A Reaction–Diffusion–Reaction System for Forming Periodic Precipitation Bands of Cu-Fe-Based Prussian Blue Analogues
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Gel Samples and Optical Microscope Observation
2.3. Fe Kα Intensity Distribution Measurements of Gel Samples
2.4. SEM Observation of Crystallites in the Precipitation Patterns
3. Results
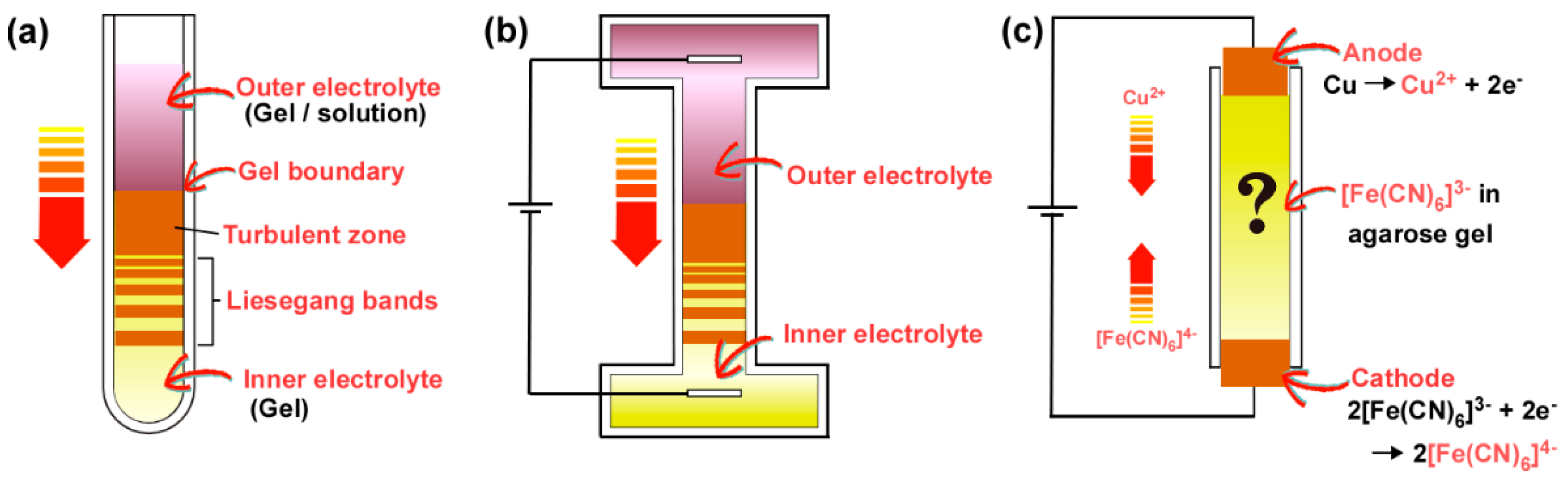
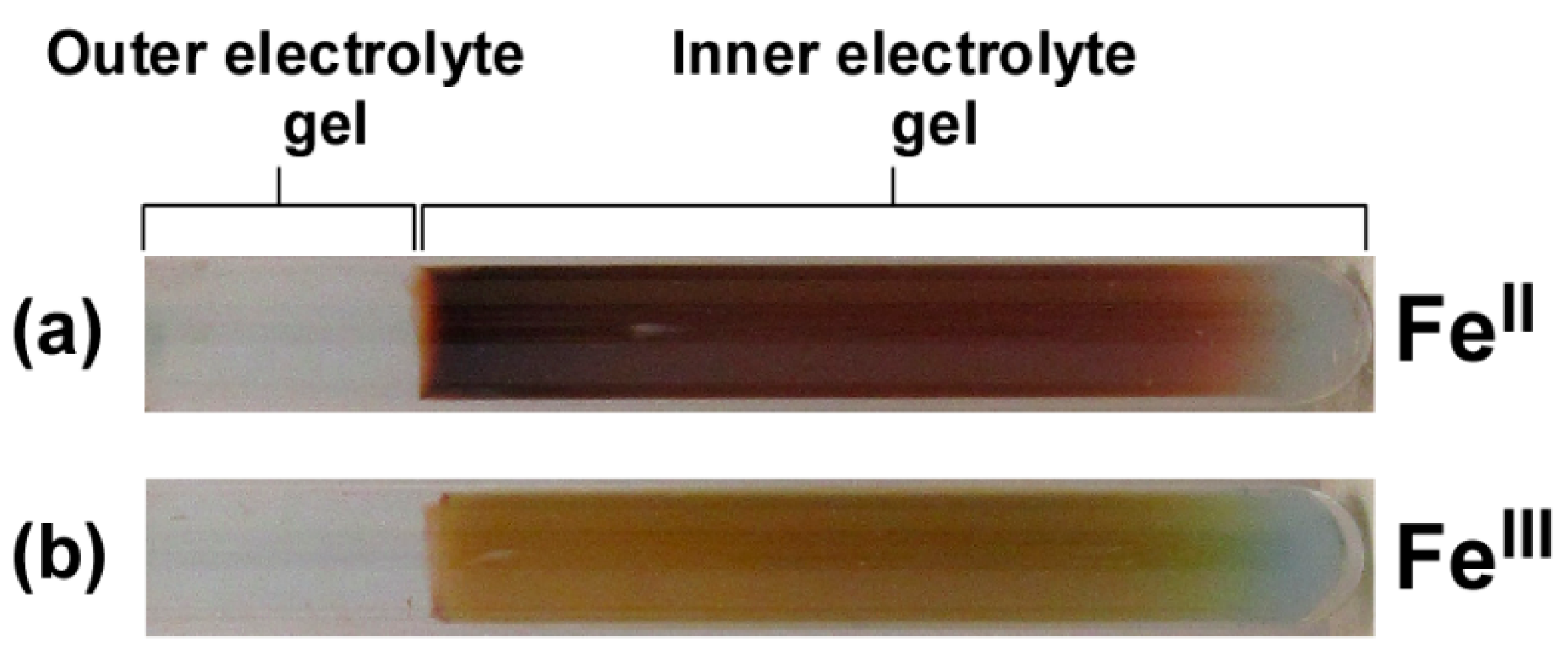
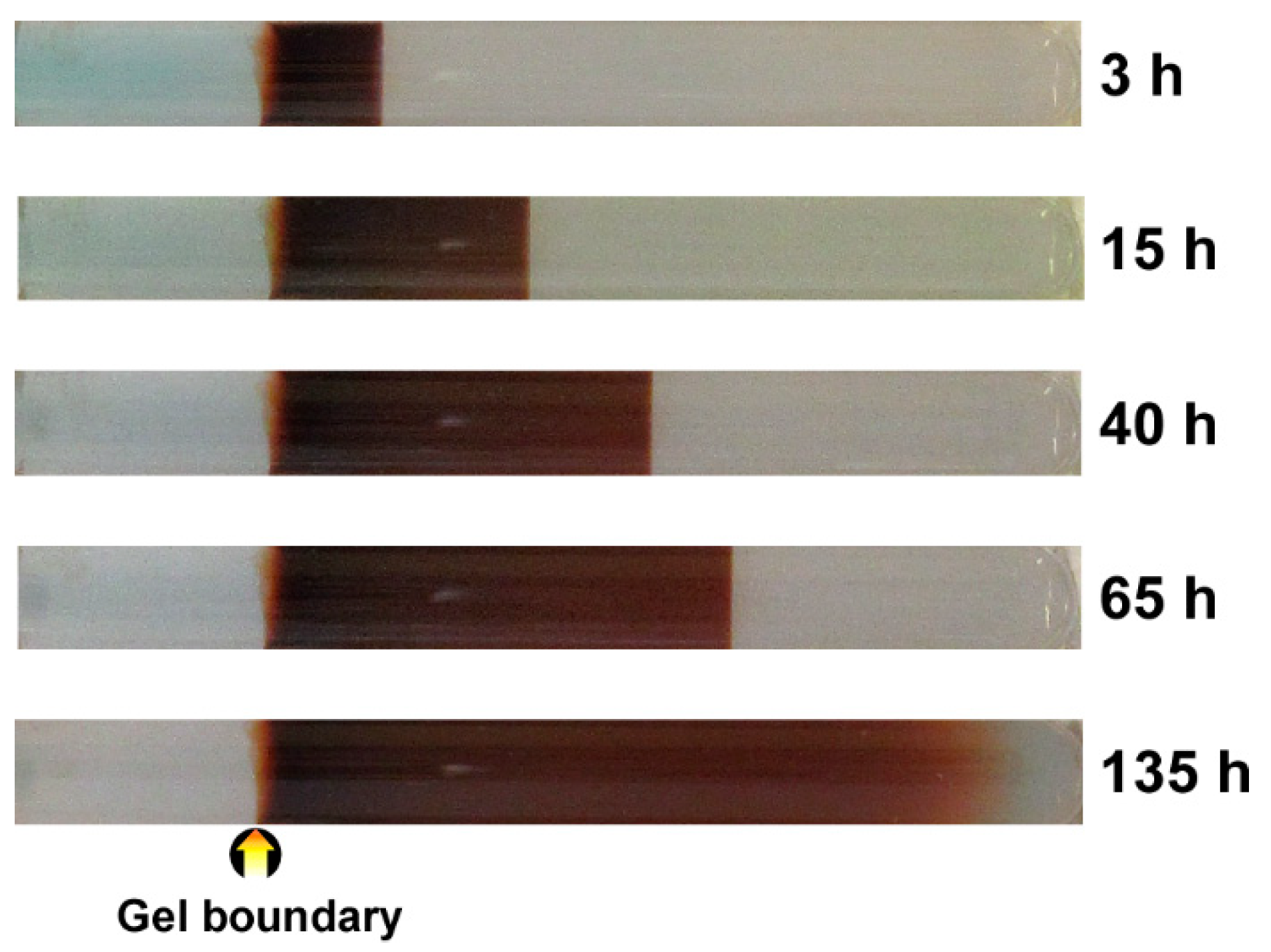
3.1. Precipitation Patterns of Cu-Fe PBA in the Conventional RD Setup for Observing Liesegang Bands
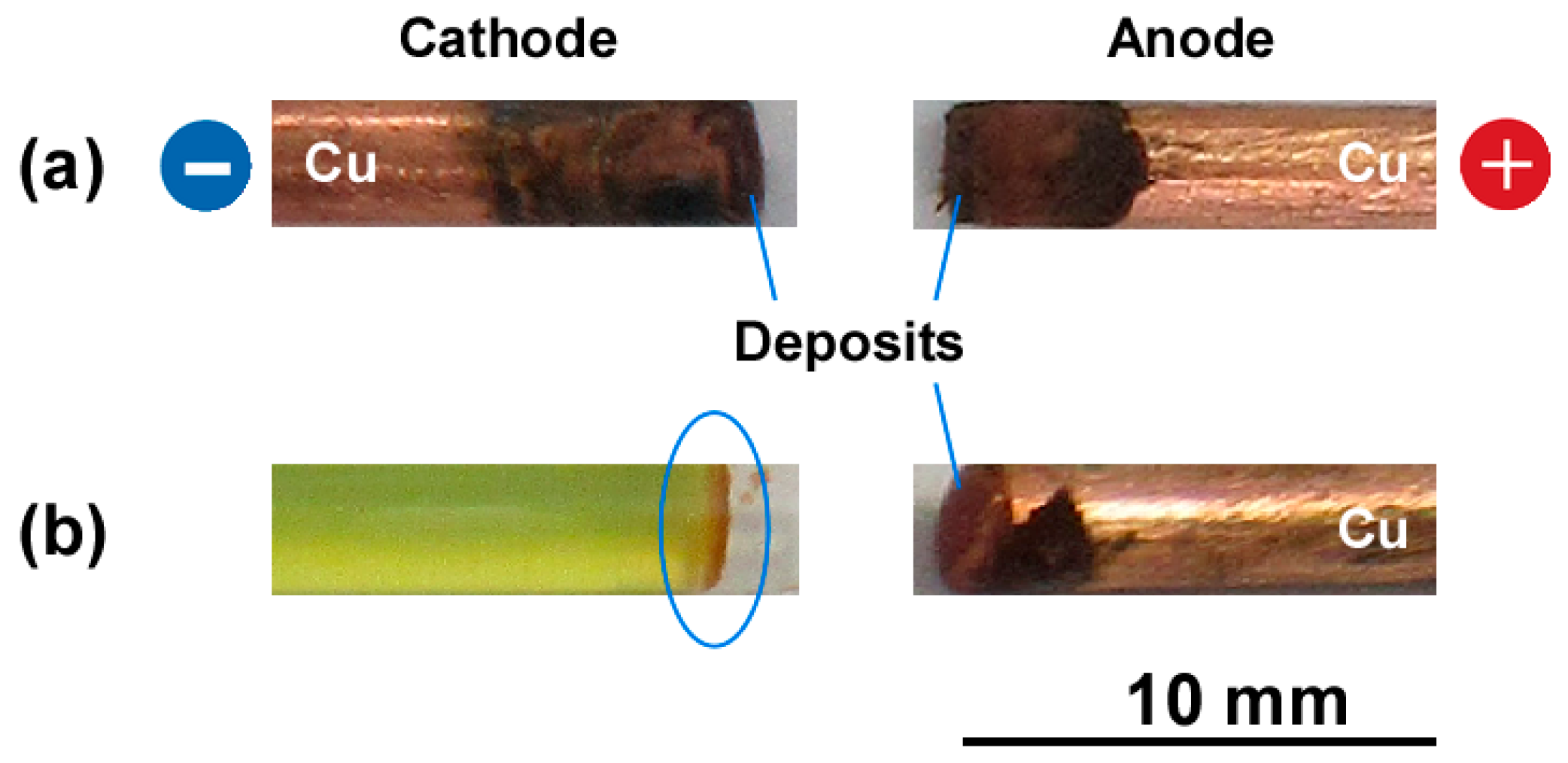
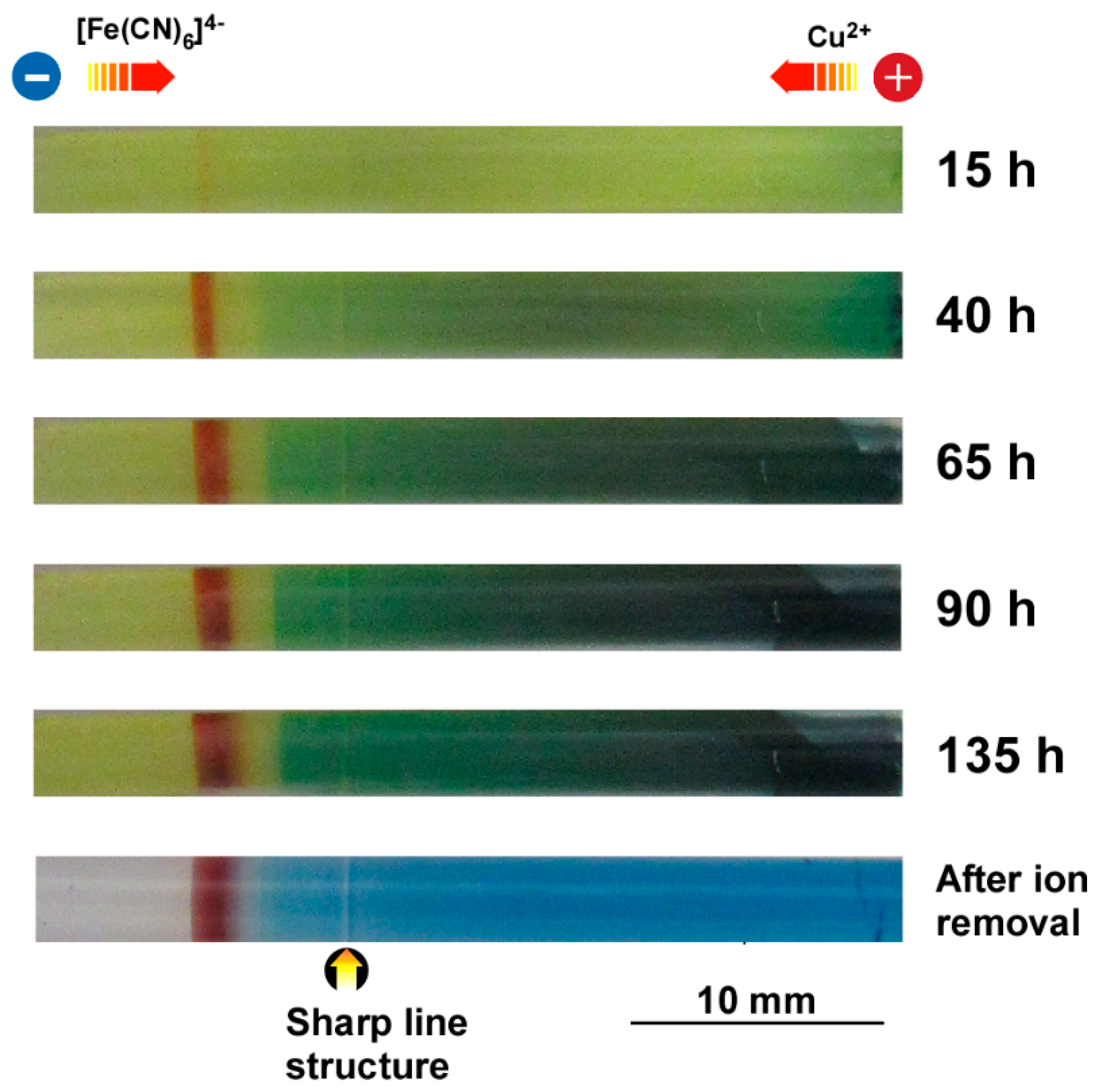
3.2. Formation of Multicolored Patterns at Constant Voltage in the RDR Setup
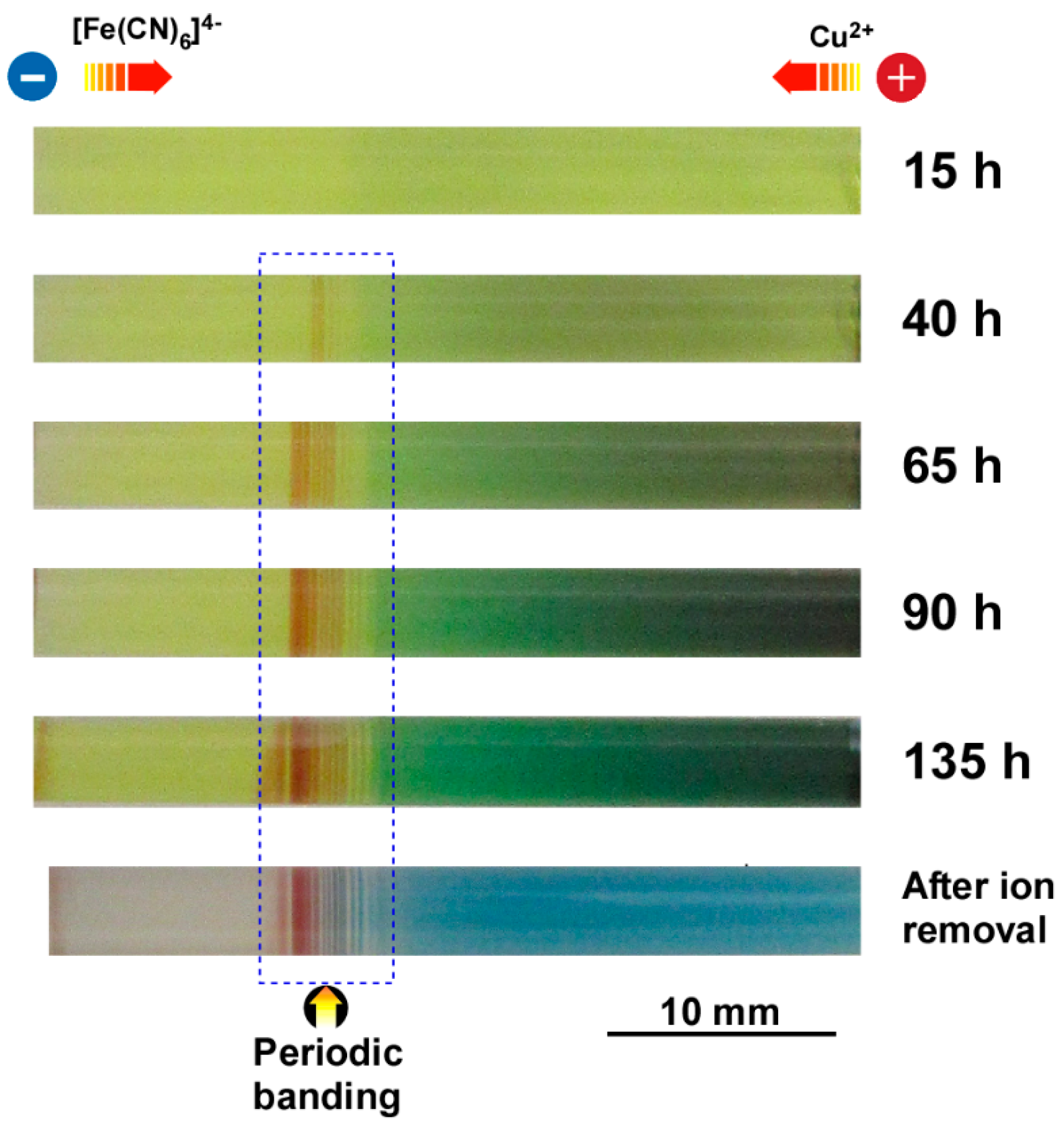
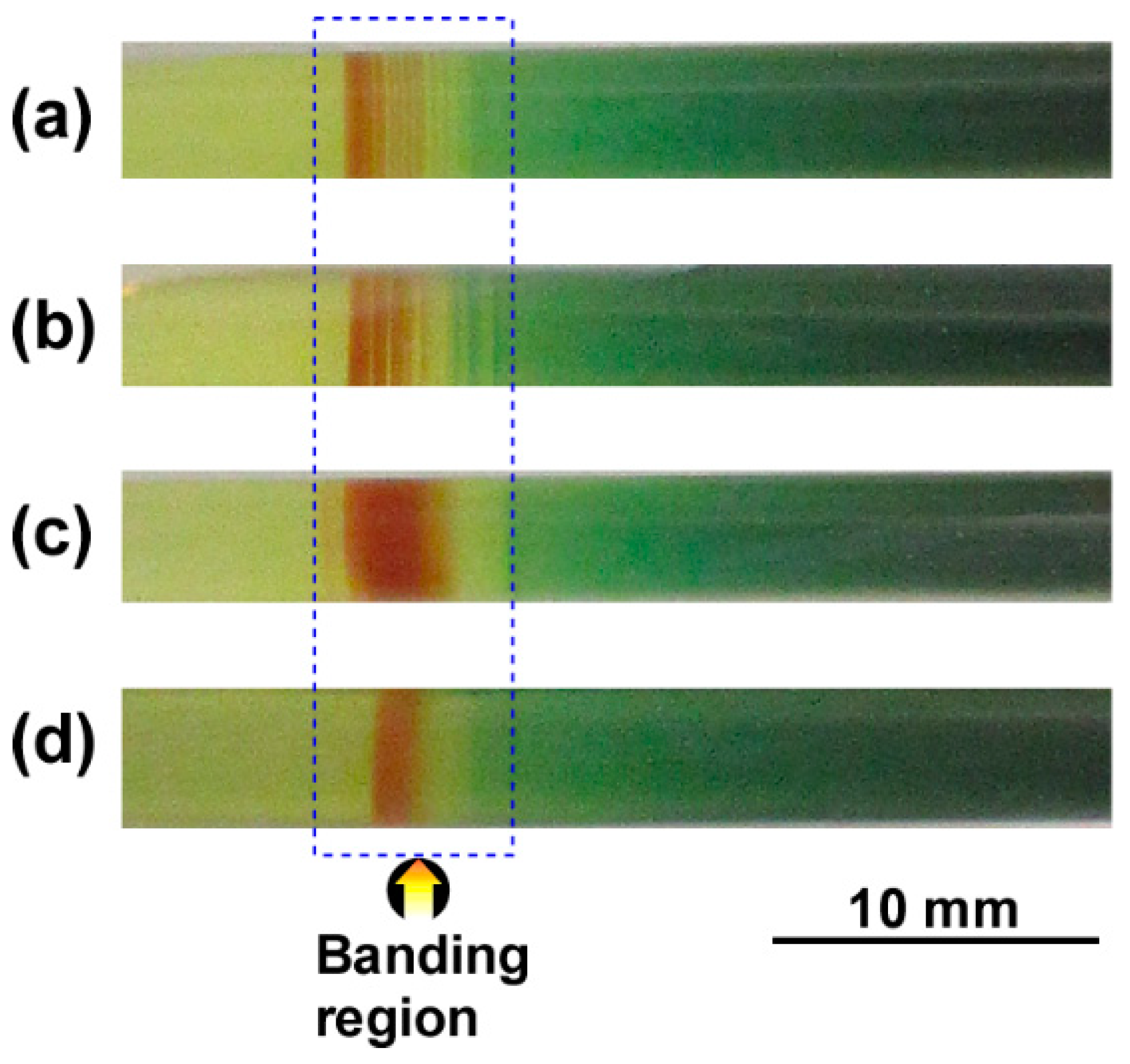
3.3. Formation of Multicolored Periodic Bands under Cyclic Alternating Voltage in the RDR Setup
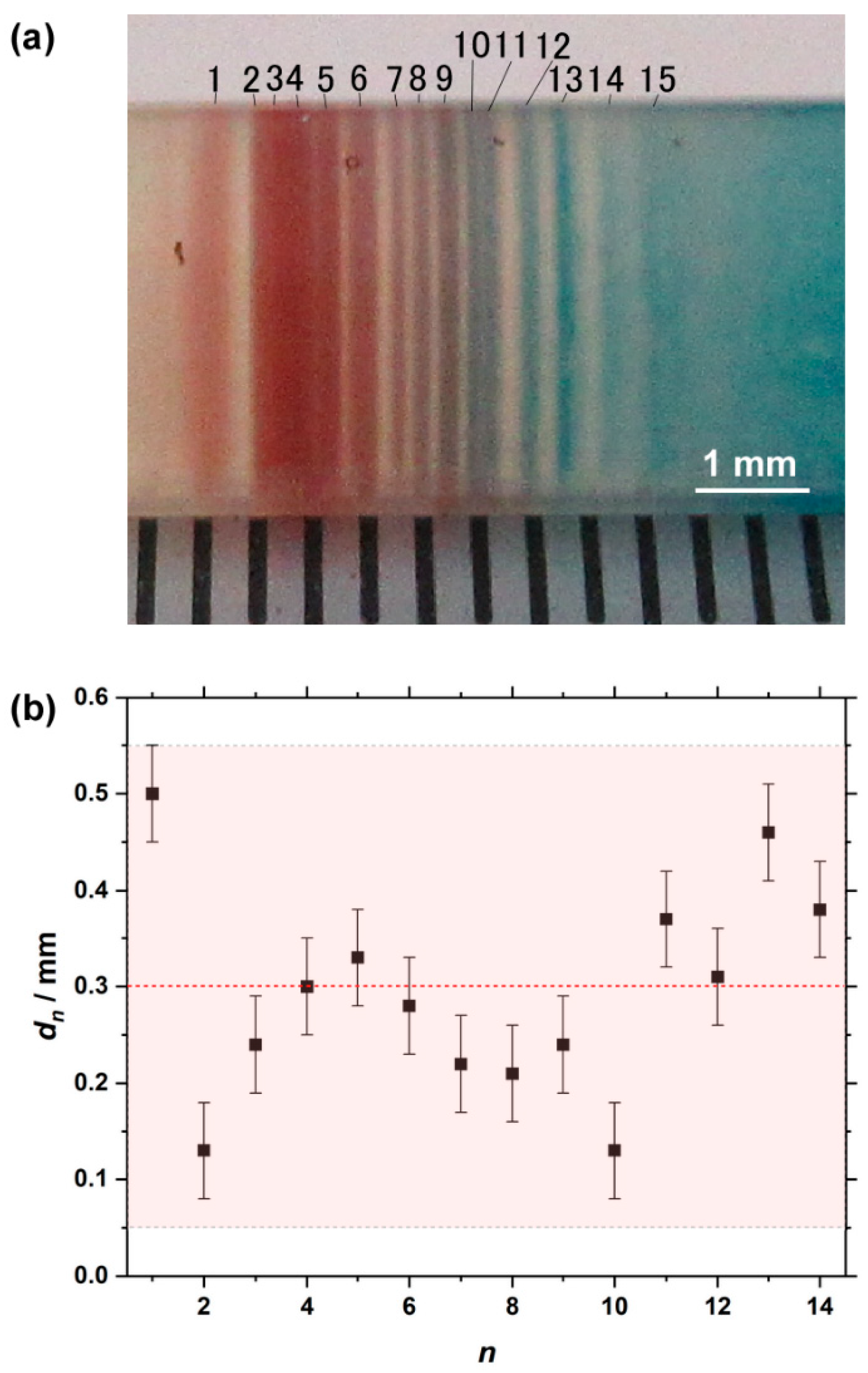
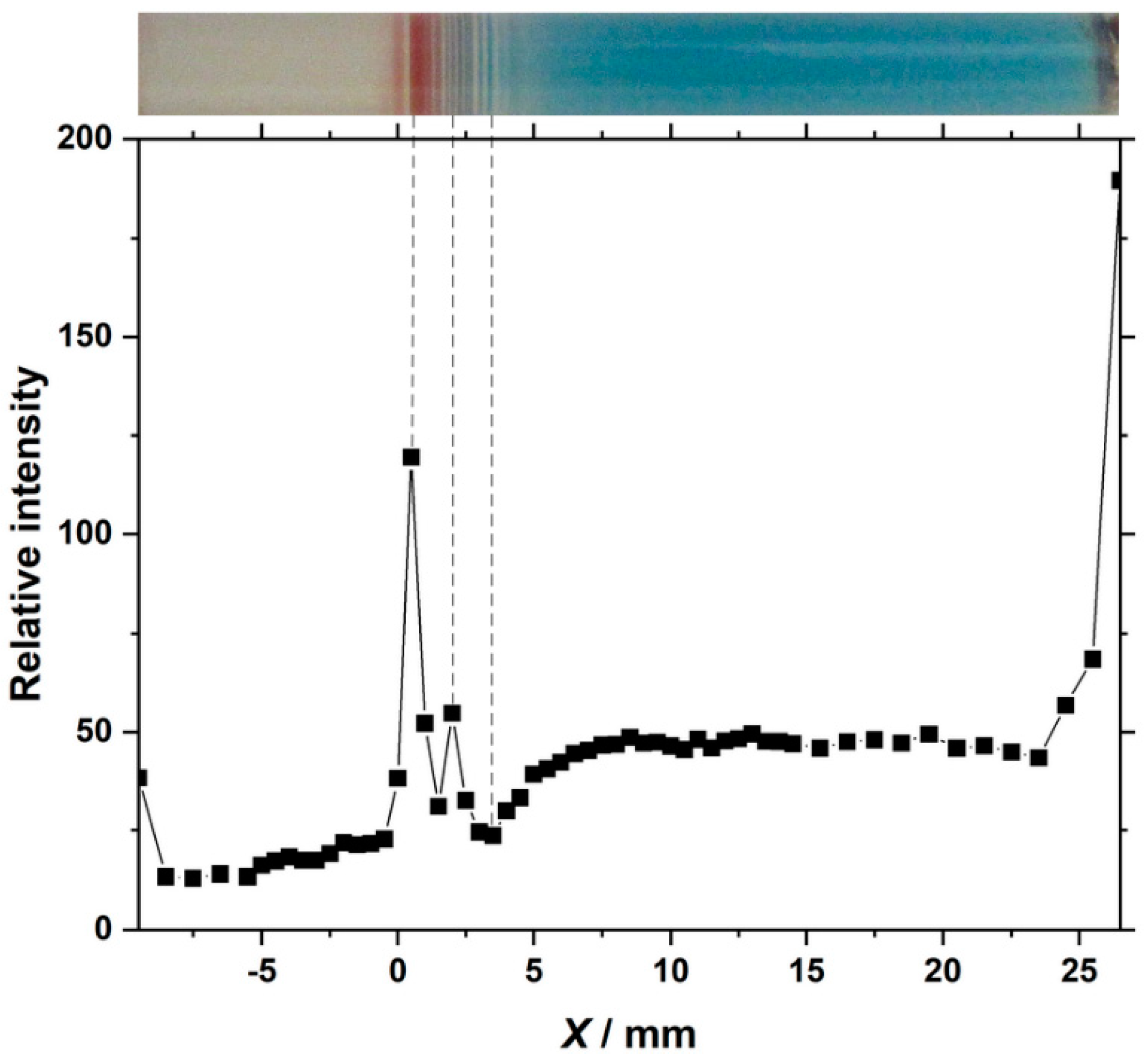
3.4. Fe Kα Intensity Distribution of the Pattern Formed under Cyclic Alternating Voltage
3.5. Microscopic Observation of the Pattern Formed under Cyclic Alternating Voltage
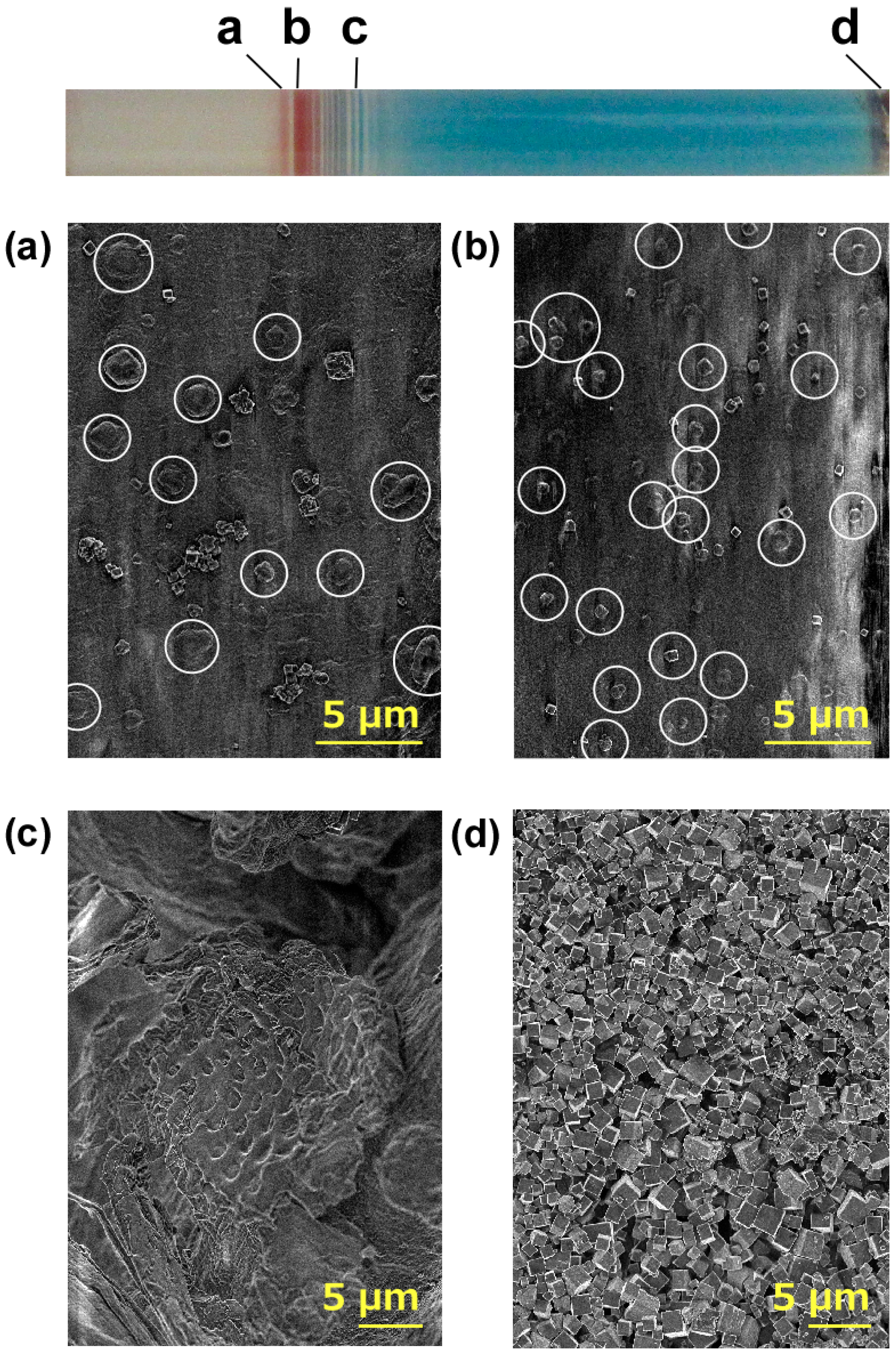
