Valorization of Waste Tires by Pyrolysis and Activation Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview
2.2. Feedstock Preparation and Characterization
2.3. Pyrolysis Process
2.4. Solid Fraction Characterization
2.5. Liquid and Gas Fraction Characterization
3. Results and Discussion
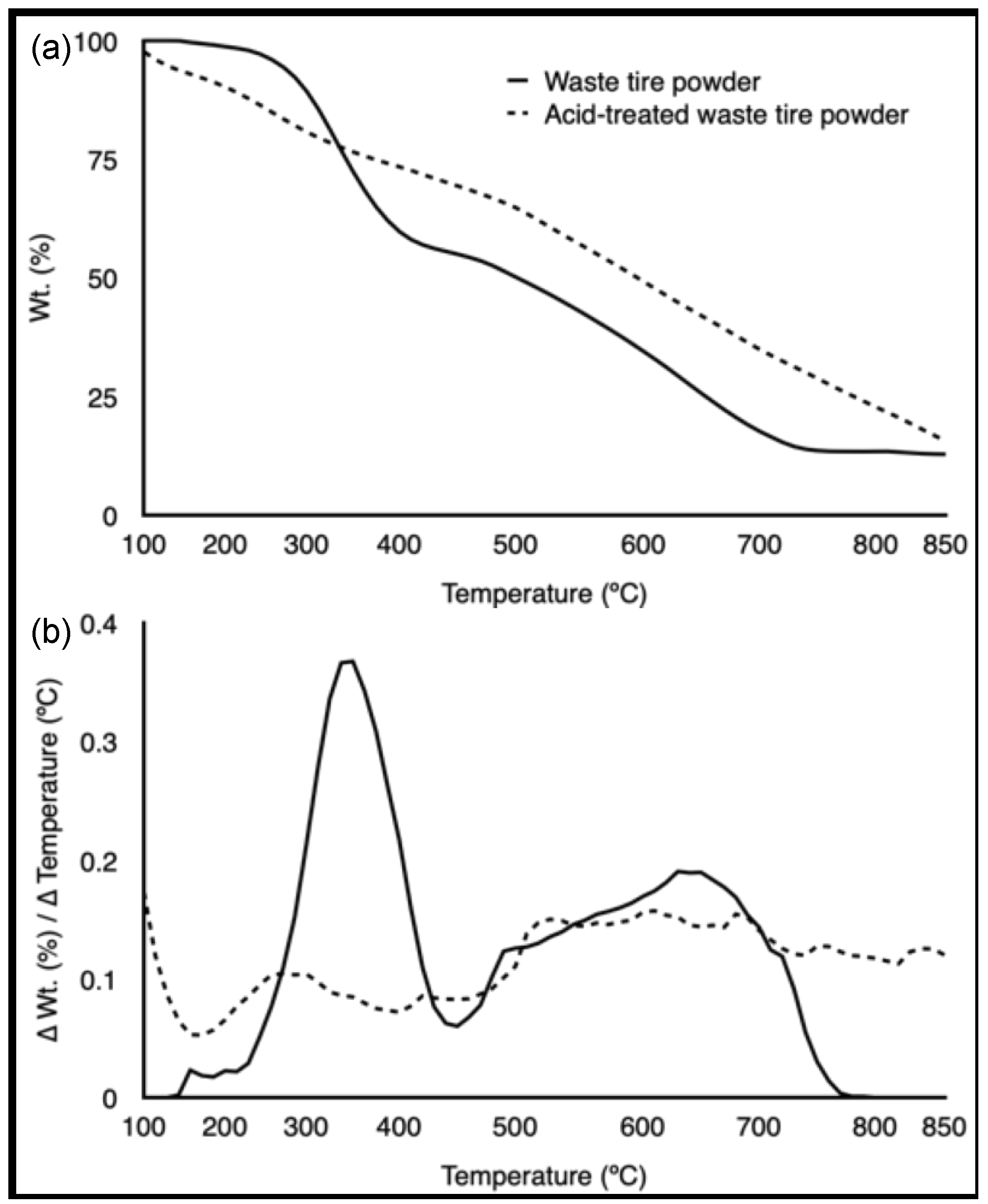
3.1. Feedstock Characterization
3.2. Pyrolysis Product Yields
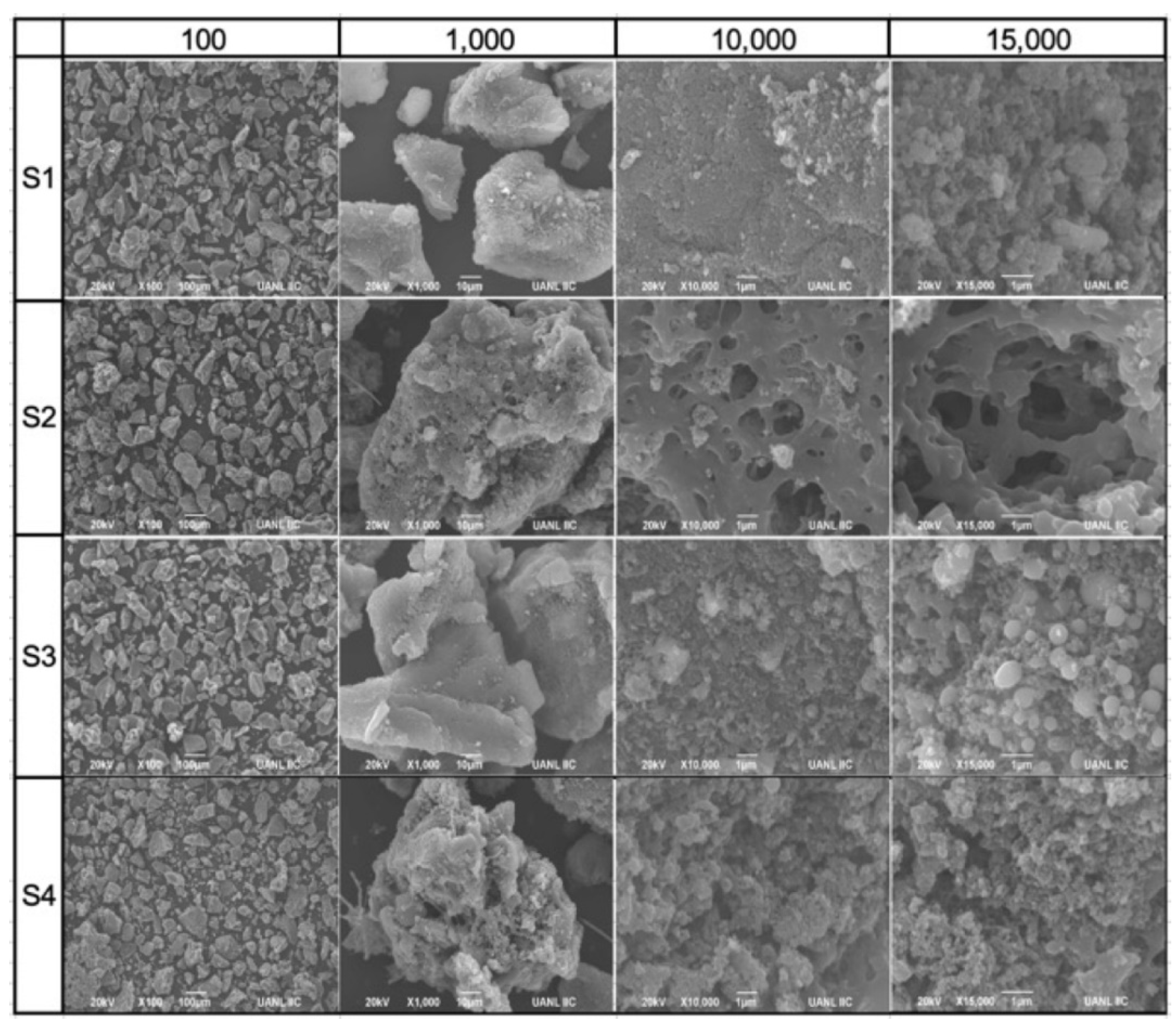
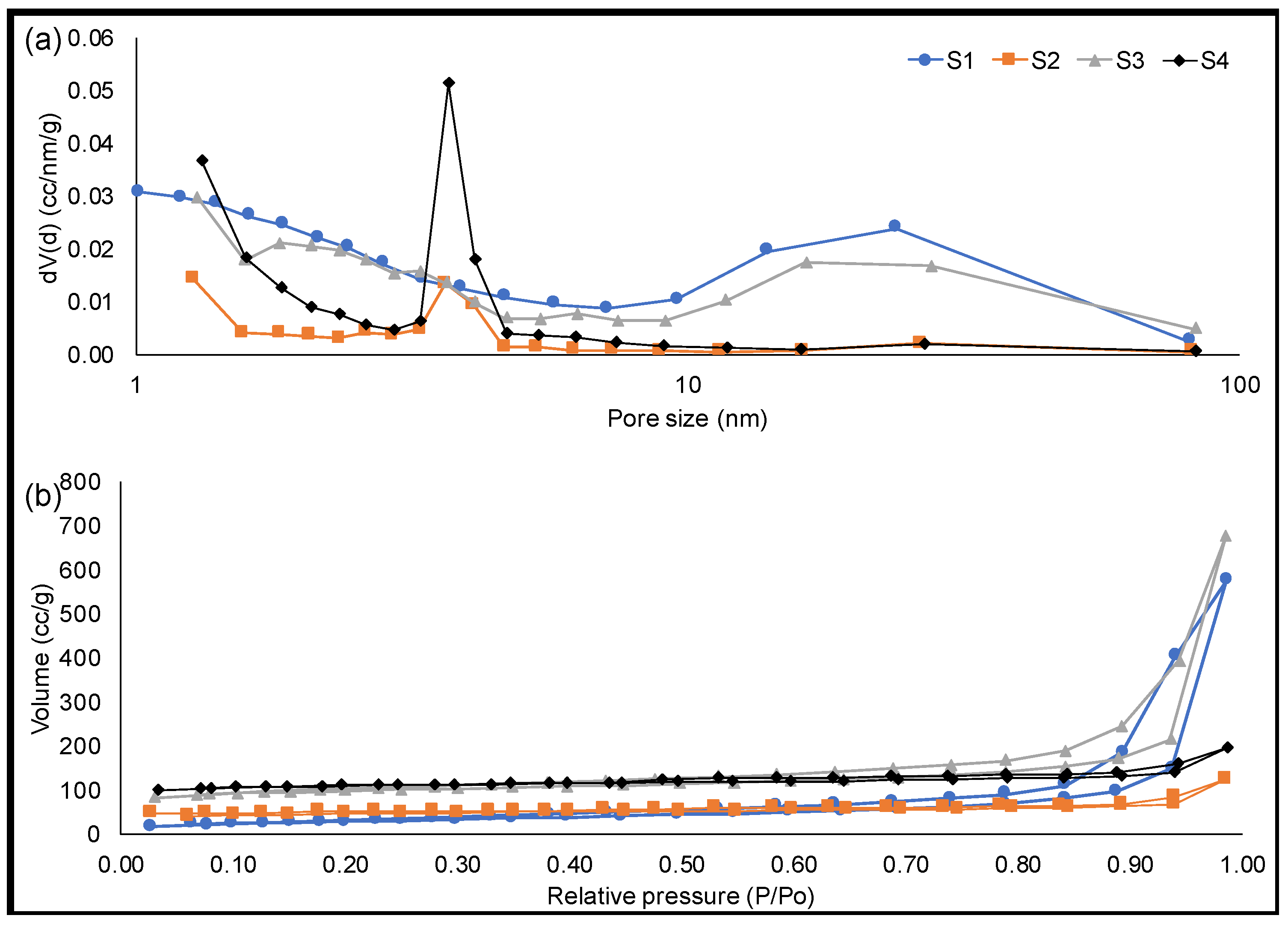
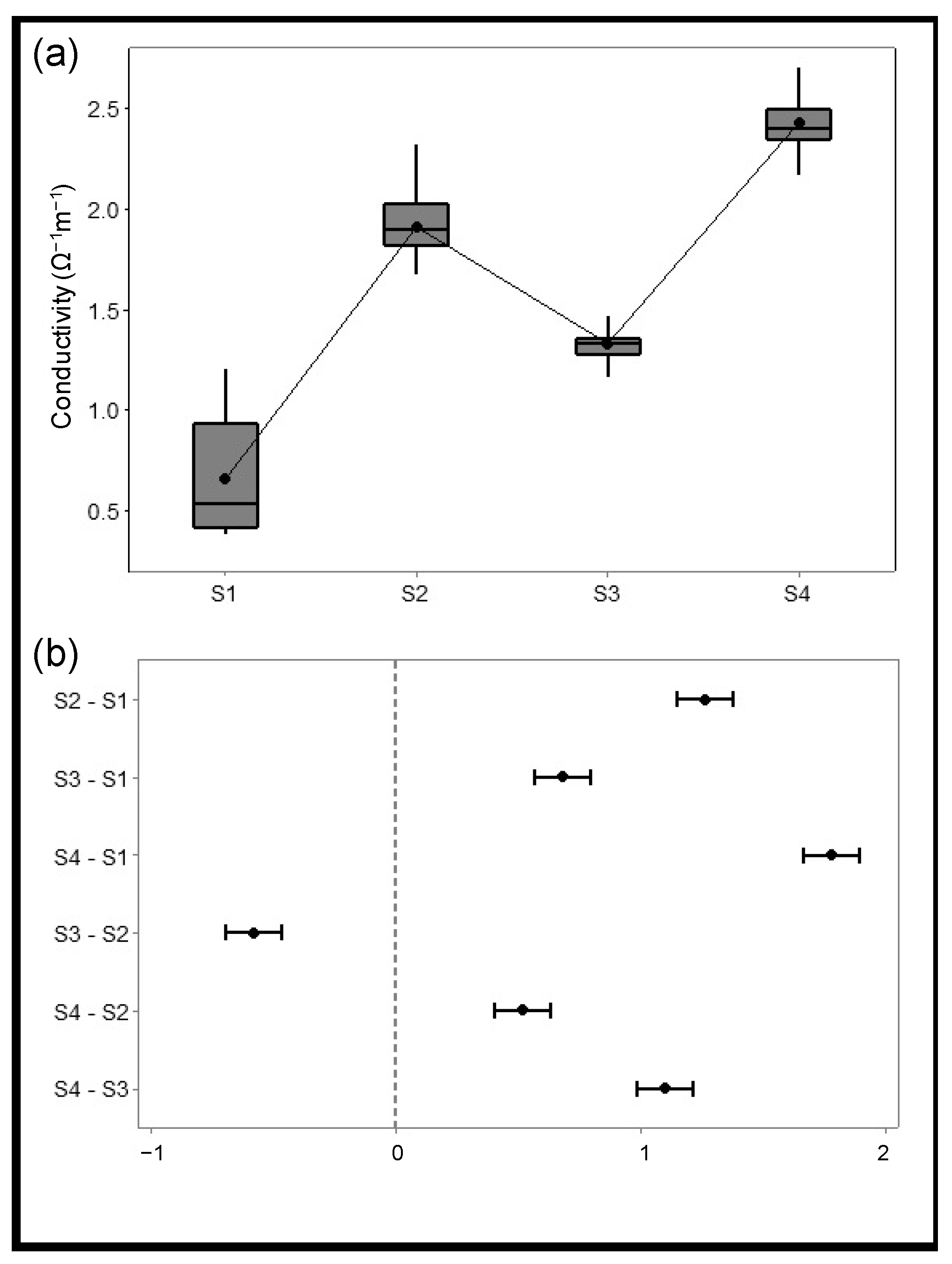
3.3. Solid Fraction Characterization
3.4. Liquid Fraction Qualitative Characterization
3.5. Gas Fraction Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nichols, W.; Smith, N. Waste Generation and Recycling Indices 2019 Overview and Findings; Verisk Maplecroft: Bath, UK, 2019. [Google Scholar]
- Ayoob, A.K.; Fadhil, A.B. Valorization of waste tires in the synthesis of an effective carbon based catalyst for biodiesel production from a mixture of non-edible oils. Fuel 2020, 264, 116754. [Google Scholar] [CrossRef]
- Machin, E.B.; Pedroso, D.T.; De Carvalho, J.A. Energetic valorization of waste tires. Renew. Sustain. Energy Rev. 2017, 68, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Martínez, J.D.; Puy, N.; Murillo, R.; García, T.; Navarro, M.V.; Mastral, A.M. Waste tyre pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 23, 179–213. [Google Scholar] [CrossRef]
- Peronard, J.P.; Ballantyne, A.G. Broadening the understanding of the role of consumer services in the circular economy: Toward a conceptualization of value creation processes. J. Clean. Prod. 2019, 239, 118010. [Google Scholar] [CrossRef]
