RF Exposure Assessment for Various Poses of Patient Assistant in Open MRI Environment
Abstract
:1. Introduction
2. Materials and Methods
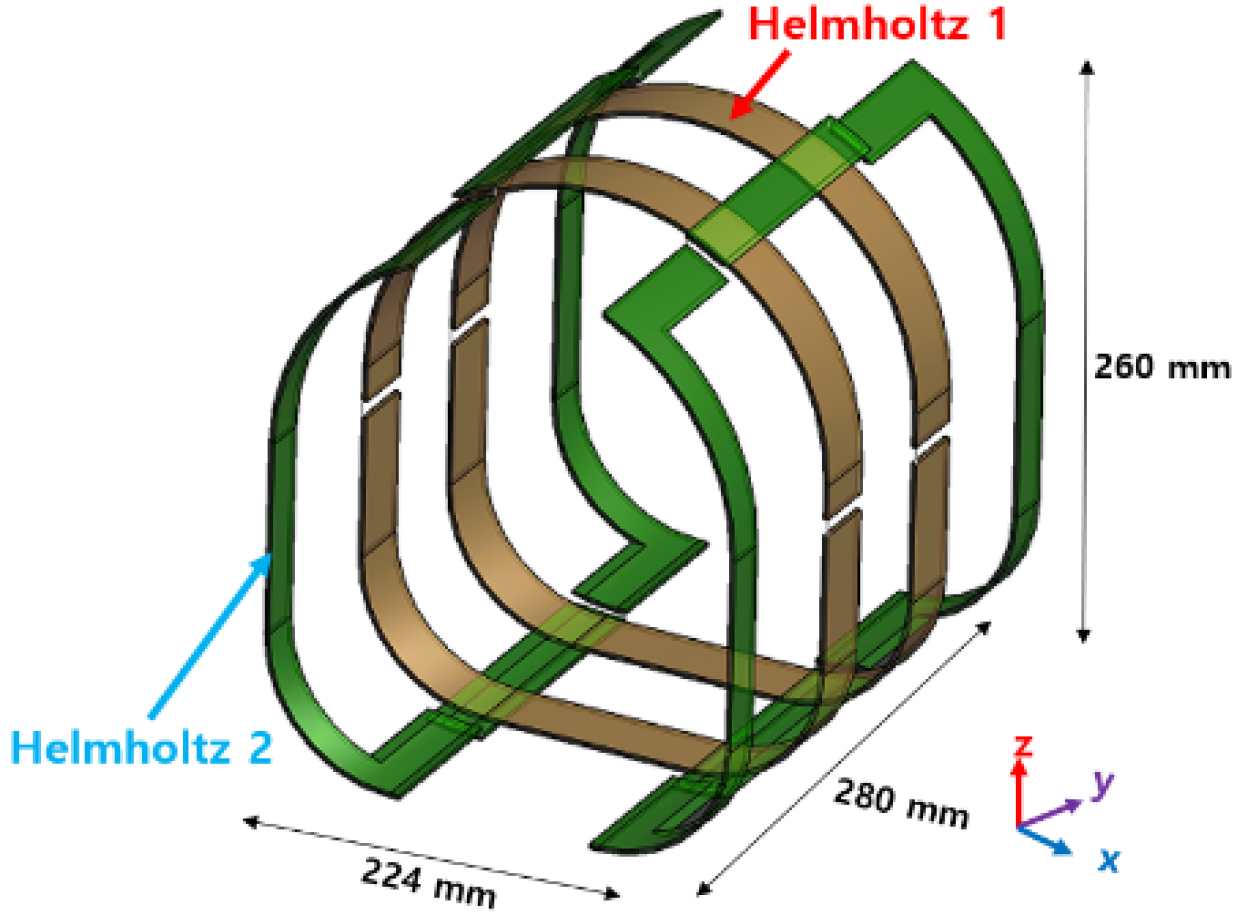
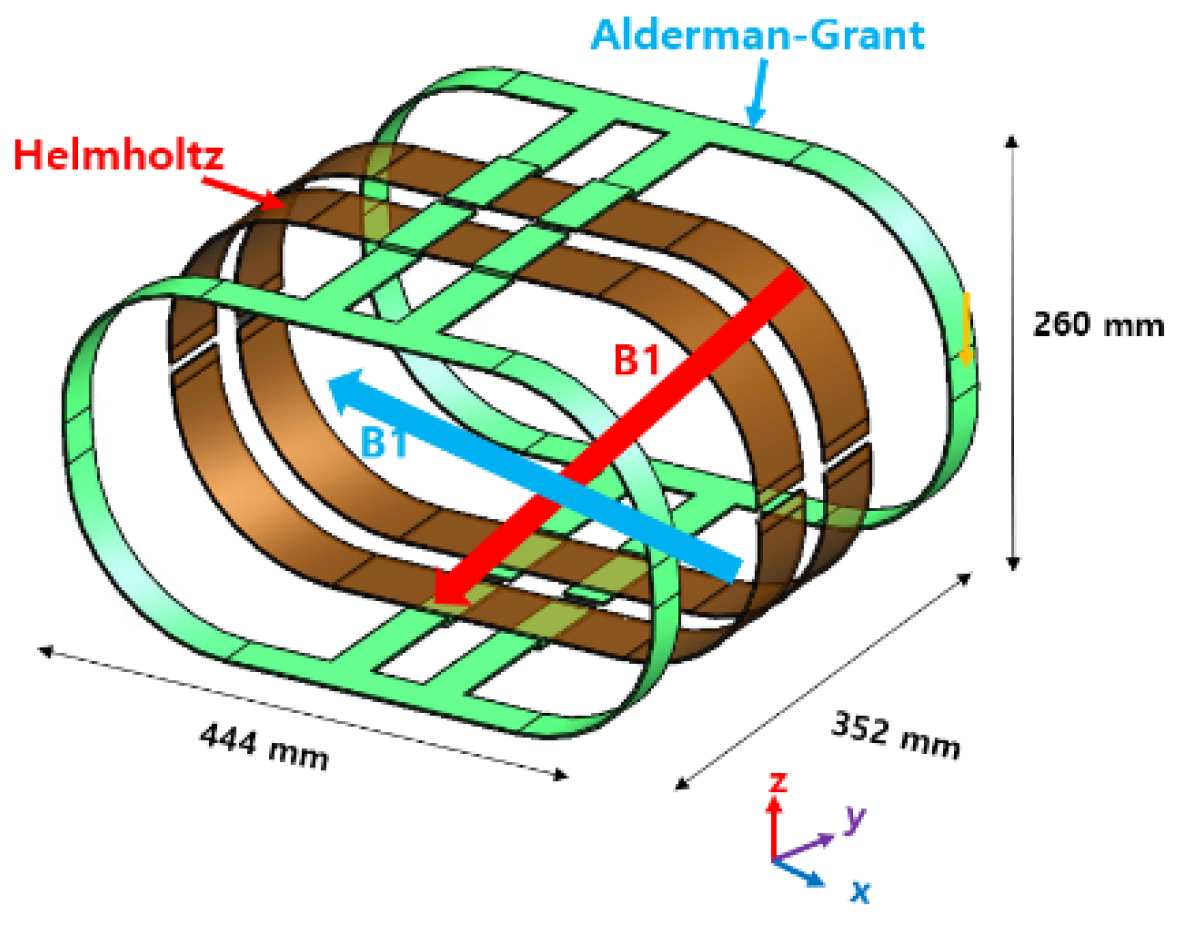
