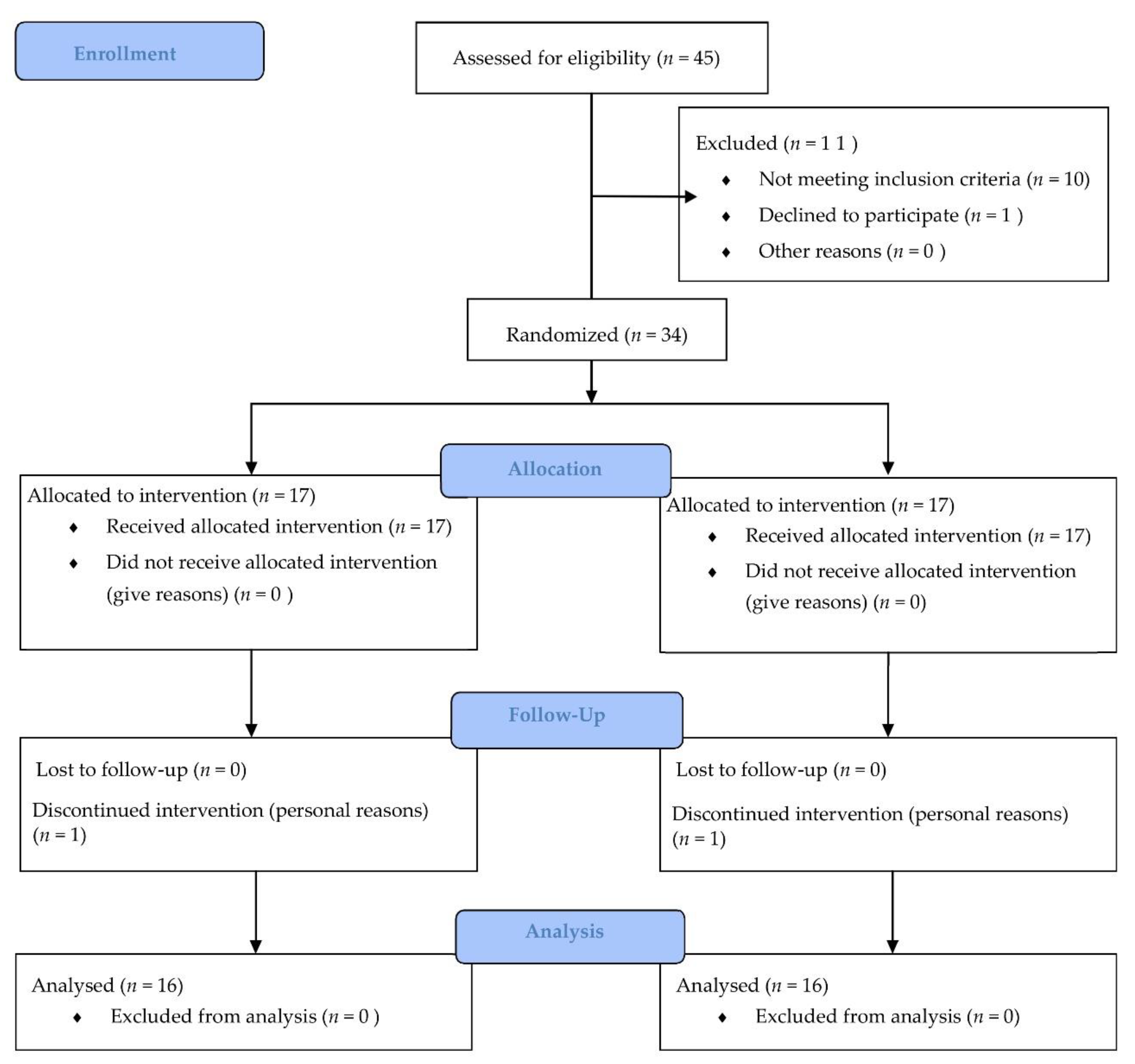
2.2. Participants
2.3. Menopause Status
2.4. Interventions
2.5. Assessments
2.5.1. Body Composition
2.5.2. Blood Pressure
2.5.3. Blood Samples
2.5.4. Cardiometabolic Risk Score
2.5.5. Fatty Liver Index
2.5.6. Physical Fitness Measures
2.6. Statistical Procedures
3. Results
3.1. Sample Characteristics
3.2. Body Composition
3.3. Clinical Outcomes
3.4. Hematological Outcomes
3.5. Physical Fitness Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bullo, V.; Bergamin, M.; Gobbo, S.; Sieverdes, J.C.; Zaccaria, M.; Neunhaeuserer, D.; Ermolao, A. The effects of Pilates exercise training on physical fitness and wellbeing in the elderly: A systematic review for future exercise prescription. Prev. Med. 2015, 75, 1–11. [Google Scholar] [CrossRef]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement From the National Strength and Conditioning Association. J. strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Chughtai, M.; Gwam, C.U.; Mohamed, N.; Khlopas, A.; Sodhi, N.; Sultan, A.A.; Bhave, A.; Mont, M.A. Impact of Physical Activity and Body Mass Index in Cardiovascular and Musculoskeletal Health: A Review. Surg. Technol. Int. 2017, 31, 213–220. [Google Scholar] [PubMed]
- Blodgett, J.; Theou, O.; Kirkland, S.; Andreou, P.; Rockwood, K. The association between sedentary behaviour, moderate-vigorous physical activity and frailty in NHANES cohorts. Maturitas 2015, 80, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Manas, A.; Del Pozo-Cruz, B.; Guadalupe-Grau, A.; Marin-Puyalto, J.; Alfaro-Acha, A.; Rodriguez-Manas, L.; Garcia-Garcia, F.J.; Ara, I. Reallocating Accelerometer-Assessed Sedentary Time to Light or Moderate- to Vigorous-Intensity Physical Activity Reduces Frailty Levels in Older Adults: An Isotemporal Substitution Approach in the TSHA Study. J. Am. Med. Dir. Assoc. 2018, 19, 185.e1–185.e6. [Google Scholar] [CrossRef] [PubMed]
- Reiner, M.; Niermann, C.; Jekauc, D.; Woll, A. Long-term health benefits of physical activity--a systematic review of longitudinal studies. BMC Public Health 2013, 13, 813. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Recommendations on Physical Activity for Health. 2015. Available online: https://www.who.int/dietphysicalactivity/factsheet_recommendations/en/#.XpA0CfV9LJE (accessed on 10 April 2020).
