Carbon Availability and Nitrogen Mineralization Control Denitrification Rates and Product Stoichiometry during Initial Maize Litter Decomposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Soil and Plant Material
2.2. Automatized Laboratory Incubation Experiment and Gas Analysis
2.3. 13CO2 Sampling, Analysis, and Calculations
2.4. 15N2O Sampling, Analysis, and Isotopocule Mapping Approach
2.5. Soil Analyses
2.6. Calculations and Statistics
3. Results
3.1. Characterization of Maize Litter
3.2. Soil N and C Content
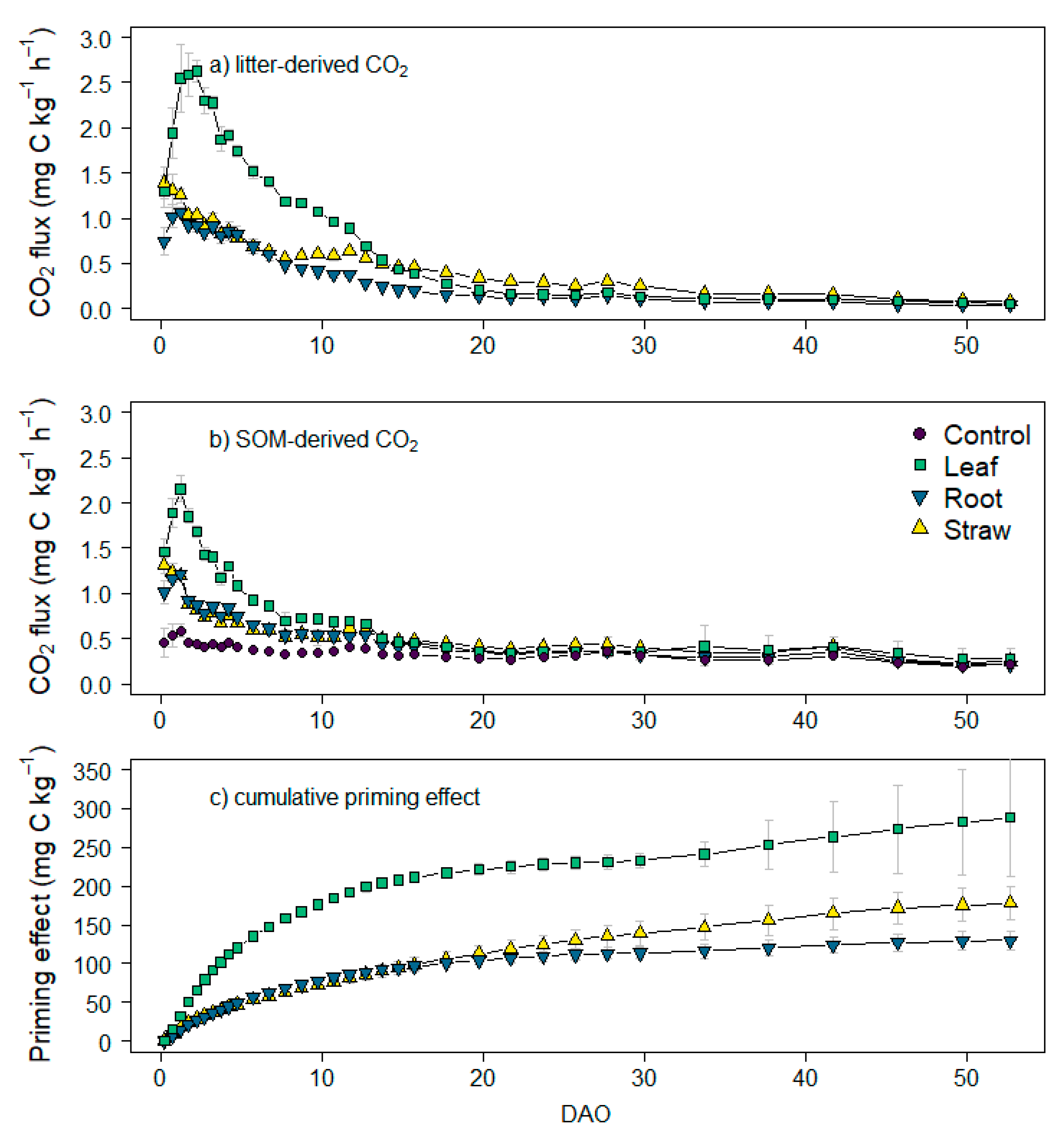
3.3. CO2 and 13CO2 Fluxes and Cumulative Emissions
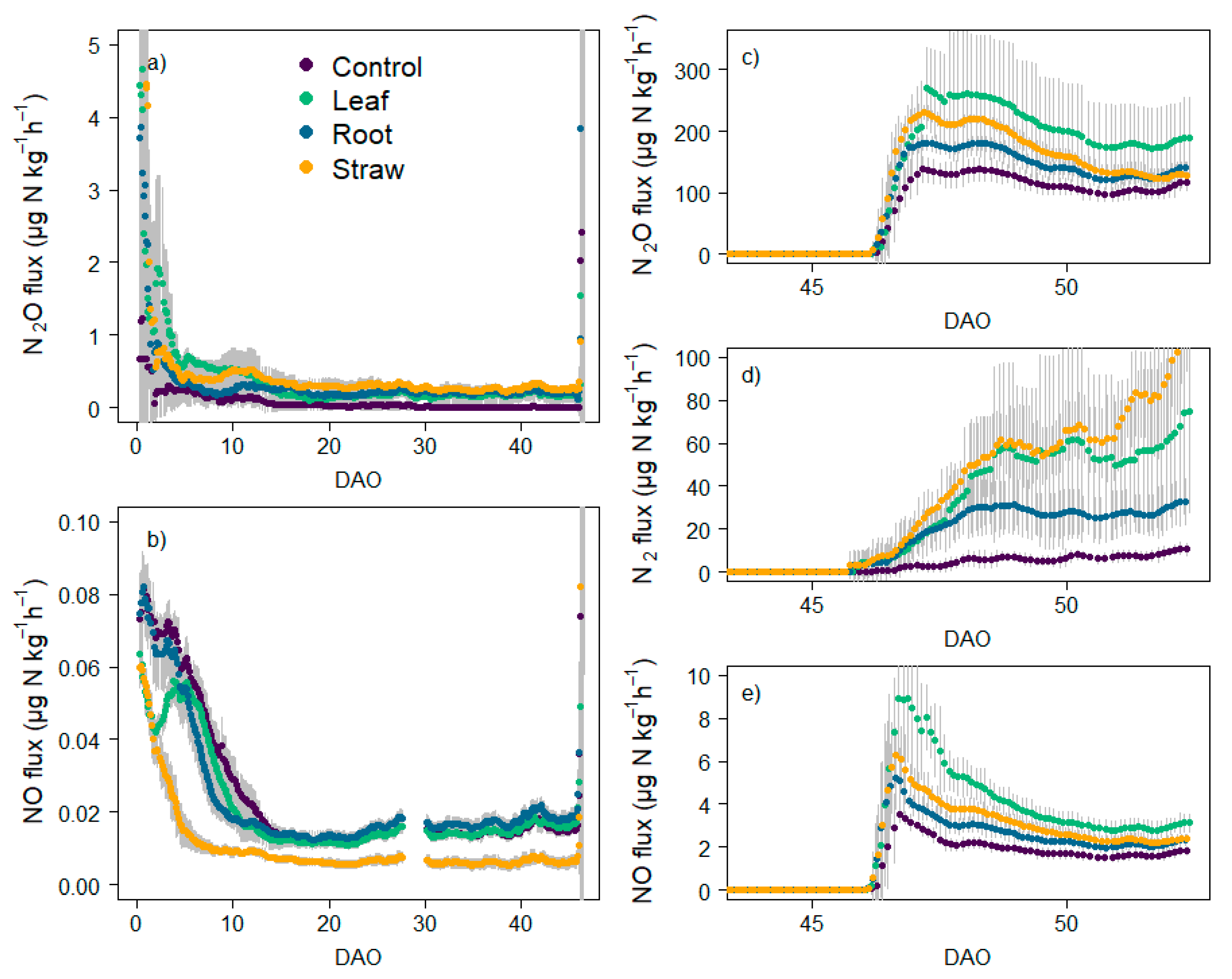
3.4. N Fluxes and Cumulative Emissions
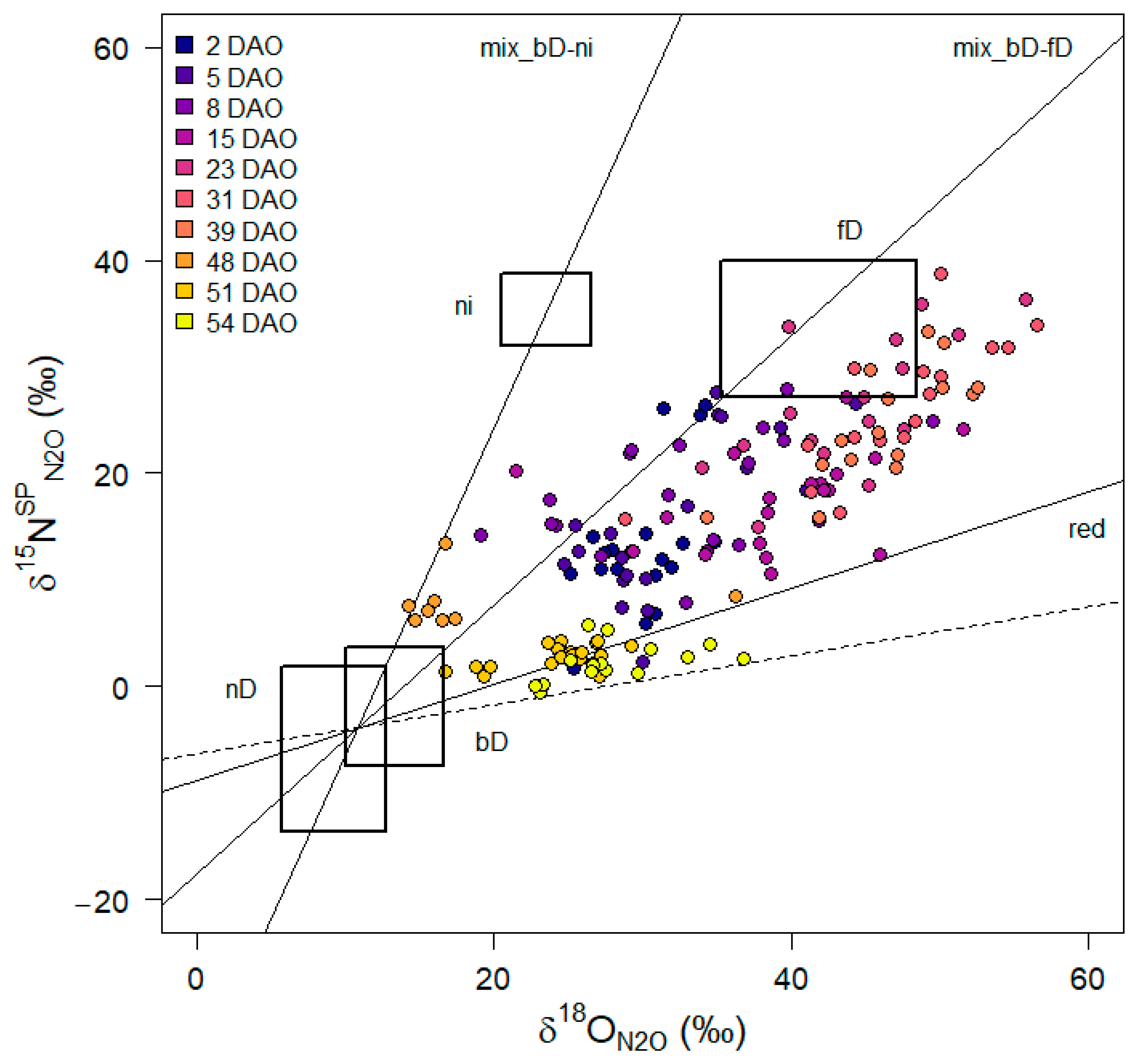
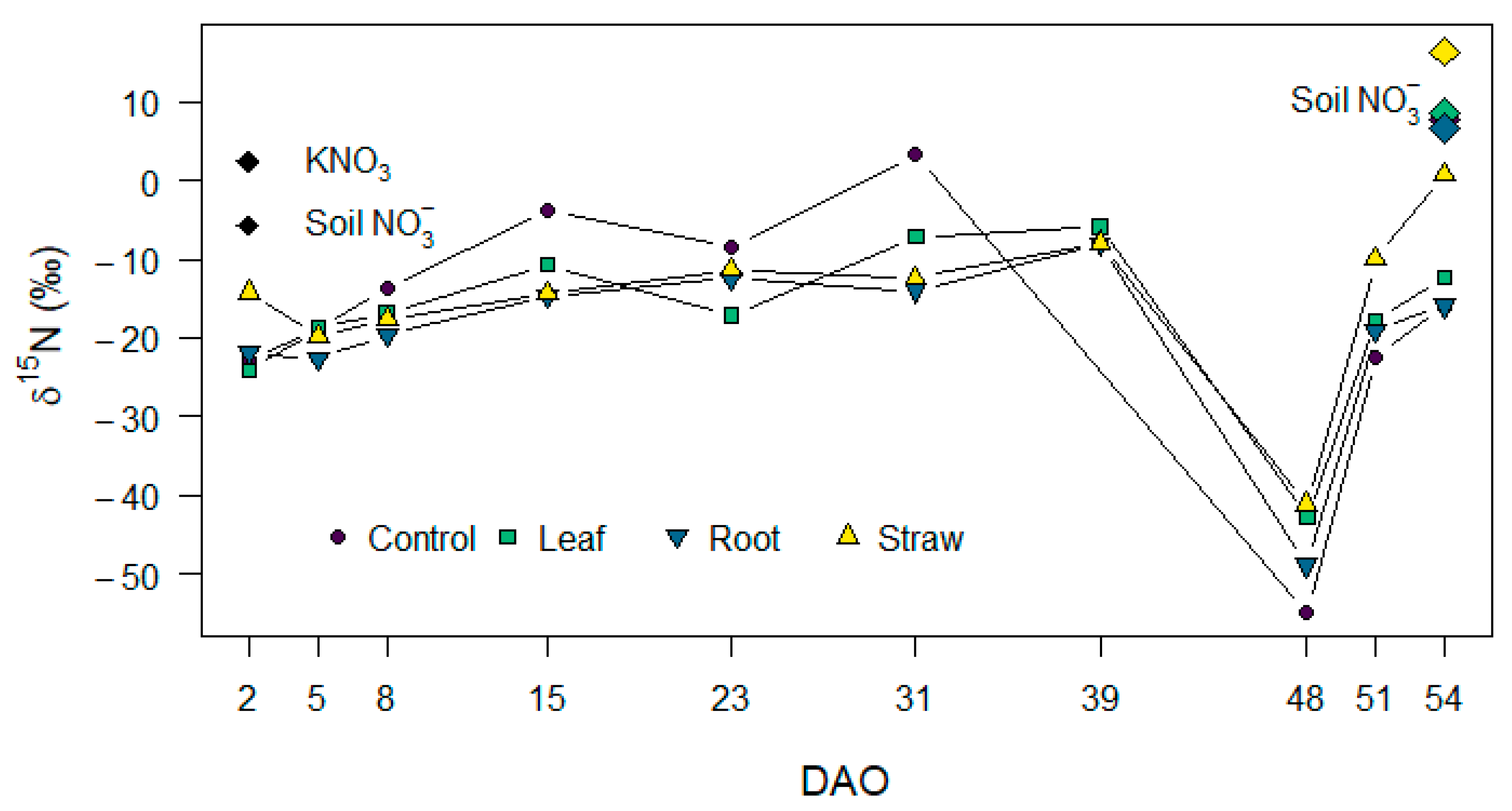
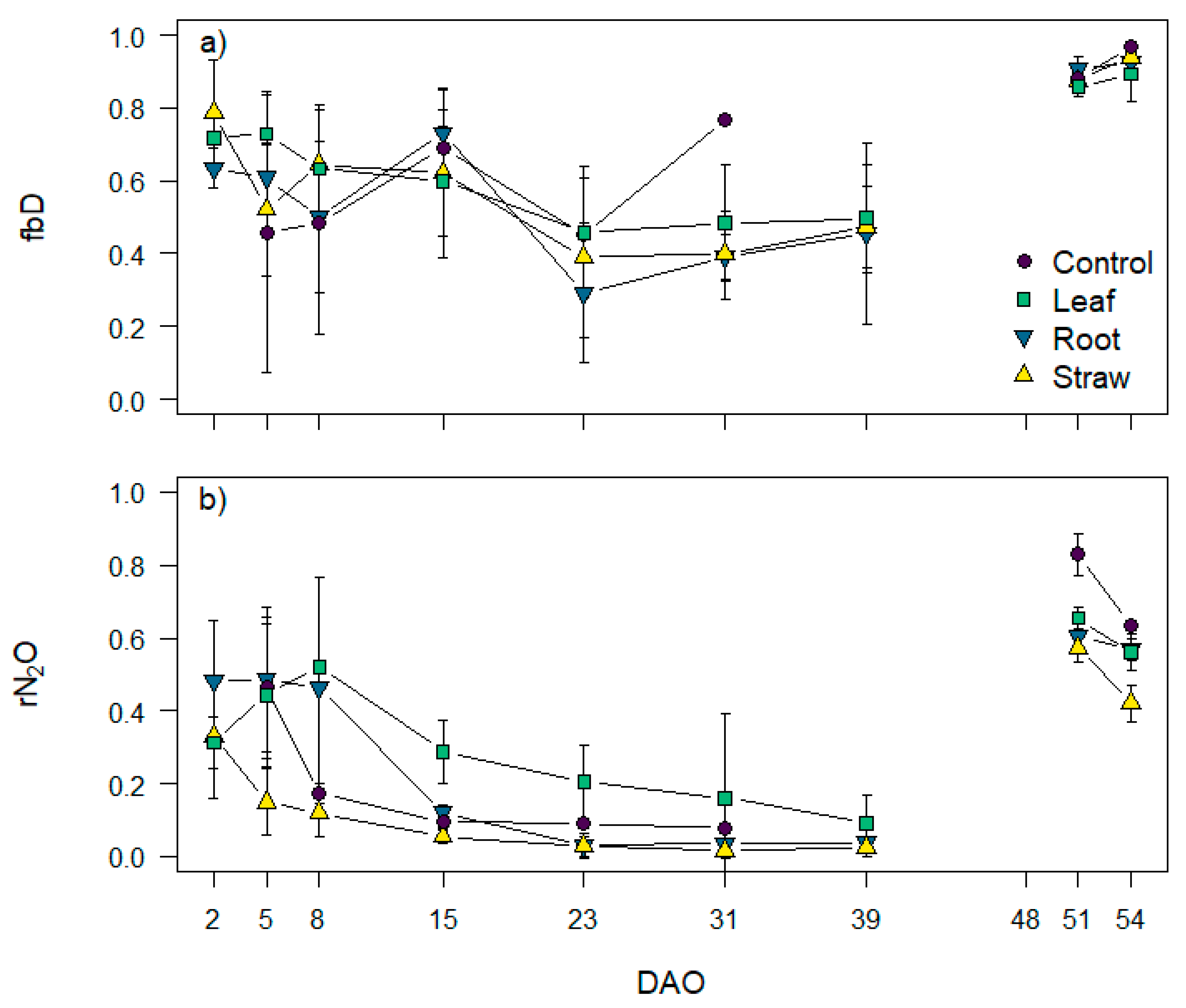
3.5. N2O Isotopocule Mapping Approach, fbD and rN2O Values
3.6. Interactions between C and N Availability and N Fluxes
4. Discussion
4.1. Maize Litter Quality Controls N Mineralization
4.2. Effect of Maize Litter Quality on CO2 Emissions and Priming Effect
4.3. Effect of Litter Quality and N Mineralization on N Emissions and Production Pathways under Oxic Atmosphere
4.4. Effect of Maize Litter Quality and Mineralization on Potential Denitrification
4.5. Interaction between C Turnover and Denitrification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baggs, E.M.; Rees, R.M.; Smith, K.A.; Vinten, A.J.A. Nitrous oxide emission from soils after incorporating crop residues. Soil Use Manag. 2000, 16, 82–87. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Hu, F.; Shi, W. Soil nitrous oxide emissions following crop residue addition: A meta-analysis. Glob. Chang. Biol. 2013, 19, 2956–2964. [Google Scholar] [CrossRef]