2.1. Numerical Modeling of RF Coils for Open MRI Systems
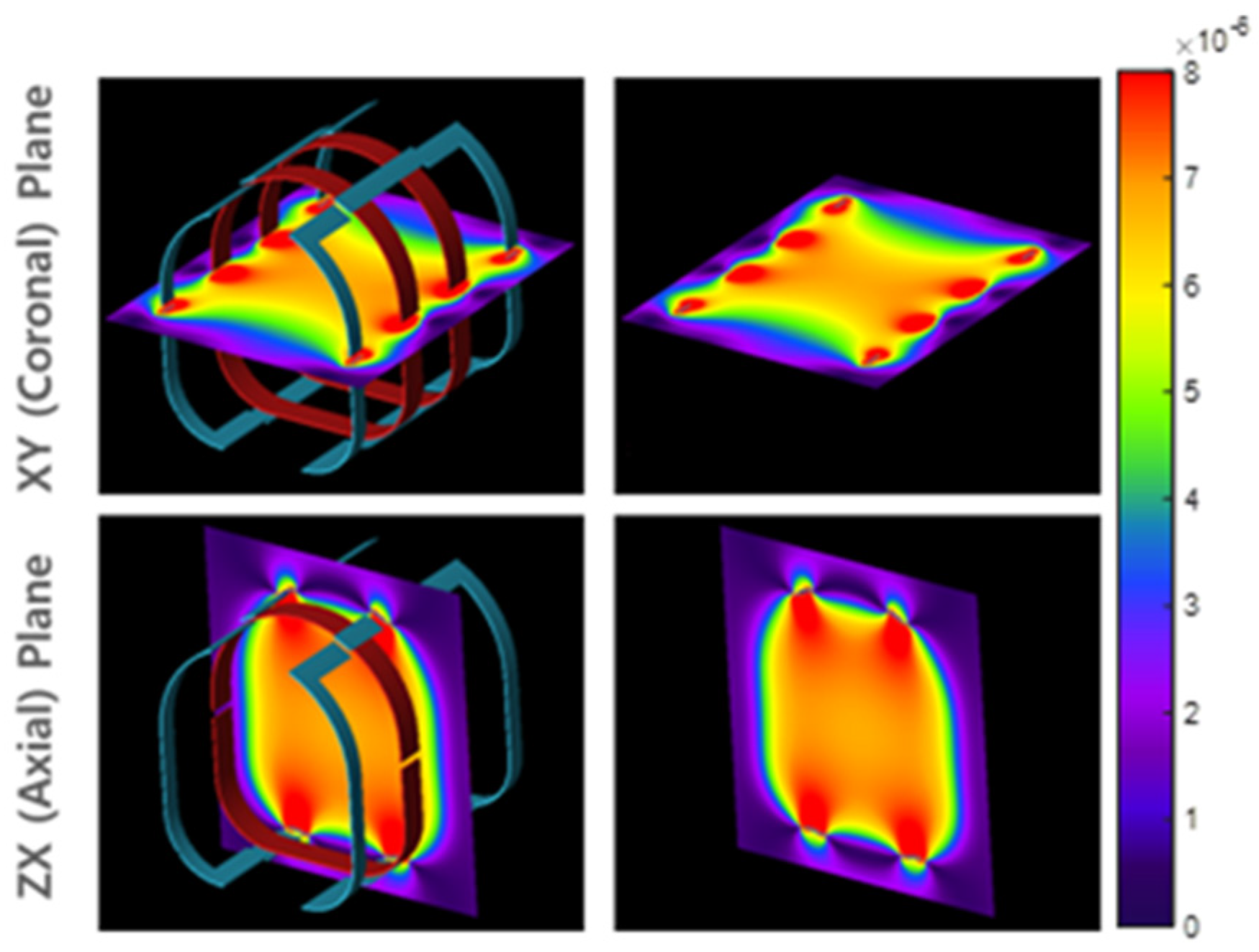