3.6. SEM Observation of the Pattern Formed under Cyclic Alternating Voltage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henisch, H. Crystals in Gels and Liesegang Rings; Cambridge University Press: Cambridge, UK, 1988; pp. 116–175. [Google Scholar]
- Nabika, H. Liesegang phenomena: Spontaneous pattern formation engineered by chemical reactions. Curr. Phys. Chem. 2015, 5, 5–20. [Google Scholar] [CrossRef]
- Nabika, H.; Itatani, M.; Lagzi, I. Pattern formation in precipitation reactions: The Liesegang phenomenon. Langmuir 2020, 36, 481–497. [Google Scholar] [CrossRef]
- Grzybowski, B.A. Chemistry in Motion: Reaction-Diffusion Systems for Micro- and Nanotechnology; John Wiley & Sons: Chichester, UK, 2009; pp. 93–163. [Google Scholar]
- Nakouzi, E.; Steinbock, O. Self-organization in precipitation reactions far from the equilibrium. Sci. Adv. 2016, 2, e1601144. [Google Scholar] [CrossRef]
- Grzybowski, B.A.; Bishop, K.J.M.; Campbell, C.J.; Fialkowski, M.; Smoukov, S.K. Micro- and nanotechnology via reaction-diffusion. Soft Matter 2005, 1, 114–128. [Google Scholar] [CrossRef]
- Grzybowski, B.A.; Campbell, C.J. Fabrication using ‘programmed’ reactions. Mater. Today 2007, 10, 38–46. [Google Scholar] [CrossRef]