- Johnson, J.M.-F.; Barbour, N.W.; Weyers, S.L. Chemical Composition of Crop Biomass Impacts Its Decomposition. Soil Sci. Soc. Am. J. 2007, 71, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Hui, D.; Luo, Y.; Zhou, G. Rates of litter decomposition in terrestrial ecosystems: Global patterns and controlling factors. J. Plant Ecol. 2008, 1, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Hu, F.; Shi, W. Plant material addition affects soil nitrous oxide production differently between aerobic and oxygen-limited conditions. Appl. Soil Ecol. 2013, 64, 91–98. [Google Scholar] [CrossRef]
- Millar, N.; Baggs, E.M. Chemical composition, or quality, of agroforestry residues influences N2O emissions after their addition to soil. Soil Biol. Biochem. 2004, 36, 935–943. [Google Scholar] [CrossRef]
- Robertson, G.P.; Groffman, P.M. Nitrogen Transformations. In Soil Microbiology, Ecology and Biochemistry; Paul, E.A., Ed.; Acwdemic Press: Burlington, MA, USA, 2015; pp. 421–446. ISBN 9780128132692. [Google Scholar]
- Velthof, G.L.; Kuikman, P.J.; Oenema, O. Nitrous oxide emission from soils amended with crop residues. Nutr. Cycl. Agroecosyst. 2002, 62, 249–261. [Google Scholar] [CrossRef]
- Burford, J.R.; Bremner, J.M. Relationships between the denitrification capacities of soils and total, water-soluble and readily decomposable soil organic matter. Soil Biol. Biochem. 1975, 7, 389–394. [Google Scholar] [CrossRef]
- Jensen, L.S.; Salo, T.; Palmason, F.; Breland, T.A.; Henriksen, T.M.; Stenberg, B.; Pedersen, A.; Lundström, C.; Esala, M. Influence of biochemical quality on C and N mineralisation from a broad variety of plant materials in soil. Plant Soil 2005, 273, 307–326. [Google Scholar] [CrossRef]
- Redin, M.; Guénon, R.; Recous, S.; Schmatz, R.; de Freitas, L.L.; Aita, C.; Giacomini, S.J. Carbon mineralization in soil of roots from twenty crop species, as affected by their chemical composition and botanical family. Plant Soil 2014, 378, 205–214. [Google Scholar] [CrossRef]