- Sun, F.; Norman, I.J.; While, A.E. Physical activity in older people: A systematic review. BMC Public Health 2013, 13, 449. [Google Scholar] [CrossRef]
- Matthews, C.E.; Chen, K.Y.; Freedson, P.S.; Buchowski, M.S.; Beech, B.M.; Pate, R.R.; Troiano, R.P. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am. J. Epidemiol. 2008, 167, 875–881. [Google Scholar] [CrossRef]
- del Pozo-Cruz, B.; Mañas, A.; Martín-García, M.; Marín-Puyalto, J.; García-García, F.J.; Rodriguez-Mañas, L.; Guadalupe-Grau, A.; Ara, I. Frailty is associated with objectively assessed sedentary behaviour patterns in older adults: Evidence from the Toledo Study for Healthy Aging (TSHA). PLoS ONE 2017, 12, e0183911. [Google Scholar] [CrossRef]
- Pano-Rodriguez, A.; Beltran-Garrido, J.V.; Hernandez-Gonzalez, V.; Reverter-Masia, J. Effects of Whole-Body Electromyostimulation on Physical Fitness in Postmenopausal Women: A Randomized Controlled Trial. Sensors 2020, 20, 1482. [Google Scholar] [CrossRef]
- Fitzpatrick, E.; Dhawan, A. Noninvasive biomarkers in non-alcoholic fatty liver disease: Current status and a glimpse of the future. World J. Gastroenterol. 2014, 20, 10851–10863. [Google Scholar] [CrossRef]
- Koot, B.G.P.; van der Baan-Slootweg, O.H.; Bohte, A.E.; Nederveen, A.J.; van Werven, J.R.; Tamminga-Smeulders, C.L.J.; Merkus, M.P.; Schaap, F.G.; Jansen, P.L.M.; Stoker, J.; et al. Accuracy of prediction scores and novel biomarkers for predicting nonalcoholic fatty liver disease in obese children. Obesity 2013, 21, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Kahl, S.; Strassburger, K.; Nowotny, B.; Livingstone, R.; Kluppelholz, B.; Kessel, K.; Hwang, J.-H.; Giani, G.; Hoffmann, B.; Pacini, G.; et al. Comparison of liver fat indices for the diagnosis of hepatic steatosis and insulin resistance. PLoS ONE 2014, 9, e94059. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, D.; Kim, H.J.; Lee, C.-H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Cho, S.-H.; Sung, M.-W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.S.D. Health benefits of physical activity: The evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef]
- Zhou, K.; Cen, J. The fatty liver index (FLI) and incident hypertension: A longitudinal study among Chinese population. Lipids Health Dis. 2018, 17, 214. [Google Scholar] [CrossRef]
- Balducci, S.; Zanuso, S.; Cardelli, P.; Salvi, L.; Bazuro, A.; Pugliese, L.; Maccora, C.; Iacobini, C.; Conti, F.G.; Nicolucci, A.; et al. Effect of high- versus low-intensity supervised aerobic and resistance training on modifiable cardiovascular risk factors in type 2 diabetes; the Italian Diabetes and Exercise Study (IDES). PLoS ONE 2012, 7, e49297. [Google Scholar]
- Paluch, A.E.; Church, T.S.; Blair, S.N. Effect of an Intensive Exercise Intervention Strategy on Modifiable Cardiovascular Risk Factors in Subjects with Type 2 Diabetes Mellitus. Curr. Cardiovasc. Risk Rep. 2011, 5, 481. [Google Scholar] [CrossRef]
- Green, D.J.; Hopman, M.T.E.; Padilla, J.; Laughlin, M.H.; Thijssen, D.H.J. Vascular Adaptation to Exercise in Humans: Role of Hemodynamic Stimuli. Physiol. Rev. 2017, 97, 495–528. [Google Scholar] [CrossRef]
- Gliemann, L.; Hellsten, Y. The exercise timing hypothesis: Can exercise training compensate for the reduction in blood vessel function after menopause if timed right? J. Physiol. 2019, 597, 4915–4925. [Google Scholar] [CrossRef] [PubMed]
- Pierce, G.L.; Donato, A.J.; LaRocca, T.J.; Eskurza, I.; Silver, A.E.; Seals, D.R. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell 2011, 10, 1032–1037. [Google Scholar] [CrossRef]
- Santos-Parker, J.R.; Strahler, T.R.; Vorwald, V.M.; Pierce, G.L.; Seals, D.R. Habitual aerobic exercise does not protect against micro- or macrovascular endothelial dysfunction in healthy estrogen-deficient postmenopausal women. J. Appl. Physiol. 2017, 122, 11–19. [Google Scholar] [CrossRef]
- McKinney, E.S.; James, S.R.; Murray, S.S.; Nelson, K.A.J. Maternal-Child Nursing; Elsevier: London, UK, 2017; ISBN 9780323401708. [Google Scholar]
- Nyberg, M.; Egelund, J.; Mandrup, C.M.; Nielsen, M.B.; Mogensen, A.S.; Stallknecht, B.; Bangsbo, J.; Hellsten, Y. Early Postmenopausal Phase Is Associated With Reduced Prostacyclin-Induced Vasodilation That Is Reversed by Exercise Training: The Copenhagen Women Study. Hypertension 2016, 68, 1011–1020. [Google Scholar] [CrossRef]
- Tunstall-Pedoe, H.; Kuulasmaa, K.; Amouyel, P.; Arveiler, D.; Rajakangas, A.M.; Pajak, A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation 1994, 90, 583–612. [Google Scholar] [CrossRef]
- Egelund, J.; Jorgensen, P.G.; Mandrup, C.M.; Fritz-Hansen, T.; Stallknecht, B.; Bangsbo, J.; Nyberg, M.; Hellsten, Y. Cardiac Adaptations to High-Intensity Aerobic Training in Premenopausal and Recent Postmenopausal Women: The Copenhagen Women Study. J. Am. Heart Assoc. 2017, 6, e005469. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef]
- Hwang, C.-L.; Yoo, J.-K.; Kim, H.-K.; Hwang, M.-H.; Handberg, E.M.; Petersen, J.W.; Christou, D.D. Novel all-extremity high-intensity interval training improves aerobic fitness, cardiac function and insulin resistance in healthy older adults. Exp. Gerontol. 2016, 82, 112–119. [Google Scholar] [CrossRef]
- Osawa, Y.; Azuma, K.; Tabata, S.; Katsukawa, F.; Ishida, H.; Oguma, Y.; Kawai, T.; Itoh, H.; Okuda, S.; Matsumoto, H. Effects of 16-week high-intensity interval training using upper and lower body ergometers on aerobic fitness and morphological changes in healthy men: A preliminary study. Open Access J. Sport. Med. 2014, 5, 257–265. [Google Scholar] [CrossRef]
- Sculthorpe, N.F.; Herbert, P.; Grace, F. One session of high-intensity interval training (HIIT) every 5 days, improves muscle power but not static balance in lifelong sedentary ageing men: A randomized controlled trial. Medicine 2017, 96, e6040. [Google Scholar] [CrossRef]
- Hurst, C.; Weston, K.L.; Weston, M. The effect of 12 weeks of combined upper- and lower-body high-intensity interval training on muscular and cardiorespiratory fitness in older adults. Aging Clin. Exp. Res. 2019, 31, 661–671. [Google Scholar] [CrossRef]
- Dehail, P.; Duclos, C.; Barat, M. Electrical stimulation and muscle strengthening. Ann. Readapt. Med. Phys. 2008, 51, 441–451. [Google Scholar] [CrossRef]
- Wiemspro-Electrostimulation for Fitness. Available online: https://wiemspro.com (accessed on 22 July 2019).