- Das, I.; Pushkarna, A.; Chand, S. Electrical field effect on periodic precipitation and chemical waves in gel media in batch and continuous-flow reactors. J. Colloid Interface Sci. 1992, 150, 178–186. [Google Scholar] [CrossRef]
- Lagzi, I. Formation of Liesegang patterns in an electric field. Phys. Chem. Chem. Phys. 2002, 4, 1268–1270. [Google Scholar] [CrossRef]
- Shreif, Z.; Mandalian, L.; Abi-Haydar, A.; Sultan, R. Taming ring morphology in 2D Co(OH)2 Liesegang patterns. Phys. Chem. Chem. Phys. 2004, 6, 3461–3466. [Google Scholar] [CrossRef]
- Bena, I.; Droz, M.; Lagzi, I.; Martens, K.; Rácz, Z.; Volford, A. Designed patterns: Flexible control of precipitation through electric currents. Phys. Rev. Lett. 2008, 101, 075701. [Google Scholar] [CrossRef]
- Karam, T.; Sultan, R. Effect of an alternating current electric field on Co(OH)2 periodic precipitation. Chem. Phys. 2013, 412, 7–12. [Google Scholar] [CrossRef]
- Heard, D.M.; Lennox, A.J.J. Electrode materials in modern organic electrochemistry. Angew. Chem. Int. Ed. 2020, 59, 18866–18884. [Google Scholar] [CrossRef]