- Silver, W.L.; Miya, R.K. Global patterns in root decomposition: Comparisons of climate and litter quality effects. Oecologia 2001, 129, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Rummel, P.S.; Pfeiffer, B.; Pausch, J.; Well, R.; Schneider, D.; Dittert, K. Maize root and shoot litter quality controls short-term CO2 and N2O emissions and bacterial community structure of arable soil. Biogeosciences 2020, 17, 1181–1198. [Google Scholar] [CrossRef] [Green Version]
- Surey, R.; Schimpf, C.M.; Sauheitl, L.; Mueller, C.W.; Rummel, P.S.; Dittert, K.; Kaiser, K.; Böttcher, J.; Mikutta, R. Potential denitrification stimulated by water-soluble organic carbon from plant residues during initial decomposition. Soil Biol. Biochem. 2020. [Google Scholar] [CrossRef]
- Birouste, M.; Kazakou, E.; Blanchard, A.; Roumet, C. Plant traits and decomposition: Are the relationships for roots comparable to those for leaves? Ann. Bot. 2012, 109, 463–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 2002, 34, 139–162. [Google Scholar] [CrossRef]
- Six, J.; Jastrow, J.D. Organic Matter Turnover. In Encyclopedia of Soil Science; Lal, R., Ed.; Dekker: New York, NY, USA, 2002. [Google Scholar]
- Kuzyakov, Y.; Bol, R. Sources and mechanisms of priming effect induced in two grassland soils amended with slurry and sugar. Soil Biol. Biochem. 2006, 38, 747–758. [Google Scholar] [CrossRef]
- Rinkes, Z.L.; DeForest, J.L.; Grandy, A.S.; Moorhead, D.L.; Weintraub, M.N. Interactions between leaf litter quality, particle size, and microbial community during the earliest stage of decay. Biogeochemistry 2014, 117, 153–168. [Google Scholar] [CrossRef]
- Kravchenko, A.N.; Toosi, E.R.; Guber, A.K.; Ostrom, N.E.; Yu, J.; Azeem, K.; Rivers, M.L.; Robertson, G.P. Hotspots of soil N2O emission enhanced through water absorption by plant residue. Nat. Geosci. 2017, 10, 496–500. [Google Scholar] [CrossRef]