- Alsaleh, A.; Sattler, M.L. Waste Tire Pyrolysis: Influential Parameters and Product Properties. Curr. Sustain. Energy Rep. 2014, 1, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Aylón, E.; Fernández-Colino, A.; Murillo, R.; Navarro, M.V.; García, T.; Mastral, A.M. Valorisation of waste tyre by pyrolysis in a moving bed reactor. Waste Manag. 2010, 30, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Juma, M.; Koreňová, Z.; Markoš, J.; Jelemensky, L.; Bafrnec, M. Pyrolysis and combustion of scrap tire. Pet. Coal 2006, 48, 15–26. [Google Scholar]
- Kar, Y. Catalytic pyrolysis of car tire waste using expanded perlite. Waste Manag. 2011, 31, 1772–1782. [Google Scholar] [CrossRef]
- Kyari, M.; Cunliffe, A.; Williams, P.T. Characterization of oils, gases, and char in relation to the pyrolysis of different brands of scrap automotive tires. Energy Fuels 2005, 19, 1165–1173. [Google Scholar] [CrossRef]
- Gauthier-Maradei, P.; Valderrama, Y.C.; Nabarlatz, D. Mathematical Model of Scrap Tire Rubber Pyrolysis in a Non-isothermal Fixed Bed Reactor: Definition of a Chemical Mechanism and Determination of Kinetic Parameters. Waste Biomass Valori. 2019, 10, 561–573. [Google Scholar] [CrossRef]
- Gauthier-Maradei, P.; Ruiz, C.P.T.; Capron, M. Oil and Aromatic Yield Maximization During Pyrolysis of Scrap Tire Rubber. Waste Biomass Valori. 2019, 10, 3723–3733. [Google Scholar] [CrossRef]
- Koreňová, Z.; Juma, M.; Annus, J.; Markoš, J.; Jelemenský, L. Kinetics of pyrolysis and properties of carbon black from a scrap tire. Chem. Pap. 2006, 60, 422–426. [Google Scholar] [CrossRef]
- Martínez, J.D.; Cardona-Uribe, N.; Murillo, R.; García, T.; López, J.M. Carbon black recovery from waste tire pyrolysis by demineralization: Production and application in rubber compounding. Waste Manag. 2019, 85, 574–584. [Google Scholar] [CrossRef] [Green Version]
- Maroufi, S.; Mayyas, M.; Sahajwalla, V. Nano-carbons from waste tyre rubber: An insight into structure and morphology. Waste Manag. 2017, 69, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, M.A.; Serio, M.A. Pyrolysis of Scrap Tires: Can It Be profitable? Chemtech: Rio de Janeiro, Brazil, 1996. [Google Scholar]