- Bard, A.J.; Parsons, R.; Jordan, J. Standard Potentials in Aqueous Solution; Dekker: New York, NY, USA, 1985; p. 827. [Google Scholar]
- Wessells, C.D.; Huggins, R.A.; Cui, Y. Copper hexacyanoferrate battery electrodes with long cycle life and high power. Nat. Commun. 2011, 2, 550. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J.B. Prussian blue: A new framework of electrode materials for sodium batteries. Chem. Commun. 2012, 48, 6544–6546. [Google Scholar] [CrossRef] [PubMed]
- Kaye, S.S.; Long, J.R. The role of vacancies in the hydrogen storage properties of Prussian blue analogues. Catal. Today 2007, 120, 311–316. [Google Scholar] [CrossRef]
- Svensson, G.; Grins, J.; Eklöf, D.; Eriksson, L.; Wardecki, D.; Thoral, C.; Bodoignet, L. Influence of the presence of different alkali cations and the amount of Fe(CN)6 vacancies on CO2 adsorption on copper hexacyanoferrates. Materials 2019, 12, 3371. [Google Scholar] [CrossRef] [PubMed]
- Verdaguer, M.; Girolami, G. Magnetic Prussian blue analogs. In Magnetism: Molecules to Materials V; Miller, J.S., Drillon, M., Eds.; Wiley–VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; pp. 283–346. [Google Scholar]