- Arcand, M.M.; Congreves, K.A. Elucidating microbial carbon utilization and nitrous oxide dynamics with 13C-substrates and N2O isotopomers in contrasting horticultural soils. Appl. Soil Ecol. 2020, 147, 103401. [Google Scholar] [CrossRef]
- Lewicka-Szczebak, D.; Augustin, J.; Giesemann, A.; Well, R. Quantifying N2O reduction to N2 based on N2O isotopocules-validation with independent methods (helium incubation and 15N gas flux method). Biogeosciences 2017, 14, 711–732. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Harris, E.; Lewicka-Szczebak, D.; Barthel, M.; Blomberg, M.R.A.; Harris, S.J.; Johnson, M.S.; Lehmann, M.F.; Liisberg, J.; Müller, C.; et al. What can we learn from N2O isotope data?–Analytics, processes and modelling. Rapid Commun. Mass Spectrom. 2020, 34, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mengutay, M.; Ceylan, Y.; Kutman, U.B.; Cakmak, I. Adequate magnesium nutrition mitigates adverse effects of heat stress on maize and wheat. Plant Soil 2013, 368, 57–72. [Google Scholar] [CrossRef]
- Köster, J.R.; Well, R.; Dittert, K.; Giesemann, A.; Lewicka-Szczebak, D.; Mühling, K.H.; Herrmann, A.; Lammel, J.; Senbayram, M. Soil denitrification potential and its influence on N2O reduction and N2O isotopomer ratios. Rapid Commun. Mass Spectrom. 2013, 27, 2363–2373. [Google Scholar] [CrossRef]
- Scholefield, D.; Hawkins, J.M.B.B.; Jackson, S.M. Development of a helium atmosphere soil incubation technique for direct measurement of nitrous oxide and dinitrogen fluxes during denitrification. Soil Biol. Biochem. 1997, 29, 1345–1352. [Google Scholar] [CrossRef]
- Senbayram, M.; Well, R.; Bol, R.; Chadwick, D.R.; Jones, D.L.; Wu, D. Interaction of straw amendment and soil NO3− content controls fungal denitrification and denitrification product stoichiometry in a sandy soil. Soil Biol. Biochem. 2018, 126, 204–212. [Google Scholar] [CrossRef]