- López, F.A.; Centeno, T.A.; Rodríguez, O.; Alguacil, F.J. Preparation and characterization of activated carbon from the char produced in the thermolysis of granulated scrap tyres. J. Air Waste Manag. Assoc. 2013, 63, 534–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaminsky, W.; Mennerich, C. Pyrolysis of synthetic tire rubber in a fluidised-bed reactor to yield 1,3-butadiene, styrene and carbon black. J. Anal. Appl. Pyrolysis 2001, 58–59, 803–811. [Google Scholar] [CrossRef]
- Ko, D.; Mui, E.; Lau, K.; McKay, G. Production of activated carbons from waste tire-Process design and economical analysis. Waste Manag. 2004, 24, 875–888. [Google Scholar] [CrossRef]
- López, G.; Olazar, M.; Artetxe, M.; Amutio, M.; Elordi, G.; Bilbao, J. Steam activation of pyrolytic tyre char at different temperatures. J. Anal. Appl. Pyrolysis 2009, 85, 539–543. [Google Scholar] [CrossRef]
- Teng, H.; Lin, Y.C.; Hsu, L.Y. Production of activated carbons from pyrolysis of waste tires impregnated with potassium hydroxide. J. Air Waste Manag. Assoc. 2011, 50, 1940–1946. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Yao, Q.; Wen, S.; Chi, Y.; Yan, J.; Li, S.; Yao, Q. Properties of Pyrolytic Chars and Activated Carbons Derived from Pilot-Scale Pyrolysis of Used Tires. J Air Waste Manag. Assoc. 2005, 55, 1315–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergna, D.; Varila, T.; Romar, H.; Lassi, U. Comparison of the Properties of Activated Carbons Produced in One-Stage and Two-Stage Processes. C 2018, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Dimpe, K.M.; Ngila, J.C.; Nomngongo, P.N. Application of waste tyre-based activated carbon for the removal of heavy metals in wastewater. Cogent Eng. 2017, 4, 1–11. [Google Scholar] [CrossRef]
- Shilpa; Kumar, R.; Sharma, A. Morphologically tailored activated carbon derived from waste tires as high-performance anode for Li-ion battery. J. Appl. Electrochem. 2018, 48, 1–13. [Google Scholar] [CrossRef]