- Kemmler, W.; Bebenek, M.; Engelke, K.; von Stengel, S. Impact of whole-body electromyostimulation on body composition in elderly women at risk for sarcopenia: The Training and ElectroStimulation Trial (TEST-III). Age 2014, 36, 395–406. [Google Scholar] [CrossRef]
- Filipovic, A.; Kleinoder, H.; Pluck, D.; Hollmann, W.; Bloch, W.; Grau, M.; Kleinöder, H.; Plück, D.; Hollmann, W.; Bloch, W.; et al. Influence of Whole-Body Electrostimulation on Human Red Blood Cell Deformability. J. Strength Cond. Res. 2015, 29, 2570–2578. [Google Scholar] [CrossRef]
- Dörmann, U.; Wirtz, N.; Micke, F.; Morat, M.; Kleinöder, H.; Donath, L. The Effects of Superimposed Whole-Body Electromyostimulation During Short-Term Strength Training on Physical Fitness in Physically Active Females: A Randomized Controlled Trial. Front. Physiol. 2019, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Grimm, A.; Bebenek, M.; Kohl, M.; von Stengel, S.; Weissenfels, A.; Teschler, M.; Willert, S.; Bebenek, M.; Shojaa, M.; et al. Effects of Combined Whole-Body Electromyostimulation and Protein Supplementation on Local and Overall Muscle/Fat Distribution in Older Men with Sarcopenic Obesity: The Randomized Controlled Franconia Sarcopenic Obesity (FranSO) Study. Calcif. Tissue Int. 2018, 103, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Weissenfels, A.; Teschler, M.; Willert, S.; Bebenek, M.; Shojaa, M.; Kohl, M.; Freiberger, E.; Sieber, C.; von Stengel, S. Whole-body electromyostimulation and protein supplementation favorably affect sarcopenic obesity in community-dwelling older men at risk: The randomized controlled FranSO study. Clin. Interv. Aging 2017, 12, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Micke, F.; Kleinöder, H.; Dörmann, U.; Wirtz, N.; Donath, L. Effects of an Eight-Week Superimposed Submaximal Dynamic Whole-Body Electromyostimulation Training on Strength and Power Parameters of the Leg Muscles: A Randomized Controlled Intervention Study. Front. Physiol. 2018, 9, 1719. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, A.; DeMarees, M.; Grau, M.; Hollinger, A.; Seeger, B.; Schiffer, T.; Bloch, W.; Gehlert, S. Superimposed Whole-Body Electrostimulation Augments Strength Adaptations and Type II Myofiber Growth in Soccer Players During a Competitive Season. Front. Physiol. 2019, 10, 1187. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, K.; Sieber, C.; Von Stengel, S.; Kohl, M.; Freiberger, E.; Jakob, F.; Lell, M.; Engelke, K.; Kemmler, W. Impact of whole body electromyostimulation on cardiometabolic risk factors in older women with sarcopenic obesity: The randomized controlled FORMOsA-sarcopenic obesity study. Clin. Interv. Aging 2016, 11, 1697–1706. [Google Scholar] [CrossRef]
- Kemmler, W.; Kohl, M.; Freiberger, E.; Sieber, C.; von Stengel, S. Effect of whole-body electromyostimulation and/or protein supplementation on obesity and cardiometabolic risk in older men with sarcopenic obesity: The randomized controlled FranSO trial. BMC Geriatr. 2018, 18, 70. [Google Scholar] [CrossRef] [PubMed]
- Pano-Rodriguez, A.; Beltran-Garrido, J.V.; Hernández-González, V.; Reverter-Masia, J. Effects of whole-body ELECTROMYOSTIMULATION on health and performance: A systematic review. BMC Complement. Altern. Med. 2019, 19, 87. [Google Scholar] [CrossRef] [PubMed]
- Amaro-gahete, F.J.; Delao, A.; Jurado-fasoli, L.; Dote-montero, M.; Gutiérrez, Á.; Ruiz, J.R.; Castillo, M.J. Changes in Physical Fitness After 12 Weeks of Structured Concurrent Exercise Training, High Intensity Interval Training, or Whole-Body Electromyostimulation Training in Sedentary Middle-Aged Adults: A Randomized Controlled Trial. Front. Physiol. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pano-Rodriguez, A.; Beltran-Garrido, J.V.; Hernandez-Gonzalez, V.; Reverter-Masia, J. Effects of Whole Body Electromyostimulation on Physical Fitness and Health in Postmenopausal Women: A Study Protocol for a Randomized Controlled Trial. Front. Public Health 2020, 8, 313. [Google Scholar] [CrossRef]
- Pano-Rodriguez, A.; Beltran-Garrido, J.V.; Hernandez-Gonzalez, V.; Nasarre-Nacenta, N.; Reverter-Masia, J. Impact of Whole Body Electromyostimulation on Velocity, Power and Body Composition in Postmenopausal Women: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 4982. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, 18–27. [Google Scholar] [CrossRef]
- Moniker Privacy Services Random Team Generator—Split a List into Random Groups. Available online: https://www.randomlists.com/team-generator (accessed on 26 November 2019).