- Wang, R.; Willibald, G.; Feng, Q.; Zheng, X.; Liao, T.; Brüggemann, N.; Butterbach-Bahl, K. Measurement of N2, N2O, NO, and CO2 emissions from soil with the gas-flow-soil-core technique. Environ. Sci. Technol. 2011, 45, 6066–6072. [Google Scholar] [CrossRef] [PubMed]
- Lewicka-Szczebak, D.; Well, R.; Köster, J.R.; Fuß, R.; Senbayram, M.; Dittert, K.; Flessa, H. Experimental determinations of isotopic fractionation factors associated with N2O production and reduction during denitrification in soils. Geochim. Cosmochim. Acta 2014, 134, 55–73. [Google Scholar] [CrossRef]
- Toyoda, S.; Yoshida, N. Determination of Nitrogen Isotopomers of Nitrous. Anal. Chem. 1999, 71, 4711–4718. [Google Scholar] [CrossRef]
- Buchen, C.; Lewicka-Szczebak, D.; Flessa, H.; Well, R. Estimating N2O processes during grassland renewal and grassland conversion to maize cropping using N2O isotopocules. Rapid Commun. Mass Spectrom. 2018, 32, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Röckmann, T.; Kaiser, J.; Brenninkmeijer, C.A.M.; Brand, W.A. Gas chromatography/isotope-ratio mass spectrometry method for high-precision position-dependent 15N and 18O measurements of atmospheric nitrous oxide. Rapid Commun. Mass Spectrom. 2003, 17, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Lewicka-Szczebak, D. Mapping approach model after Lewicka-Szczebak et al. (2017)-detailed description of calculation procedures. RG 2018. [Google Scholar] [CrossRef]
- Casciotti, K.L.; Sigman, D.M.; Hastings, M.G.; Böhlke, J.K.; Hilkert, A. Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Anal. Chem. 2002, 74, 4905–4912. [Google Scholar] [CrossRef]
- Sigman, D.M.; Casciotti, K.L.; Andreani, M.; Barford, C.; Galanter, M.; Böhlke, J.K. A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Anal. Chem. 2001, 73, 4145–4153. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing 2019. R version 3.6.0 -“Planting of a Tree”; R Foundation for Statistical Computing: Vienna, Austria, 2019.