- Naskar, A.K.; Bi, Z.; Li, Y.; Akato, S.K.; Saha, D.; Chi, M.; Bridges, C.A.; Paranthaman, M.P. Tailored recovery of carbons from waste tires for enhanced performance as anodes in lithium-ion batteries. RSC Adv. 2014, 4, 38213–38221. [Google Scholar] [CrossRef]
- Juma, M.; Koreňová, Z.; Markoš, J.; Jelemensky, L.; Bafrnec, M. Experimental study of pyrolysis and combustion of scrap tire. Polym. Adv. Technol. 2007, 18, 144–148. [Google Scholar] [CrossRef]
- Sathiskumar, C.; Karthikeyan, S. Recycling of waste tires and its energy storage application of by-products—A review. Sustain. Mater. Technol. 2019, 22, e00125. [Google Scholar] [CrossRef]
- Ayoob, A.K.; Fadhil, A.B. Biodiesel production through transesterification of a mixture of non-edible oils over lithium supported on activated carbon derived from scrap tires. Energy Convers. Manag. 2019, 201, 112149. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar]
- Ansbæk, T.; Petersen, D.H.; Hansen, O.; Larsen, J.B.; Hansen, T.M.; Bøggild, P. Fundamental size limitations of micro four-point probes. Microelectron. Eng. 2009, 86, 987–990. [Google Scholar] [CrossRef]
- Ruiz-Gómez, N.; Quispe, V.; Ábrego, J.; Atienza-Martínez, M.; Murillo, M.B.; Gea, G. Co-pyrolysis of sewage sludge and manure. Waste Manag. 2017, 59, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Susa, D.; Haydary, J. Sulphur distribution in the products of waste tire pyrolysis. Chem. Pap. 2013, 67, 1521–1526. [Google Scholar] [CrossRef]
- Cunliffe, A.M.; Williams, P.T. Composition of oils derived from the batch pyrolysis of tyres. J. Anal. Appl. Pyrolysis 1998, 44, 131–152. [Google Scholar] [CrossRef]
- Larsen, M.B.; Schultz, L.; Glarborg, P.; Skaarup-Jensen, L.; Dam-Johansen, K.; Frandsen, F.; Henriksen, U. Devolatilization characteristics of large particles of tyre rubber under combustion conditions. Fuel 2006, 85, 1335–1345. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, J.S.; Kim, J.R.; Kim, S.D. Pyrolysis of waste tires with partial oxidation in a fluidized-bed reactor. Energy 1995, 20, 969–976. [Google Scholar] [CrossRef]