- Sjöström, M.; Oja, P.; Hagströmer, M.; Smith, B.J.; Bauman, A. Health-enhancing physical activity across European Union countries: The Eurobarometer study. J. Public Health 2006, 14, 291–300. [Google Scholar] [CrossRef]
- Kovanen, V.; Aukee, P.; Kokko, K.; Finni, T.; Tarkka, I.M.; Tammelin, T.; Kujala, U.M.; Sipilä, S.; Laakkonen, E.K. Design and protocol of Estrogenic Regulation of Muscle Apoptosis (ERMA) study with 47 to 55-year-old women’s cohort: Novel results show menopause-related differences in blood count. Menopause 2018, 25, 1020–1032. [Google Scholar] [CrossRef]
- Aragão-Santos, J.C.; de Resende-Neto, A.G.; Costa Nogueira, A.; Feitosa-Neta, M.L.; Albuquerque Brandão, L.H.; da Silva Chaves, L.M.; da Silva-Grigoletto, M.E. The effects of functional and traditional strength training on different parameters of strength elderly women: A trial randomized and controlled. J. Sport. Med. Phys. Fit. 2018, 59, 380–386. [Google Scholar] [CrossRef]
- Gonzalez-Badillo, J.J.; Sanchez-Medina, L. Movement velocity as a measure of loading intensity in resistance training. Int. J. Sports Med. 2010, 31, 347–352. [Google Scholar] [CrossRef]
- Wirtz, N.; Wahl, P.; Kleinöder, H.; Wechsler, K.; Achtzehn, S.; Mester, J.; Kleinder, H.; Wechsler, K.; Achtzehn, S.; Mester, J. Acute metabolic, hormonal, and psychological responses to strength training with superimposed EMS at the beginning and the end of a 6 week training period. J. Musculoskelet. Neuronal Interact. 2015, 15, 325–332. [Google Scholar]
- Reed, J.L.; Pipe, A.L. The talk test: A useful tool for prescribing and monitoring exercise intensity. Curr. Opin. Cardiol. 2014, 29, 475–480. [Google Scholar] [CrossRef]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar]
- Filipovic, A.; Klein Der, H.; Rmann, U.D.; Mester, J. Electromyostimulation—a Systematic Review of the Influence of Training Regimens and Stimulation Parameters on Effectiveness in Electromyostimulation Training of Selected Strength Parameters. J. strength Cond. Res. 2011, 11, 3218–3238. [Google Scholar] [CrossRef] [PubMed]
- Doucet, B.M.; Lam, A.; Griffin, L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J. Biol. Med. 2012, 85, 201–215. Available online: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3375668&tool=pmcentrez&rendertype=abstract (accessed on 3 June 2021). [PubMed]
- A Guide to the Use of Electrical Stimulation in Pediatric Nurodisbility/Association of Paediatric Chartered Physiotherapists. Available online: https://apcp.csp.org.uk/content/guide-use-electrical-stimulation-paedaitric-neurodisability (accessed on 19 December 2019).
- Deley, G.; Babault, N. Could low-frequency electromyostimulation training be an effective alternative to endurance training? An overview in one adult. J. Sport. Sci. Med. 2014, 13, 444–450. [Google Scholar]
- Petrofsky, J. The effect of the subcutaneous fat on the transfer of current through skin and into muscle. Med. Eng. Phys. 2008, 30, 1168–1176. [Google Scholar] [CrossRef]
- Kemmler, W.; Schliffka, R.; Mayhew, J.L.; von Stengel, S. Effects of whole-body electromyostimulation on resting metabolic rate, body composition, and maximum strength in postmenopausal women: The Training and ElectroStimulation Trial. J. strength Cond. Res. 2010, 24, 1880–1887. [Google Scholar] [CrossRef]
- Alvero Cruz, J.E. Al Protocolo de valoración de la composición corporal para el reconocimiento médico-deportivo. Documento de Consenso del Grupo Español de Cineantropometría de la Federacion Española de Medicina del Deporte. Arch. Med. Deport. 2009, 26, 166–179. [Google Scholar]
- International Society for Advancement of Kinanthropometry. International Strandars for Anthropometric Assessment: ISAK; International Society for Advancement of Kinanthropometry: Potchefstroom, South Africa, 2001. [Google Scholar]
- Whelton, P.K.; Williams, B. The 2018 European Society of Cardiology/European Society of Hypertension and 2017 American College of Cardiology/American Heart Association Blood Pressure Guidelines: More Similar Than Different. JAMA 2018, 320, 1749–1750. [Google Scholar] [CrossRef] [PubMed]
- Stefanska, A.; Bergmann, K.; Sypniewska, G. Metabolic Syndrome and Menopause: Pathophysiology, Clinical and Diagnostic Significance. Adv. Clin. Chem. 2015, 72, 1–75. [Google Scholar]
- Onat, A.; Karadeniz, Y.; Tusun, E.; Yuksel, H.; Kaya, A. Advances in understanding gender difference in cardiometabolic disease risk. Expert Rev. Cardiovasc. Ther. 2016, 14, 513–523. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Huang, X.; Xu, M.; Chen, Y.; Peng, K.; Huang, Y.; Wang, P.; Ding, L.; Lin, L.; Xu, Y.; Chen, Y.; et al. Validation of the Fatty Liver Index for Nonalcoholic Fatty Liver Disease in Middle-Aged and Elderly Chinese. Medicine 2015, 94, e1682. [Google Scholar] [CrossRef] [PubMed]
- Sagarra-Romero, L.; Vicente-Rodriguez, G.; Pedrero-Chamizo, R.; Vila-Maldonado, S.; Gusi, N.; Villa-Vicente, J.G.; Espino, L.; Gonzalez-Gross, M.; Casajus, J.A.; Ara, I.; et al. Is Sitting Time Related with Physical Fitness in Spanishelderly Population? The EXERNET Multicenter Study. J. Nutr. Health Aging 2019, 23, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Colado, J.C.; Triplett, N.T. Effects of a short-term resistance program using elastic bands versus weight machines for sedentary middle-aged women. J. Strength Cond. Res. 2008, 22, 1441–1448. [Google Scholar] [CrossRef]
- Bajerska, J.; Chmurzynska, A.; Muzsik, A.; Krzyzanowska, P.; Madry, E.; Malinowska, A.M.; Walkowiak, J. Weight loss and metabolic health effects from energy-restricted Mediterranean and Central-European diets in postmenopausal women: A randomized controlled trial. Sci. Rep. 2018, 8, 11170. [Google Scholar] [CrossRef]