- Li, W.-J.; Han, C.; Cheng, G.; Chou, S.-L.; Liu, H.-K.; Dou, S.-X. Chemical properties, structural properties, and energy storage applications of Prussian blue analogues. Small 2019, 15, 1900470. [Google Scholar] [CrossRef]
- Azhar, A.; Li, Y.; Cai, Z.; Zakaria, M.B.; Masud, M.K.; Hossain, M.S.; Kim, J.; Zhang, W.; Na, J.; Yamauchi, Y.; et al. Nanoarchitectonics: A new materials horizon for Prussian blue and its analogues. Bull. Chem. Soc. Jpn. 2019, 92, 875–904. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Duan, Y.; Liu, J.-D.; Chen, Y.; Liu, X.-K.; Liu, W.; Ma, T.; Li, Y.; Zheng, X.-S.; Yao, T.; et al. Unconventional CN vacancies suppress iron-leaching in Prussian blue analogue pre-catalyst for boosted oxygen evolution catalysis. Nat. Commun. 2019, 10, 2799. [Google Scholar] [CrossRef]
- Hayashi, H.; Aoki, S.; Suzuki, T. Spontaneous precipitation pattern formation by crystallites of Mn-Fe-based Prussian blue analogues in agarose gel. RSC Adv. 2019, 9, 36240–36247. [Google Scholar] [CrossRef]
- Hayashi, H.; Takaishi, M. Low-cost, high-performance sample cell for X-ray spectroscopy of solutions and gels made from plastic straw. Anal. Sci. 2019, 35, 651–657. [Google Scholar] [CrossRef]