- Mikulova, Z.; Sedenkova, I.; Matejova, L.; Vecer, M.; Dombek, V. Study of carbon black obtained by pyrolysis of waste scrap tyres. J. Therm. Anal. Calorim. 2013, 111, 1475–1481. [Google Scholar] [CrossRef]
- Choi, G.G.; Jung, S.H.; Oh, S.J.; Kim, J.S. Total utilization of waste tire rubber through pyrolysis to obtain oils and CO2 activation of pyrolysis char. Fuel Process. Technol. 2014, 123, 57–64. [Google Scholar] [CrossRef]
- Kwon, E.; Castaldi, M.J. Fundamental understanding of the thermal degradation mechanisms of waste tires and their air pollutant generation in a N2 atmosphere. Environ. Sci. Technol. 2009, 43, 5996–6002. [Google Scholar] [CrossRef]
- Williams, P.T.; Besler, S. Pyrolysis-thermogravimetric analysis of tyres and tyre components. Fuel 1995, 74, 1277–1283. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Choi, H.S.; Park, H.C.; Hwang, J.G.; Yoo, H.S.; Lee, B.K.; Upadhyay, M. Influence of process conditions on product yield of waste tyre pyrolysis—A review. Korean J. Chem. Eng. 2016, 33, 2268–2286. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, H.; Cao, Q.; Jin, L.; Wang, F. Upgrading pyrolytic residue from waste tires to commercial carbon black. Waste Manag. Res. 2018, 36, 436–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, H.M.; Chen, H.; Chen, Y.Z.; Li, P.; Zhang, J.Q.; Zhao, X. Simple synthesis of porous carbon materials for high-performance supercapacitors. J. Appl. Electrochem. 2016, 46, 703–712. [Google Scholar] [CrossRef]
- Zhang, W.; Zou, Y.; Yu, C.; Zhong, W. Nitrogen-enriched compact biochar-based electrode materials for supercapacitors with ultrahigh volumetric performance. J. Power Sources 2019, 439, 227067. [Google Scholar] [CrossRef]