- Aranda-Ramírez, P.; López-Jurado, M.; Llopis-González, J.; Ruiz-Cabello-Turmo, P.; del Mar Fernández-Martínez, M. The effectiveness of an education program and nutritional intervention, integrated health and physical activity in postmenopausal women. Nutr. Hosp. 2016, 33, 359–367. [Google Scholar]
- Mataix, J.; Lopez-Frias, M.; Martinez-de-Victoria, E.; Lopez-Jurado, M.; Aranda, P.; Llopis, J. Factors associated with obesity in an adult Mediterranean population: Influence on plasma lipid profile. J. Am. Coll. Nutr. 2005, 24, 456–465. [Google Scholar] [CrossRef]
- Cornellana, M.J.; Harvey, X.; Carballo, A.; Khartchenko, E.; Llaneza, P.; Palacios, S.; Mendoza, N. Sexual health in Spanish postmenopausal women presenting at outpatient clinics. Climacteric 2017, 20, 164–170. [Google Scholar] [CrossRef]
- Hita-Contreras, F.; Zagalaz-Anula, N.; Martinez-Amat, A.; Cruz-Diaz, D.; Sanchez-Montesinos, I.; Aibar-Almazan, A.; Lomas-Vega, R. Sleep quality and its association with postural stability and fear of falling among Spanish postmenopausal women. Menopause 2018, 25, 62–69. [Google Scholar] [CrossRef]
- Keating, S.E.; Machan, E.A.; O’Connor, H.T.; Gerofi, J.A.; Sainsbury, A.; Caterson, I.D.; Johnson, N.A. Continuous exercise but not high intensity interval training improves fat distribution in overweight adults. J. Obes. 2014, 2014, 834865. [Google Scholar] [CrossRef]
- Olinto, M.T.A.; Nacul, L.C.; Gigante, D.P.; Costa, J.S.D.; Menezes, A.M.B.; Macedo, S. Waist circumference as a determinant of hypertension and diabetes in Brazilian women: A population-based study. Public Health Nutr. 2004, 7, 629–635. [Google Scholar] [CrossRef]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.-Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Birlauf, A. Einfluss eines Elektromyostimulations-trainings auf die körperzusammensetzung bei älteren männern mit metabolischem syndrom. Dtsch. Z. Sportmed. 2010, 61, 117–123. [Google Scholar]
- Kemmler, W.; Birlauf, A.; von Stengel, S. Effects of Whole-Body-Electromyostimulation on Body Composition and Cardiac Risk Factors in Elderly Men with the Metabolic Syndrome. The TEST-II Study. Dtsch. Z. Sportmed. 2010, 61, 117–123. [Google Scholar]
- Kemmler, W.; von Stengel, S.; Engelke, K.; Häberle, L.; Mayhew, J.L.; Kalender, W.A. Exercise, Body Composition, and Functional Ability. A Randomized Controlled Trial. Am. J. Prev. Med. 2010, 38, 279–287. [Google Scholar] [CrossRef]
- Kemmler, W.; Schliffka, R.; Mayhew, J.L.; von Stengel, S. Effects of whole body electromyostimulation on resting metabolic rate, body composition in postmenopausal women. J. Strength Cond. Res. 2013, 7, 1880–1887. [Google Scholar]
- Kemmler, W.; Teschler, M.; Weißenfels, A.; Bebenek, M.; Fröhlich, M.; Kohl, M.; von Stengel, S.; Weissenfels, A.; Bebenek, M.; Frohlich, M.; et al. Effects of whole-body electromyostimulation versus high-intensity resistance exercise on body composition and strength: A randomized controlled study. Evid. Based Complement. Altern. Med. 2016, 2016, 9236809. [Google Scholar] [CrossRef]
- Filipovic, A.; Grau, M.; Kleinoeder, H.; Zimmer, P.; Hollmann, W.; Bloch, W. Effects of a Whole-Body Electrostimulation Program on Strength, Sprinting, Jumping, and Kicking Capacity in Elite Soccer Players. J. Sport. Sci. Med. 2016, 15, 639–648. [Google Scholar]
- Wirtz, N.; Zinner, C.; Doermann, U.; Kleinoeder, H.; Mester, J. Effects of Loaded Squat Exercise with and without Application of Superimposed EMS on Physical Performance. J. Sport. Sci. Med. 2016, 15, 26–33. [Google Scholar]
- Pescatello, L.S.; MacDonald, H.V.; Lamberti, L.; Johnson, B.T. Exercise for Hypertension: A Prescription Update Integrating Existing Recommendations with Emerging Research. Curr. Hypertens. Rep. 2015, 17, 87. [Google Scholar] [CrossRef]
- Whelton, S.P.; Chin, A.; Xin, X.; He, J. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Ann. Intern. Med. 2002, 136, 493–503. [Google Scholar] [CrossRef]
- Dimeo, F.; Pagonas, N.; Seibert, F.; Arndt, R.; Zidek, W.; Westhoff, T.H. Aerobic exercise reduces blood pressure in resistant hypertension. Hypertens 2012, 60, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Kastner, A.; Braun, M.; Meyer, T. Two Cases of Rhabdomyolysis After Training With Electromyostimulation by 2 Young Male Professional Soccer Players. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2015, 25, e71–e73. [Google Scholar] [CrossRef] [PubMed]
- Stollberger, C.; Finsterer, J. Side effects of and contraindications for whole-body electro-myo-stimulation: A viewpoint. BMJ Open Sport Exerc. Med. 2019, 5, e000619. [Google Scholar] [CrossRef]
- Hong, J.Y.; Hyeok Oh, J.; Shin, J.-H. Rhabdomyolysis caused by knee push-ups with whole body electromyostimulation. Br. J. Hosp. Med. 2016, 77, 542–543. [Google Scholar] [CrossRef] [PubMed]
- Teschler, M.; Mooren, F.C. (Whole-Body) Electromyostimulation, Muscle Damage, and Immune System: A Mini Review. Front. Physiol. 2019, 10, 1461. [Google Scholar] [CrossRef] [PubMed]
- Teschler, M.; Weissenfels, A.; Froehlich, M.; Kohl, M.; Bebenek, M.; von Stengel, S.; Kemmler, W. (Very) high creatine kinase (CK) levels after Whole-Body Electromyostimulation. Are there implications for health? Int. J. Clin. Exp. Med. 2016, 9, 22841–22850. [Google Scholar]
- Castagno, E.; Lupica, M.; Viola, S.; Savino, F.; Miniero, R. Creatin-kinase elevation after accidental ingestion of almotriptan in an 18-month-old girl. Minerva Pediatr. 2014, 66, 95–97. [Google Scholar]
- Khan, F.Y. Rhabdomyolysis: A review of the literature. Neth. J. Med. 2009, 67, 272–283. [Google Scholar]
- Quiroga, E.V.; Arze, S.A. Rabdomiolisis, mioglobinuria e injuria renal aguda inducida por el ejercicio: Reporte de un caso en el Centro Médico Boliviano Belga. Gac. Médica Boliv. 2014, 37, 27–30. [Google Scholar]