- Hayashi, H.; Sato, Y.; Aoki, S.; Takaishi, M. In situ XRF analysis of Cs adsorption by the precipitation bands of Prussian blue analogues formed in agarose gels. J. Anal. At. Spectrom. 2019, 34, 979–985. [Google Scholar] [CrossRef]
- Hayashi, H.; Abe, H. An X-ray Spectroscopic study of Co-Fe-based Prussian blue analog gels. Bull. Chem. Soc. Jpn. 2016, 89, 1510–1517. [Google Scholar] [CrossRef]
- Hayashi, H.; Abe, H. A combined X-ray spectroscopic study on the multicolored pattern formation in gels containing FeCl3 and K3[Fe(CN)6]. J. Anal. At. Spectrom. 2016, 31, 912–923. [Google Scholar] [CrossRef]
- Hayashi, H.; Abe, H. X-ray spectroscopic analysis of Liesegang patterns in Mn-Fe-based Prussian blue analogs. J. Anal. At. Spectrom. 2016, 31, 1658–1672. [Google Scholar] [CrossRef]
- Hayashi, H.; Abe, H. Gel-state dependencies of brown patterns of Mn-Fe-based Prussian blue analogues studied by combined X-ray spectroscopies. Bull. Chem. Soc. Jpn. 2017, 90, 807–819. [Google Scholar] [CrossRef]
- Hayashi, H.; Sato, Y.; Abe, H. X-ray spectroscopic analysis of stochastic, periodic precipitation in Co-Fe-based Prussian blue analogues. J. Anal. At. Spectrom. 2018, 33, 957–966. [Google Scholar] [CrossRef]