- Parant, H.; Muller, G.; Le Mercier, T.; Tarascon, J.M.; Poulin, P.; Colin, A. Flowing suspensions of carbon black with high electronic conductivity for flow applications: Comparison between carbons black and exhibition of specific aggregation of carbon particles. Carbon 2017, 119, 10–20. [Google Scholar] [CrossRef]
- Xu, S.; Lai, D.; Zeng, X.; Zhang, L.; Han, Z.; Cheng, J.; Wu, R.; Mašek, O.; Xu, G. Pyrolysis characteristics of waste tire particles in fixed-bed reactor with internals. Carbon Resour. Convers. 2018, 1, 228–237. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Ma, L.; Chang, J. Vacuum pyrolysis of waste tires with basic additives. Waste Manag. 2008, 28, 2301–2310. [Google Scholar] [CrossRef]
- Danon, B.; Van Der Gryp, P.; Schwarz, C.E.; Görgens, J.F. A review of dipentene (dl-limonene) production from waste tire pyrolysis. J. Anal. Appl. Pyrolysis 2015, 112, 1–13. [Google Scholar] [CrossRef]
- Mkhize, N.M.; van der Gryp, P.; Danon, B.; Görgens, J.F. Effect of temperature and heating rate on limonene production from waste tyre pyrolysis. J. Anal. Appl. Pyrolysis 2016, 120, 314–320. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Wu, L.; Wang, T.; Zhao, J.; Zhang, Y.; Men, Z.; Mao, H. Occurrence of benzothiazole and its derivates in tire wear, road dust, and roadside soil. Chemosphere 2018, 201, 310–317. [Google Scholar] [CrossRef]
- Aydin, H.; Ilkiliç, C. Optimization of fuel production from waste vehicle tires by pyrolysis and resembling to diesel fuel by various desulfurization methods. Fuel 2012, 102, 605–612. [Google Scholar] [CrossRef]
- Nkosi, N.; Muzenda, E. A review and discussion of waste tyre pyrolysis and derived products. In Proceedings of the World Congress on Engineering 2014, London, UK, 2–4 July 2014; Volume 2. [Google Scholar]
- Corgnale, C.; Gorensek, M.B.; Summers, W.A. Review of sulfuric acid decomposition processes for sulfur-based thermochemical hydrogen production cycles. Processes 2020, 8, 1383. [Google Scholar] [CrossRef]