- Arslan, E.; Can, S.; Demirkan, E. Effect of short-term aerobic and combined training program on body composition, lipids profile and psychological health in premenopausal women. Sci. Sports 2017, 32, 106–113. [Google Scholar] [CrossRef]
- Del Pozo-Cruz, J.; Magaña, M.; Ballesteros, M.; Porras, M.; Bíes, E.R.; Navas, P.; López-Lluch, G. Influencia de la capacidad funcional sobre el perfil lipídico, daño muscular y perfil bioquímico en personas mayores no institucionalizadas. Rev. Andaluza Med. Deport. 2013, 6, 57–65. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Xu, D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017, 16, 132. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, D.; Wang, R.; Fu, W.; Zhang, S. Relationship between Muscle Mass/Strength and Hepatic Fat Content in Post-Menopausal Women. Medicina 2019, 55, 629. [Google Scholar] [CrossRef]
- Wang, C.; Bai, L. Sarcopenia in the elderly: Basic and clinical issues. Geriatr. Gerontol. Int. 2012, 12, 388–396. [Google Scholar] [CrossRef]
- Wong, V.W.-S.; Wong, G.L.-H.; Yip, G.W.-K.; Lo, A.O.-S.; Limquiaco, J.; Chu, W.C.-W.; Chim, A.M.-L.; Yu, C.-M.; Yu, J.; Chan, F.K.-L.; et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut 2011, 60, 1721–1727. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011, 43, 617–649. [Google Scholar] [CrossRef]
- Sung, K.-C.; Kim, S.H. Interrelationship between fatty liver and insulin resistance in the development of type 2 diabetes. J. Clin. Endocrinol. Metab. 2011, 96, 1093–1097. [Google Scholar] [CrossRef]
- Banitalebi, E.; Faramarzi, M.; Nasiri, S.; Mardaniyan, M.; Rabiee, V. Effects of different exercise modalities on novel hepatic steatosis indices in overweight women with type 2 diabetes. Clin. Mol. Hepatol. 2019, 25, 294–304. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Kozakova, M.; Hojlund, K.; Flyvbjerg, A.; Favuzzi, A.; Mitrakou, A.; Balkau, B. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology 2009, 49, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, I.; Buezo, I.; Exposito, C.; Pera, G.; Rodriguez, L.; Aluma, A.; Auladell, M.A.; Toran, P.; Caballeria, L. Non-invasive markers of fibrosis in the diagnosis of non-alcoholic fatty liver disease. Gastroenterol. Hepatol. 2014, 37, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Balducci, S.; Cardelli, P.; Pugliese, L.; D’Errico, V.; Haxhi, J.; Alessi, E.; Iacobini, C.; Menini, S.; Bollanti, L.; Conti, F.G.; et al. Volume-dependent effect of supervised exercise training on fatty liver and visceral adiposity index in subjects with type 2 diabetes The Italian Diabetes Exercise Study (IDES). Diabetes Res. Clin. Pract. 2015, 109, 355–363. [Google Scholar] [CrossRef]
- Barsalani, R.; Riesco, E.; Lavoie, J.-M.; Dionne, I.J. Effect of exercise training and isoflavones on hepatic steatosis in overweight postmenopausal women. Climacteric 2013, 16, 88–95. [Google Scholar] [CrossRef]
- Byambasukh, O.; Zelle, D.; Corpeleijn, E. Physical Activity, Fatty Liver, and Glucose Metabolism Over the Life Course: The Lifelines Cohort. Am. J. Gastroenterol. 2019, 114, 907–915. [Google Scholar] [CrossRef]
- Rossi, F.E.; Fortaleza, A.C.S.; Neves, L.M.; Buonani, C.; Picolo, M.R.; Diniz, T.A.; Kalva-Filho, C.A.; Papoti, M.; Lira, F.S.; Freitas Junior, I.F. Combined Training (Aerobic Plus Strength) Potentiates a Reduction in Body Fat but Demonstrates No Difference on the Lipid Profile in Postmenopausal Women When Compared With Aerobic Training With a Similar Training Load. J. Strength Cond. Res. 2016, 30, 226–234. [Google Scholar] [CrossRef]
- Amaro-Gahete, F.J.; De-la-O, A.; Jurado-Fasoli, L.; Martinez-Tellez, B.; Ruiz, J.R.; Castillo, M.J. Exercise Training as a Treatment for Cardiometabolic Risk in Sedentary Adults: Are Physical Activity Guidelines the Best Way to Improve Cardiometabolic Health? The FIT-AGEING Randomized Controlled Trial. J. Clin. Med. 2019, 8, 2097. [Google Scholar] [CrossRef]
- Bondarev, D.; Laakkonen, E.K.; Finni, T.; Kokko, K.; Kujala, U.M.; Aukee, P.; Kovanen, V.; Sipila, S. Physical performance in relation to menopause status and physical activity. Menopause 2018, 25, 1432–1441. [Google Scholar] [CrossRef]
- Schink, K.; Herrmann, H.J.; Schwappacher, R.; Meyer, J.; Orlemann, T.; Waldmann, E.; Wullich, B.; Kahlmeyer, A.; Fietkau, R.; Lubgan, D.; et al. Effects of whole-body electromyostimulation combined with individualized nutritional support on body composition in patients with advanced cancer: A controlled pilot trial. BMC Cancer 2018, 18, 1–17. [Google Scholar] [CrossRef]
- Orgaz Gallego, M.P.; Bermejo Lopez, P.; Tricio Armero, M.A.; Abellan Aleman, J.; Solera Albero, J.; Tarraga Lopez, P.J. Metabolic Syndrome and its Components in Spanish Postmenopausal Women. Nutr. Hosp. 2015, 32, 656–666. [Google Scholar]
- Cuadros, J.L.; Fernandez-Alonso, A.M.; Cuadros, A.M.; Chedraui, P.; Perez-Lopez, F.R. Body mass index and its correlation to metabolic and hormone parameters in postmenopausal Spanish women. Gynecol. Endocrinol. 2011, 27, 678–684. [Google Scholar] [CrossRef]
- Hallajzadeh, J.; Khoramdad, M.; Izadi, N.; Karamzad, N.; Almasi-Hashiani, A.; Ayubi, E.; Qorbani, M.; Pakzad, R.; Hasanzadeh, A.; Sullman, M.J.M.; et al. Metabolic syndrome and its components in premenopausal and postmenopausal women: A comprehensive systematic review and meta-analysis on observational studies. Menopause 2018, 25, 1155–1164. [Google Scholar] [CrossRef]
- WHO. Diet, Nutrition and the Prevention of Chronic Diseases Report of the Joint WHO/FAO Expert Consultation. 2014. Available online: https://www.who.int/dietphysicalactivity/publications/trs916/intro/en/#.XpG6E15gunU (accessed on 11 April 2020).
- WHO/Europe. Data and Evidence—European Health for All Family of Databases (HFA-DB). Available online: http://www.euro.who.int/en/data-and-evidence/databases/european-health-for-all-family-of-databases-hfa-db (accessed on 11 April 2020).