- Hayashi, H.; Aoki, S.; Takaishi, M.; Sato, Y.; Abe, H. An XAFS study of Cs adsorption by the precipitation bands of Mn-Fe-based Prussian blue analogues spontaneously formed in agarose gel. Phys. Chem. Chem. Phys. 2019, 21, 22553–22562. [Google Scholar] [CrossRef]
- Estelrich, J.; Busquets, M.A. Prussian blue: A safe pigment with zeolitic-like activity. Int. J. Mol. Sci. 2021, 22, 780. [Google Scholar] [CrossRef]
- Sadek, S.; Sultan, R. Liesegang patterns in nature: A diverse scenery across the sciences. In Precipitation Patterns in Reaction-Diffusion Systems; Lagzi, I., Ed.; Research Signpost: Kerala, India, 2010; pp. 1–43. [Google Scholar]
- Izsák, F.; Lagzi, I. Models of Liesegang pattern formation. In Precipitation Patterns in Reaction-Diffusion Systems; Lagzi, I., Ed.; Research Signpost: Kerala, India, 2010; pp. 207–217. [Google Scholar]
- Chacron, M.; L’Heureux, I. A new model of periodic precipitation incorporating nucleation, growth and ripening. Phys. Lett. A 1999, 263, 70–77. [Google Scholar] [CrossRef]
- L’Heureux, I.; Bektursunova, R. Modeling Liesegang periodic precipitation patterns in geochemical systems. In Precipitation Patterns in Reaction-Diffusion Systems; Lagzi, I., Ed.; Research Signpost: Kerala, India, 2010; pp. 45–71. [Google Scholar]