- Blackmon, H.; Adams, R.H. evobiR: Comparative and Population Genetic Analyses 2015. R Package Version 1.1; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Zeileis, A.; Grothendieck, G. zoo: S3 Infrastructure for Regular and Irregular Time Series. J. Stat. Softw. 2005, 14, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Garnier, S. viridisLite: Default Color Maps from “matplotlib” (Lite Version) 2018. R Package Version 0.3.0; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Höper, H. Carbon and nitrogen mineralisation rates of fens in Germany used for agriculture. In Wetlands in Central Europe; Broll, G., Merbach, W., Pfeiffer, E.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 149–164. ISBN 978-3-662-05054-5. [Google Scholar]
- Kristensen, H.L.; Debosz, K.; McCarty, G.W. Short-term effects of tillage on mineralization of nitrogen and carbon in soil. Soil Biol. Biochem. 2003, 35, 979–986. [Google Scholar] [CrossRef]
- Machinet, G.E.; Bertrand, I.; Chabbert, B.; Recous, S. Decomposition in soil and chemical changes of maize roots with genetic variations affecting cell wall quality. Eur. J. Soil Sci. 2009, 60, 176–185. [Google Scholar] [CrossRef]
- Mary, B.; Fresneau, C.; Morel, J.L.; Mariotti, A. C and N cycling during decomposition of root mucilage, roots and glucose in soil. Soil Biol. Biochem. 1993, 25, 1005–1014. [Google Scholar] [CrossRef]
- Machinet, G.E.; Bertrand, I.; Barrière, Y.; Chabbert, B.; Recous, S. Impact of plant cell wall network on biodegradation in soil: Role of lignin composition and phenolic acids in roots from 16 maize genotypes. Soil Biol. Biochem. 2011, 43, 1544–1552. [Google Scholar] [CrossRef]
- Knicker, H. Solid state CPMAS 13C and 15N NMR spectroscopy in organic geochemistry and how spin dynamics can either aggravate or improve spectra interpretation. Org. Geochem. 2011, 42, 867–890. [Google Scholar] [CrossRef]
- Cheng, W.; Tsuruta, H.; Chen, G.; Yagi, K. N2O and NO production in various Chinese agricultural soils by nitrification. Soil Biol. Biochem. 2004, 36, 953–963. [Google Scholar] [CrossRef]
- Skiba, U.; Fowler, D.; Smith, K.A. Nitric oxide emissions from agricultural soils in temperate and tropical climates: Sources, controls and mitigation options. Nutr. Cycl. Agroecosyst. 1997, 48, 139–153. [Google Scholar] [CrossRef]
- Chen, H.; Mothapo, N.V.; Shi, W. Fungal and bacterial N2O production regulated by soil amendments of simple and complex substrates. Soil Biol. Biochem. 2015, 84, 116–126. [Google Scholar] [CrossRef]
- Hayatsu, M.; Tago, K.; Saito, M. Various players in the nitrogen cycle: Diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci. Plant Nutr. 2008, 54, 33–45. [Google Scholar] [CrossRef]
- Lavrent’ev, R.B.; Zaitsev, S.A.; Sudnitsyn, I.I.; Kurakov, A.V. Nitrous oxide production by fungi in soils under different moisture levels. Moscow Univ. Soil Sci. Bull. 2008, 63, 178–183. [Google Scholar] [CrossRef]
- Laughlin, R.J.; Stevens, R.J. Evidence for Fungal Dominance of Denitrification and Codenitrification in a Grassland Soil. Soil Sci. Soc. Am. J. 2002, 66, 1540–1548. [Google Scholar] [CrossRef]
- Wu, D.; Senbayram, M.; Well, R.; Brüggemann, N.; Pfeiffer, B.; Loick, N.; Stempfhuber, B.; Dittert, K.; Bol, R. Nitrification inhibitors mitigate N2O emissions more effectively under straw-induced conditions favoring denitrification. Soil Biol. Biochem. 2017, 104, 197–207. [Google Scholar] [CrossRef]
- Zhong, L.; Bowatte, S.; Newton, P.C.D.; Hoogendoorn, C.J.; Luo, D. An increased ratio of fungi to bacteria indicates greater potential for N2O production in a grazed grassland exposed to elevated CO2. Agric. Ecosyst. Environ. 2018, 254, 111–116. [Google Scholar] [CrossRef]
- Zumft, W.G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 1997, 61, 533–616. [Google Scholar] [CrossRef] [PubMed]
- Firestone, M.K.; Smith, M.S.; Firestone, R.B.; Tiedje, J.M. The Influence of Nitrate, Nitrite, and Oxygen on the Composition of the Gaseous Products of Denitrification in Soil. Soil Sci. Soc. Am. J. 1979, 43, 1140–1144. [Google Scholar] [CrossRef]