- Messier, V.; Rabasa-Lhoret, R.; Barbat-Artigas, S.; Elisha, B.; Karelis, A.D.; Aubertin-Leheudre, M. Menopause and sarcopenia: A potential role for sex hormones. Maturitas 2011, 68, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Trombetti, A.; Reid, K.F.; Hars, M.; Herrmann, F.R.; Pasha, E.; Phillips, E.M.; Physiology, E.; Human, J.M. Age-associated declines in muscle mass, strength, power, and physical performance: Impact on fear of falling and quality of life. Osteoporos. Int. 2016, 27, 463–471. [Google Scholar] [CrossRef]
- McKinnon, N.B.; Connelly, D.M.; Rice, C.L.; Hunter, S.W.; Doherty, T.J. Neuromuscular contributions to the age-related reduction in muscle power: Mechanisms and potential role of high velocity power training. Ageing Res. Rev. 2017, 35, 147–154. [Google Scholar] [CrossRef]
- Kemmler, W.; von Stengel, S. Whole-body electromyostimulation as a means to impact muscle mass and abdominal body fat in lean, sedentary, older female adults: Subanalysis of the TEST-III trial. Clin. Interv. Aging 2013, 8, 1353–1364. [Google Scholar] [CrossRef]
- Von Stengel, S.; Bebenek, M.; Engelke, K.; Kemmler, W. Whole-body electromyostimulation to fight osteopenia in elderly females: The randomized controlled training and electrostimulation trial (TEST-III). J. Osteoporos. 2015, 2015, 643520. [Google Scholar] [CrossRef]
- Eskurza, I.; Donato, A.J.; Moreau, K.L.; Seals, D.R.; Tanaka, H. Changes in maximal aerobic capacity with age in endurance-trained women: 7-yr follow-up. J. Appl. Physiol. 2002, 92, 2303–2308. [Google Scholar] [CrossRef]
- Bouchard, D.R.; Heroux, M.; Janssen, I. Association between muscle mass, leg strength, and fat mass with physical function in older adults: Influence of age and sex. J. Aging Health 2011, 23, 313–328. [Google Scholar] [CrossRef]
- Ruiz, J.R.; Segura-Jimenez, V.; Ortega, F.B.; Alvarez-Gallardo, I.C.; Camiletti-Moiron, D.; Aparicio, V.A.; Carbonell-Baeza, A.; Femia, P.; Munguia-Izquierdo, D.; Delgado-Fernandez, M. Objectively measured sedentary time and physical activity in women with fibromyalgia: A cross-sectional study. BMJ Open 2013, 3, e002722. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Gallardo, I.C.; Carbonell-Baeza, A.; Segura-Jimenez, V.; Soriano-Maldonado, A.; Intemann, T.; Aparicio, V.A.; Estevez-Lopez, F.; Camiletti-Moiron, D.; Herrador-Colmenero, M.; Ruiz, J.R.; et al. Physical fitness reference standards in fibromyalgia: The al-Andalus project. Scand. J. Med. Sci. Sports 2017, 27, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Gowans, S.E.; deHueck, A. Pool exercise for individuals with fibromyalgia. Curr. Opin. Rheumatol. 2007, 19, 168–173. [Google Scholar] [CrossRef] [PubMed]
| Outcome | All | EX+WB-EMS | EX | p-Value |
|---|---|---|---|---|
| Age (years) | 61.59 ± 3.95 | 62.94 ± 3.32 | 60.25 ± 4.17 | 0.053 |
| Body mass (kg) | 67.44 ± 10.84 | 67.11 ± 11.84 | 67.78 ± 10.12 | 0.866 |
| Height (cm) | 158.32 ± 5.28 | 156.69 ± 5.02 | 159.94 ± 5.18 | 0.081 |
| Body mass index (kg/m2) | 26.91 ± 4.11 | 27.29 ± 4.25 | 26.54 ± 4.08 | 0.614 |
| Outcome | Group | Week 0 | Week 10 | MD [95% CI] | p (Time) | p (Group × Time) |
|---|---|---|---|---|---|---|
| Weight (kg) | All | 67.44 ± 10.84 | 67.15 ± 11.04 | 0.29 [−0.27, 0.85] | 0.295 | 0.991 |
| EX + WB-EMS | 67.11 ± 11.84 | 66.82 ± 12.10 | 0.29 [−0.80, 1,38] | |||
| EX | 67.78 ± 10.12 | 67.49 ± 10.27 | 0.29 [−0.80, 1,38] | |||
| BMI (kg/m2) | All | 26.91 ± 4.11 | 26.81 ± 4.16 | 0.10 [−0.13, 0.34] | 0.367 | 0.853 |
| EX + WB-EMS | 27.29 ± 4.25 | 27.16 ± 4.28 | 0.13 [−0.33, 0.58] | |||
| EX | 26.54 ± 4.08 | 26.46 ± 4.15 | 0.08 [−0.37, 0.54] | |||
| Waist (cm) | All | 82.02 ± 16.99 | 83.86 ± 10.50 | −1.83 [−6.85, 2.84] | 0.428 | 0.283 |
| EX + WB-EMS | 84.13 ± 10.88 | 83.47 ± 10.11 | 0.66 [−8.48, 9.81] | |||
| EX | 79.90 ± 21.64 | 84.24 ± 11.19 | −4.34 [−13.48, 4.80] |
| Outcome | Group | Week 0 | Week 10 | Estimated Mean Difference [95% CI] | p (Time) | p (Group × Time) |
|---|---|---|---|---|---|---|
| Blood pressure | ||||||
| Systolic blood pressure (mm Hg) | All | 121.14 ± 16.80 | 127.00 ± 20.76 | 6.88 [0.80, 12.96] | 0.028 | 0.516 |
| EX + WB-EMS | 122.64 ± 16.90 | 131.46 ± 21.88 | 8.82 [−4.05, 21.69] | |||
| EX | 119.60 ± 18.24 | 124.53 ± 18.00 | 4.93 [−6.09, 15.95] | |||
| Diastolic blood pressure (mm Hg) | All | 73.32 ± 7.89 | 76.70 ± 7.29 | 3.60 [1.03, 6.17] | 0.008 | 0.211 |
| EX + WB-EMS | 74.00 ± 7.94 | 76.00 ± 6.40 | 2.00 [−3.43, 7.43] | |||
| EX | 72.33 ± 8.40 | 77.53 ± 7.47 | 5.20 [0.55, 9,85] | |||
| Mean blood pressure (mm Hg) | All | 89.26 ± 9.61 | 93.47 ± 10.49 | 4.69 [1.95, 7.43] | 0.002 | 0.755 |
| EX + WB-EMS | 90.21 ± 8.81 | 94.49 ± 9.94 | 4.27 [−1.53, 10.07] | |||
| EX | 88.09 ± 10.89 | 93.20 ± 9.70 | 5.11 [0.14, 10.08] | |||
| Liver function | ||||||
| ALT (IU/L) | All | 24.72 ± 16.04 | 21.28 ± 13.39 | −3.44 [−10.31, 3.43] | 0.315 | 0.203 |