- Hayashi, H.; Aoki, S.; Abe, H. Magnetic-field-induced painting-out of precipitation bands of Mn-Fe-based Prussian blue analogues in water-glass gels. ACS Omega 2018, 3, 4494–4501. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, H.; Suzuki, T. A Reaction–Diffusion–Reaction System for Forming Periodic Precipitation Bands of Cu-Fe-Based Prussian Blue Analogues. Appl. Sci. 2021, 11, 5000. https://doi.org/10.3390/app11115000
Hayashi H, Suzuki T. A Reaction–Diffusion–Reaction System for Forming Periodic Precipitation Bands of Cu-Fe-Based Prussian Blue Analogues. Applied Sciences. 2021; 11(11):5000. https://doi.org/10.3390/app11115000
Chicago/Turabian StyleHayashi, Hisashi, and Tomoko Suzuki. 2021. "A Reaction–Diffusion–Reaction System for Forming Periodic Precipitation Bands of Cu-Fe-Based Prussian Blue Analogues" Applied Sciences 11, no. 11: 5000. https://doi.org/10.3390/app11115000
APA StyleHayashi, H., & Suzuki, T. (2021). A Reaction–Diffusion–Reaction System for Forming Periodic Precipitation Bands of Cu-Fe-Based Prussian Blue Analogues. Applied Sciences, 11(11), 5000. https://doi.org/10.3390/app11115000