- Schlüter, S.; Henjes, S.; Zawallich, J.; Bergaust, L.; Horn, M.; Ippisch, O.; Vogel, H.-J.; Dörsch, P. Denitrification in Soil Aggregate Analogues-Effect of Aggregate Size and Oxygen Diffusion. Front. Environ. Sci. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Wu, D.; Well, R.; Cárdenas, L.M.; Fuß, R.; Lewicka-Szczebak, D.; Köster, J.R.; Brüggemann, N.; Bol, R. Quantifying N2O reduction to N2 during denitrification in soils via isotopic mapping approach: Model evaluation and uncertainty analysis. Environ. Res. 2019, 179, 1–6. [Google Scholar] [CrossRef]
- Ostrom, N.E.; Piit, A.; Sutka, R.; Ostrom, P.H.; Grandy, A.S.; Huizinga, K.M.; Robertson, G.P. Isotopologue effects during N2O reduction in soils and in pure cultures of denitrifiers. J. Geophys. Res. Biogeosci. 2007, 112, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bergstermann, A.; Cárdenas, L.; Bol, R.; Gilliam, L.; Goulding, K.; Meijide, A.; Scholefield, D.; Vallejo, A.; Well, R. Effect of antecedent soil moisture conditions on emissions and isotopologue distribution of N2O during denitrification. Soil Biol. Biochem. 2011, 43, 240–250. [Google Scholar] [CrossRef]
- Cardenas, L.M.; Bol, R.; Lewicka-Szczebak, D.; Gregory, A.S.; Matthews, G.P.; Whalley, W.R.; Misselbrook, T.H.; Scholefield, D.; Well, R. Effect of soil saturation on denitrification in a grassland soil. Biogeosciences 2017, 14, 4691–4710. [Google Scholar] [CrossRef] [Green Version]
- Lewicka-Szczebak, D.; Well, R.; Bol, R.; Gregory, A.S.; Matthews, G.P.; Misselbrook, T.; Whalley, W.R.; Cardenas, L.M. Isotope fractionation factors controlling isotopocule signatures of soil-emitted N2O produced by denitrification processes of various rates. Rapid Commun. Mass Spectrom. 2015, 29, 269–282. [Google Scholar] [CrossRef] [Green Version]
- Lewicka-Szczebak, D.; Piotr Lewicki, M.; Well, R. N2O isotope approaches for source partitioning of N2O production and estimation of N2O reduction-validation with the 15N gas-flux method in laboratory and field studies. Biogeosciences 2020, 17, 5513–5537. [Google Scholar] [CrossRef]
- Azam, F.; Müller, C.; Weiske, A.; Benckiser, G.; Ottow, J.C.G. Nitrification and denitrification as sources of atmospheric nitrous oxide-Role of oxidizable carbon and applied nitrogen. Biol. Fertil. Soils 2002, 35, 54–61. [Google Scholar] [CrossRef]
- Millar, N.; Baggs, E.M. Relationships between N2O emissions and water-soluble C and N contents of agroforestry residues after their addition to soil. Soil Biol. Biochem. 2005, 37, 605–608. [Google Scholar] [CrossRef]
- Rohe, L.; Apelt, B.; Vogel, H.-J.; Well, R.; Wu, G.-M.; Schlüter, S. Denitrification in soil as a function of oxygen supply and demand at the microscale. Biogeosciences 2021, 1–32. [Google Scholar]
- Schlüter, S.; Zawallich, J.; Vogel, H.J.; Dörsch, P. Physical constraints for respiration in microbial hotspots in soil and their importance for denitrification. Biogeosciences 2019, 16, 3665–3675. [Google Scholar] [CrossRef] [Green Version]
- Firestone, M.K. Biological denitrification. In Nitrogen in Agricultural Soils, Agronomy Monograph; American Society of Agronomy, Inc.: Madison, WI, USA; Crop Science Society of America, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; Volume 22, pp. 289–326. [Google Scholar] [CrossRef]
| Dry Matter (%) | C (%) | δ 13C (‰) | N (%) | C:N | Water Soluble C (%) | Water Soluble N (%) | Water Soluble C:N | Alkyl C (%) | O/N-Alkyl C (%) | Aryl C (%) | Carboxyl C (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maize leaves | 27.9 | 46.58 | −14.70 | 3.80 | 12.27 | 8.03 | 0.69 | 11.6 | 16.05 | 63.67 | 10.96 | 9.31 |
| Maize roots | 7.8 | 40.12 | −12.97 | 2.90 | 13.82 | 8.53 | 1.22 | 7.0 | 8.08 | 80.65 | 10.19 | 1.10 |
| Maize straw | 31.4 | 43.84 | −14.11 | 0.85 | 51.36 | 8.25 | 0.39 | 21.4 | 11.48 | 69.93 | 11.23 | 7.23 |
| NO3− (mg N kg−1 dry soil) | NH4+ (mg N kg−1 dry soil) | WEOC (mg C kg−1 dry soil) | Net N Mineralization (mg N kg−1 dry soil) | δ15NNO3 of Soil NO3− (‰) | δ18ONO3 of Soil NO3− (‰) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | 102.9 ± 4.59 | 34.7 ± 3.16 | 51.4 ± 6.49 | - | −5.74 ± 0.19/2.44 ± 0.22 1 | 1.84 ± 0.29/22.95 ± 0.40 1 | ||||||
| Control | 142.1 ± 7.8 | b *** | 4.12 ± 0.27 | c *** | 56.3 ± 8.0 | b | 26.4 ± 5.5 | b | 7.80 ± 0.58 | bc | 9.21 ± 1.05 | c |
| Maize Leaves | 169.6 ± 4.4 | a *** | 5.84 ± 0.28 | a *** | 76.4 ± 3.1 | a *** | 75.5 ± 15.1 | a | 8.58 ± 0.24 | b | 9.27 ± 0.31 | c |