- Sülzle, D.; Verhoeven, M.; Terlouw, J.K.; Schwarz, H. Generation and Characterization of Sulfurous Acid (H2SO3) and of Its Radical Cation as Stable Species in the Gas Phase. Angew. Chem. Int. Ed. Engl. 1988, 27, 1533–1534. [Google Scholar] [CrossRef]



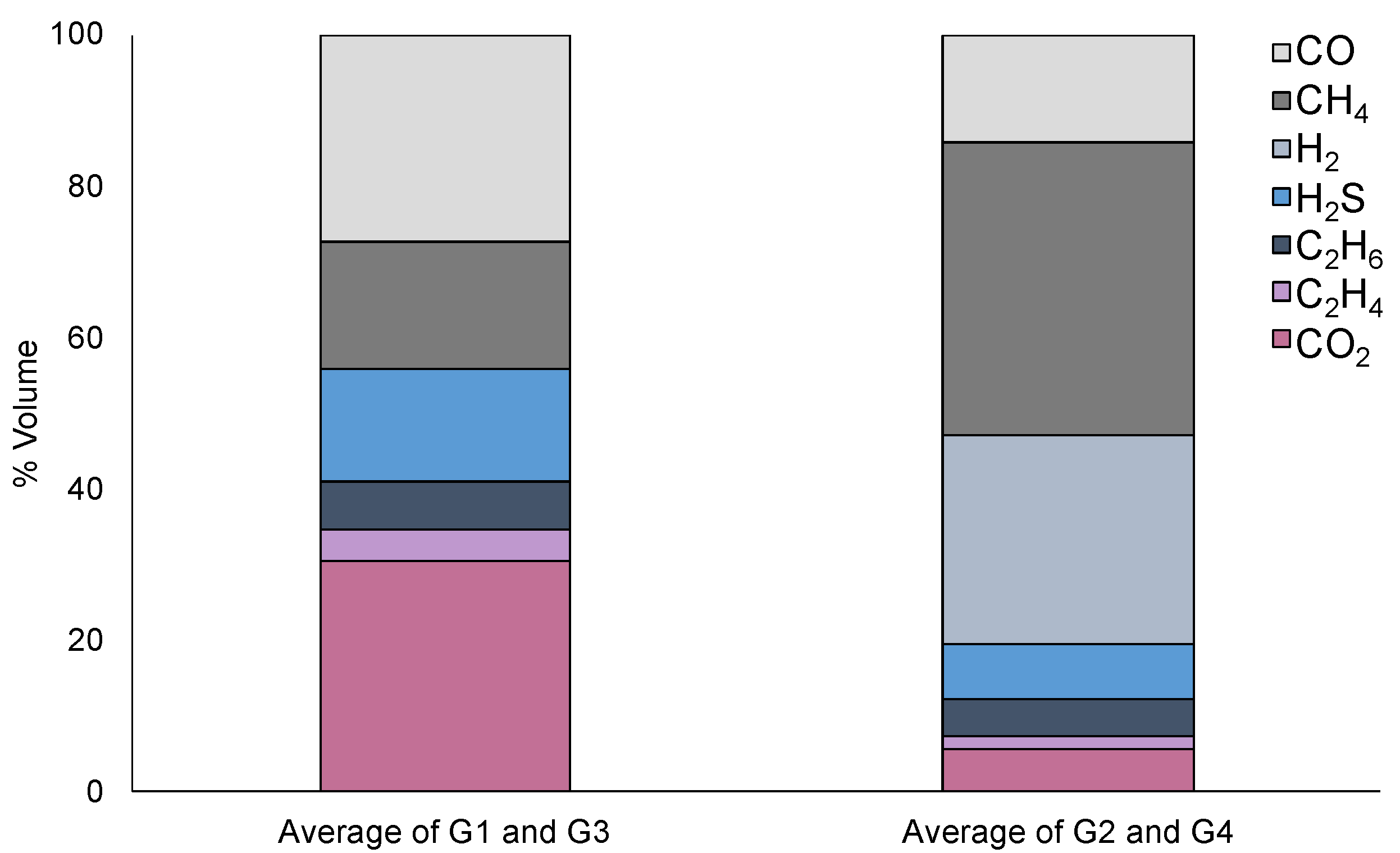
| Waste Tire Powder | Acid-Treated Waste Tire Powder | |
|---|---|---|
| Moisture (wt%) | 1.12 ± 0.09 | 1.65 ± 0.19 |
| Ash (wt%) | 6.55 ± 0.23 | 3.94 ± 0.07 |
| Volatile matter (wt%) | 67.16 ± 0.04 | 47.04 ± 0.58 |
| Fixed carbon a (wt%) | 25.16 ± 0.18 | 47.37 ± 0.46 |
| Nitrogen (wt%) | 0.32 ± 0.01 | 0.26 ± 0.25 |
| Carbon (wt%) | 81.52 ± 0.08 | 71.63 ± 0.28 |
| Hydrogen (wt%) | 7.34 ± 0.09 | 4.02 ± 0.12 |
| Sulfur (wt%) | 1.76 ± 0.09 | 6.93 ± 0.19 |
| Oxygen and other a (wt%) | 9.06 ± 0.13 | 17.16 ± 0.56 |
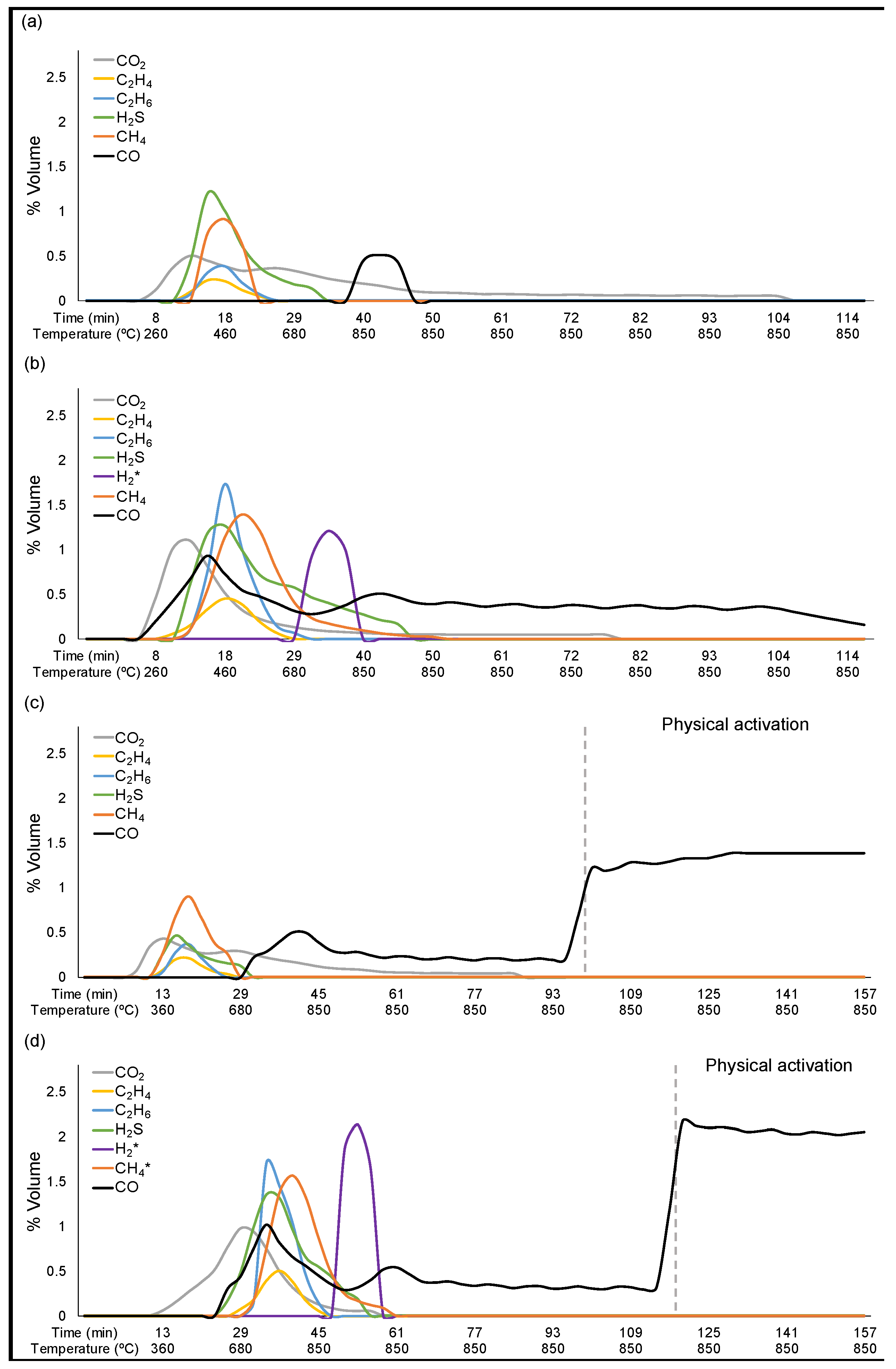
| Process | Solid (%) | Liquid (%) | Gas (%) a | |
|---|---|---|---|---|
| 1 | Pyrolysis | 34.69 ± 2.54 | 65.79 | 1.32 |
| 2 | Chemical activation and pyrolysis | 57.19 ± 0.07 | 13.59 ± 5.57 | 29.21 ± 5.50 |
| 3 | Pyrolysis and physical activation | 24.82 ± 2.32 | 60.93 | 15.89 |
| 4 | Chemical activation, pyrolysis, and physical activation | 52.22 ± 1.58 | 9.1 ± 1.10 | 38.7 ± 0.48 |
| Solid Sample | Process | Surface Area (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) | Conductivity (Ω−1 m−1) |
|---|---|---|---|---|---|
| S1 | Pyrolysis | 107.9 | 33.0 | 0.9 | 0.7 ± 0.3 |
| S2 | Chemical activation and pyrolysis | 151.5 | 5.1 | 0.2 | 1.9 ± 0.2 |
| S3 | Pyrolysis and physical activation | 313.4 | 13.4 | 1.0 | 1.3 ± 0.1 |
| S4 | Chemical activation, pyrolysis, and physical activation | 339.1 | 3.6 | 0.3 | 2.4 ± 0.1 |
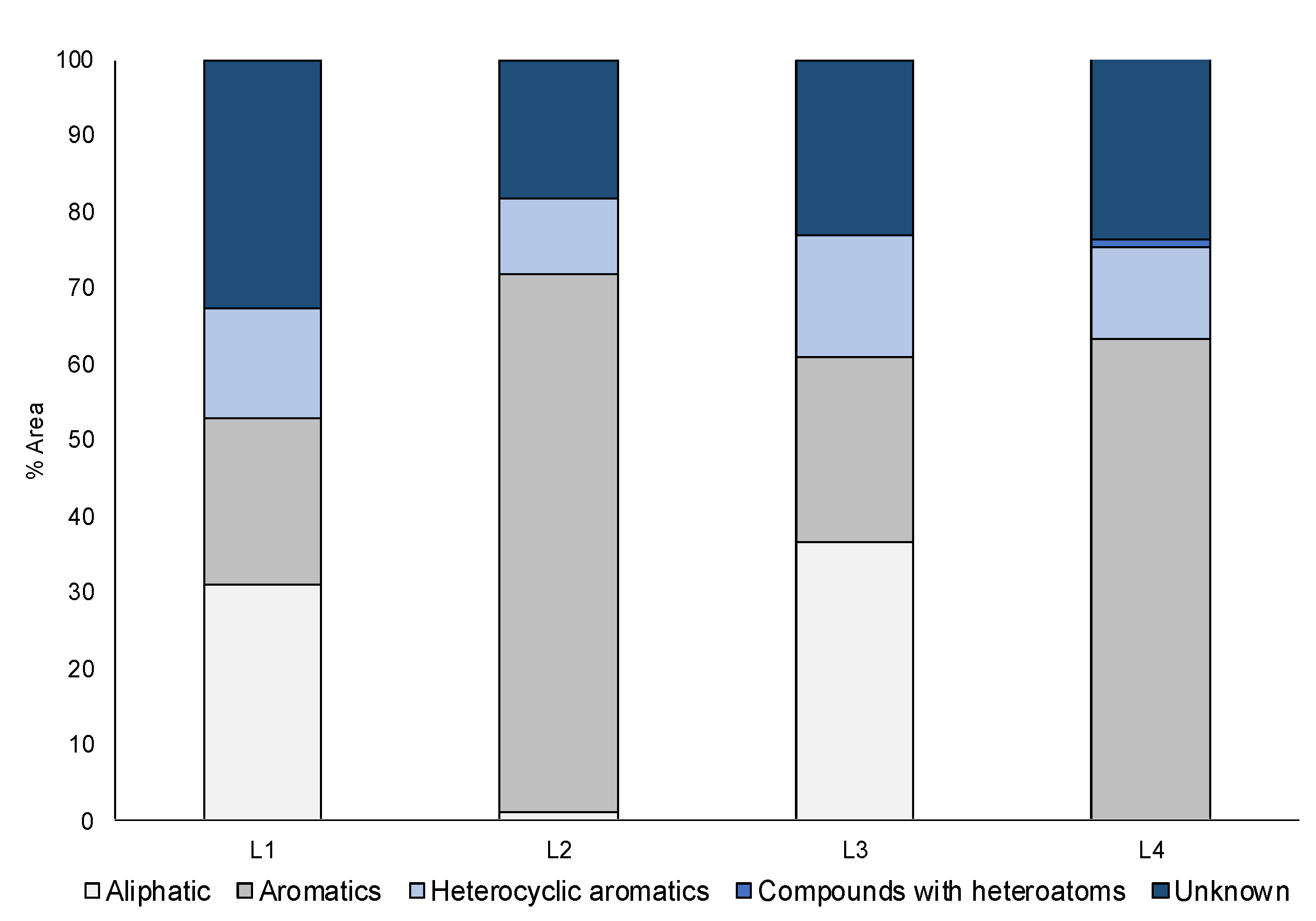
| L1 | L2 | L3 | L4 | ||||
|---|---|---|---|---|---|---|---|
| Compound | Area % | Compound | Area % | Compound | Area % | Compound | Area % |