| EX + WB-EMS | 30.81 ± 20.35 | 23.00 ± 16.96 | −7.81 [−21.25, 5.63] | |||
| EX | 18.63 ± 6.17 | 19.56 ± 8.76 | 0.94 [−12.50, 14.38] | |||
| GGT (IU/L) | All | 26.91 ± 21.74 | 22.56 ± 13.04 | −4.34 [−12.12, 3.43] | 0.263 | 0.655 |
| EX + WB-EMS | 31.46 ± 28.80 | 25.50 ± 16.55 | −6.06 [−21.26, 9.14] | |||
| EX | 22.25 ± 10.04 | 19.63 ± 7.68 | −2.63 [17.83, 12.58] | |||
| Fatty liver index | All | 30.70 ± 27.67 | 28.16 ± 25.78 | −2.55 [−6.79, 1.70] | 0.230 | 0.381 |
| EX + WB-EMS | 34.05 ± 29.11 | 29.65 ± 26.54 | −4.39 [−12.70, 3.91] | |||
| EX | 27.36 ± 26.67 | 26.66 ± 25.78 | −0.70 [−9.01, 7.61] | |||
| Markers of overtraining | ||||||
| Creatine kinase (CPK; IU/L) | All | 101.66 ± 39.33 | 132.75 ± 67.21 | 31.09 [8.74, 53.45] | 0.008 | 0.182 |
| EX + WB-EMS | 94.19 ± 41.45 | 140.25 ± 81.71 | 46.06 [2.33, 89.79] | |||
| EX | 109.13 ± 36.87 | 125.50 ± 48.72 | 16.13 [−27.61, 59.86] | |||
| Creatinine (mg/dL) | All | 0.71 ± 0.10 | 0.70 ± 0.11 | −0.00 [−0.03, 0.02] | 0.812 | 0.812 |
| EX + WB-EMS | 0.71 ± 0.10 | 0.71 ± 0.12 | ||||
| EX | 0.70 ± 0.10 | 0.70 ± 0.11 |
| Outcome | Group | Week 0 | Week 10 | Estimated Mean Difference [95% CI] | p (Time) | p (Group × Time) |
|---|---|---|---|---|---|---|
| Total cholesterol (mg/dL) | All | 222.88 ± 25.95 | 218.88 ± 31.10 | −4.00 [−14.04, 6.04] | 0.422 | 0.920 |
| EX + WB-EMS | 219.19 ± 29.90 | 215.69 ± 37.15 | −3.50 [−23.14, 16.14] | |||
| EX | 226.56 ± 21.65 | 222.06 ± 24.43 | −4.50 [−24.14, 15.14] | |||
| HDL (mmol/L) | All | 64.78 ± 12.88 | 65.88 ± 14.08 | 1.09 [−2.16, 4.34] | 0.497 | 0.547 |
| EX + WB-EMS | 66.19 ± 15.38 | 68.25 ± 15.64 | 2.06 [−4.29, 8.42] | |||
| EX | 63.38 ± 10.10 | 63.50 ± 12.37 | 0.13 [−6.23, 6.48] | |||
| LDL (mmol/L) | All | 142.45 ± 32.64 | 135.67 ± 25.70 | −6.78 [−18.05, 4.49] | 0.229 | 0.624 |
| EX + WB-EMS | 134.20 ± 22.76 | 130.15 ± 28.67 | −4.05 [−26.10, 18.00] | |||
| EX | 150.70 ± 39.23 | 141.19 ± 21.88 | −9.51 [−31.56, 12.54] | |||
| Triglycerids (mmol/L) | All | 93.84 ± 35.21 | 86.97 ± 23.22 | −6.88 [.18.28, 4.53] | 0.228 | 0.903 |
| EX + WB-EMS | 94.00 ± 41.79 | 86.44 ± 22.22 | −7.56 [−29.88, 14.75] | |||
| EX | 93.69 ± 28.56 | 87.50 ± 24.91 | −6.19 [−28.50, 16.13] | |||
| Glucose (mmol/L) | All | 90.78 ± 10.52 | 94.97 ± 10.16 | 4.19 [0.88, 7.50] | 0.015 | 0.848 |
| EX + WB-EMS | 91.38 ± 11.88 | 95.88 ± 8.39 | 4.50 [−1.97, 10.97] | |||
| EX | 90.19 ± 9.30 | 94.06 ± 11.89 | 3.88 [−2.59, 10.34] | |||
| Cardiometabolic risk score | All | −0.26 ± 0.88 | −0.07 ± 0.52 | 0.19 [−0.13, 0.51] | 0.235 | 0.574 |
| EX + WB-EMS | −0.42 ± 1.09 | −0.14 ± 0.44 | 0.28 [−0.35, 0.91] | |||
| EX | −0.10 ± 0.62 | 0.00 ± 0.59 | 0.10 [−0.53, 0.73] |
| Outcome | Group | Week 0 | Week 10 | Estimated Mean Difference [95% CI] | p (Time) | p (Group × Time) |
|---|---|---|---|---|---|---|
| Strength right arm (reps) | All | 15.53 ± 2.57 | 20.84 ± 2.65 | 5.31 [4.24, 6.38] | <0.001 | 0.031 |
| EX + WB-EMS | 14.81 ± 2.26 | 21.31 ± 3.05 | 6.50 [4.41, 8.59] | |||
| EX | 16.25 ± 2.72 | 20.375 ± 2.19 | 4.13 [2.03, 6.22] | |||
| Strength left arm (reps) | All | 15.78 ± 2.46 | 21.25 ± 2.89 | 5.47 [4.58, 6.36] | <0.001 | 0.018 |
| EX + WB-EMS | 15.06 ± 1.73 | 21.63 ± 3.074 | 6.56 [4.82, 8.31] | |||
| EX | 16.50 ± 2.90 | 20.88 ± 2.73 | 4.38 [2.63, 6.12] | |||
| 6 min walk test (m) | All | 567.90 ± 57.32 | 658.50 ± 82.74 | 90.60 [73.96, 107.24] | <0.001 | <0.001 |
| EX + WB-EMS | 561.78 ± 54.58 | 717.31 ± 59.91 # | 155.54 [122.99, 188.08] | |||
| EX | 574.03 ± 61.09 | 599.688 ± 56.41 | 25.66 [−6.88, 58.21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reverter-Masia, J.; Pano-Rodriguez, A.; Beltran-Garrido, J.V.; Lecube, A.; Sánchez, E.; Hernández-González, V. Effect of a Training Program on Hepatic Fat Content and Cardiometabolic Risk in Postmenopausal Women: The Randomized Controlled Trial. Appl. Sci. 2021, 11, 6409. https://doi.org/10.3390/app11146409
Reverter-Masia J, Pano-Rodriguez A, Beltran-Garrido JV, Lecube A, Sánchez E, Hernández-González V. Effect of a Training Program on Hepatic Fat Content and Cardiometabolic Risk in Postmenopausal Women: The Randomized Controlled Trial. Applied Sciences. 2021; 11(14):6409. https://doi.org/10.3390/app11146409
Chicago/Turabian StyleReverter-Masia, Joaquín, Alvaro Pano-Rodriguez, Jose Vicente Beltran-Garrido, Albert Lecube, Enric Sánchez, and Vicenç Hernández-González. 2021. "Effect of a Training Program on Hepatic Fat Content and Cardiometabolic Risk in Postmenopausal Women: The Randomized Controlled Trial" Applied Sciences 11, no. 14: 6409. https://doi.org/10.3390/app11146409
APA StyleReverter-Masia, J., Pano-Rodriguez, A., Beltran-Garrido, J. V., Lecube, A., Sánchez, E., & Hernández-González, V. (2021). Effect of a Training Program on Hepatic Fat Content and Cardiometabolic Risk in Postmenopausal Women: The Randomized Controlled Trial. Applied Sciences, 11(14), 6409. https://doi.org/10.3390/app11146409