| Maize Roots | 176.1 ± 6.9 | a *** | 4.85 ± 0.39 | b *** | 72.0 ± 5.7 | a *** | 69.3 ± 5.4 | a | 6.64 ± 0.62 | c | 11.49 ± 0.52 | b |
| Maize Straw | 70.6 ± 5.6 | c *** | 6.00 ± 0.60 | a *** | 71.9 ± 5.3 | a *** | −26.8 ± 5.7 | c | 16.40 ± 1.63 | a | 14.53 ± 0.34 | a |
| Adjusted R2 | p-Value | n | |
|---|---|---|---|
| Oxic incubation period | |||
| Cumulative NO+N2O emissions ~ water-soluble litter C input | 0.4401 | 0.001172 | 19 |
| Cumulative N2O emissions ~ litter C:N ratio | 0.247 | 0.03428 | 15 |
| Cumulative NO emissions ~ water-soluble litter C:N ratio | 0.8703 | 2.427 × 10−7 | 15 |
| Cumulative NO emission ~ net N mineralization | 0.5671 | 0.0001197 | 19 |
| NO+N2O flux ~ CO2 flux | 0.08023 | <2.2 × 10−16 | 1715 |
| Anoxic incubation period | |||
| Cumulative N2 emissions ~ water-soluble litter C:N ratio | 0.2553 | 0.03158 | 15 |
| Cumulative NO+N2O+N2 emissions ~ total litter C input | 0.5087 | 0.0003655 | 19 |
| N2O/(N2O+N2) ratio ~ water-soluble litter C:N ratio | 0.5061 | 1.886 × 10−6 | 19 |
| N2O/(N2O+N2) ratio ~ WEOC: NO3− ratio | 0.4127 | 0.0018 | 19 |
| NO+N2O+N2 flux ~ CO2 flux | 0.864 | <2.2 × 10−16 | 176 |
| Total incubation period | |||
| Cumulative CO2 emissions ~ total litter C input | 0.8974 | 4.84 × 10−10 | 19 |
| Cumulative CO2 emissions ~ water-soluble litter C input | 0.798 | 1.606 × 10−7 | 19 |
| Litter-derived CO2 flux ~ SOM-derived CO2 flux | 0.8838 | <2.2 × 10−16 | 495 |
| δ15NNO3 of soil NO3− ~ net N mineralization (52 DAO) | 0.729 | <2.024 × 10−6 | 19 |
| Total CO2 (mg C kg−1 dry soil) | SOM-Derived CO2 (mg C kg−1 dry soil) | Litter-Derived CO2 (mg C kg−1 dry soil) | Priming Effect (mg C kg−1 dry soil) | |||||
|---|---|---|---|---|---|---|---|---|
| Control | 359.5 ± 13.2 | d | 359.5 ± 13.2 | c | - | - | ||
| Maize Leaves | 1266.0 ± 118.8 | a | 654.8 ± 83.5 | a | 597.5 ± 33.9 | a | 288.2 ± 76.2 | a |
| Maize Roots | 749.8 ± 68.1 | c | 504.9 ± 10.7 | b | 281.6 ± 17.6 | c | 130.0 ± 12.0 | b |
| Maize Straw | 970.8 ± 34.3 | b | 561.9 ± 26.9 | b | 449.7 ± 21.1 | b | 178.4 ± 21.5 | b |
| Oxic Incubation Phase (0–46 DAO) | Anoxic Incubation Phase (47–55 DAO) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cumulative NO (µg N kg−1 dry soil) | Cumulative N2O (µg N kg−1 dry soil) | NO/N2O | Cumulative NO (mg N kg−1 dry soil) | Cumulative N2O (mg N kg−1 dry soil) | Cumulative N2 (mg N kg−1 dry soil) | N2O/(N2O+N2) | ||||||||
| Control | 24.1 ± 2.5 | a | 78.3 ± 97.2 | c | 0.37 ± 0.19 | a | 0.29 ± 0.04 | c | 16.6 ± 2.5 | c | 0.88 ± 0.33 | c | 0.95 ± 0.03 | a |
| Maize Leaves | 20.8 ± 1.4 | b | 387.2 ± 94.4 | ab | 0.05 ± 0.02 | b | 0.64 ± 0.10 | a | 29.8 ± 9.0 | a | 6.75 ± 4.28 | ab | 0.83 ± 0.04 | b |
| Maize Roots | 22.9 ± 2.8 | ab | 319.0 ± 81.0 | b | 0.07 ± 0.01 | b | 0.41 ± 0.06 | b | 21.5 ± 1.5 | bc | 3.70 ± 1.58 | bc | 0.85 ± 0.05 | b |
| Maize Straw | 10.0 ± 1.7 | c | 552.2 ± 260.7 | a | 0.02 ± 0.01 | b | 0.48 ± 0.06 | b | 24.8 ± 1.0 | ab | 8.36 ± 2.06 | ab | 0.75 ± 0.05 | c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rummel, P.S.; Well, R.; Pausch, J.; Pfeiffer, B.; Dittert, K. Carbon Availability and Nitrogen Mineralization Control Denitrification Rates and Product Stoichiometry during Initial Maize Litter Decomposition. Appl. Sci. 2021, 11, 5309. https://doi.org/10.3390/app11115309
Rummel PS, Well R, Pausch J, Pfeiffer B, Dittert K. Carbon Availability and Nitrogen Mineralization Control Denitrification Rates and Product Stoichiometry during Initial Maize Litter Decomposition. Applied Sciences. 2021; 11(11):5309. https://doi.org/10.3390/app11115309
Chicago/Turabian StyleRummel, Pauline Sophie, Reinhard Well, Johanna Pausch, Birgit Pfeiffer, and Klaus Dittert. 2021. "Carbon Availability and Nitrogen Mineralization Control Denitrification Rates and Product Stoichiometry during Initial Maize Litter Decomposition" Applied Sciences 11, no. 11: 5309. https://doi.org/10.3390/app11115309
APA StyleRummel, P. S., Well, R., Pausch, J., Pfeiffer, B., & Dittert, K. (2021). Carbon Availability and Nitrogen Mineralization Control Denitrification Rates and Product Stoichiometry during Initial Maize Litter Decomposition. Applied Sciences, 11(11), 5309. https://doi.org/10.3390/app11115309