| D-limonene | 23 | Naphthalene | 42 | D-limonene | 27 | Naphthalene | 32 |
| Benzene | 8 | Benzothiazole | 6 | Benzene | 9 | Benzothiazole | 7 |
| Quinoline | 5 | Anthracene | 6 | Benzothiazole | 5 | Pyrene | 6 |
| Benzenediamine | 5 | Benzene | 5 | Benzenediamine | 5 | Anthracene | 6 |
| Benzothiazole | 4 | Indene | 5 | Quinoline | 5 | Benzene | 5 |
| Naphthalene | 3 | Biphenyl | 4 | Styrene | 5 | Indene | 5 |
| Heptan-2-one, 6-hydroxy-5-methyl-6-vinyl | 3 | Quinoline | 4 | Xylene | 4 | Difenil | 4 |
| Styrene | 3 | Pyrene | 3 | Heptan-2-one, 6-hydroxy-5-methyl-6-vinyl | 4 | Phenanthrene | 3 |
| Other compounds a | 12 | Other compounds b | 7 | Other compounds a | 12 | Other compounds b | 9 |
| Unknown | 33 | Unknown | 18 | Unknown | 23 | Unknown | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-González, R.B.; Ruiz-Gómez, N.; Gea, G.; Vazquez-Pinon, M.; Martinez-Chapa, S.O.; Caballero, P.; Mendoza, A. Valorization of Waste Tires by Pyrolysis and Activation Processes. Appl. Sci. 2021, 11, 6342. https://doi.org/10.3390/app11146342
González-González RB, Ruiz-Gómez N, Gea G, Vazquez-Pinon M, Martinez-Chapa SO, Caballero P, Mendoza A. Valorization of Waste Tires by Pyrolysis and Activation Processes. Applied Sciences. 2021; 11(14):6342. https://doi.org/10.3390/app11146342
Chicago/Turabian StyleGonzález-González, Reyna Berenice, Nadia Ruiz-Gómez, Gloria Gea, Matias Vazquez-Pinon, Sergio O. Martinez-Chapa, Porfirio Caballero, and Alberto Mendoza. 2021. "Valorization of Waste Tires by Pyrolysis and Activation Processes" Applied Sciences 11, no. 14: 6342. https://doi.org/10.3390/app11146342
APA StyleGonzález-González, R. B., Ruiz-Gómez, N., Gea, G., Vazquez-Pinon, M., Martinez-Chapa, S. O., Caballero, P., & Mendoza, A. (2021). Valorization of Waste Tires by Pyrolysis and Activation Processes. Applied Sciences, 11(14), 6342. https://doi.org/10.3390/app11146342