2.1.1. RF Head Coil
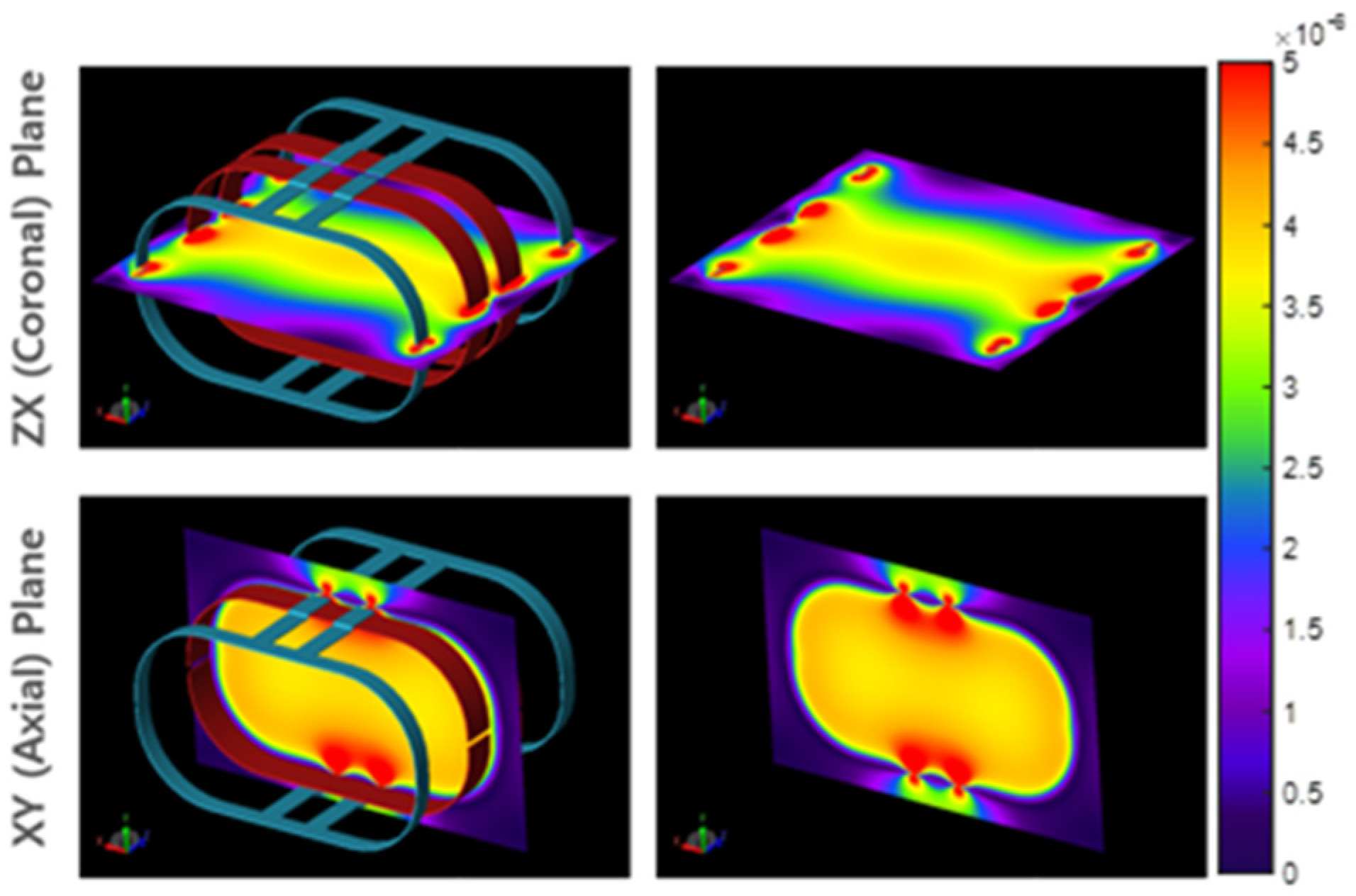
2.1.2. RF Body Coil
2.2. Human Body Model
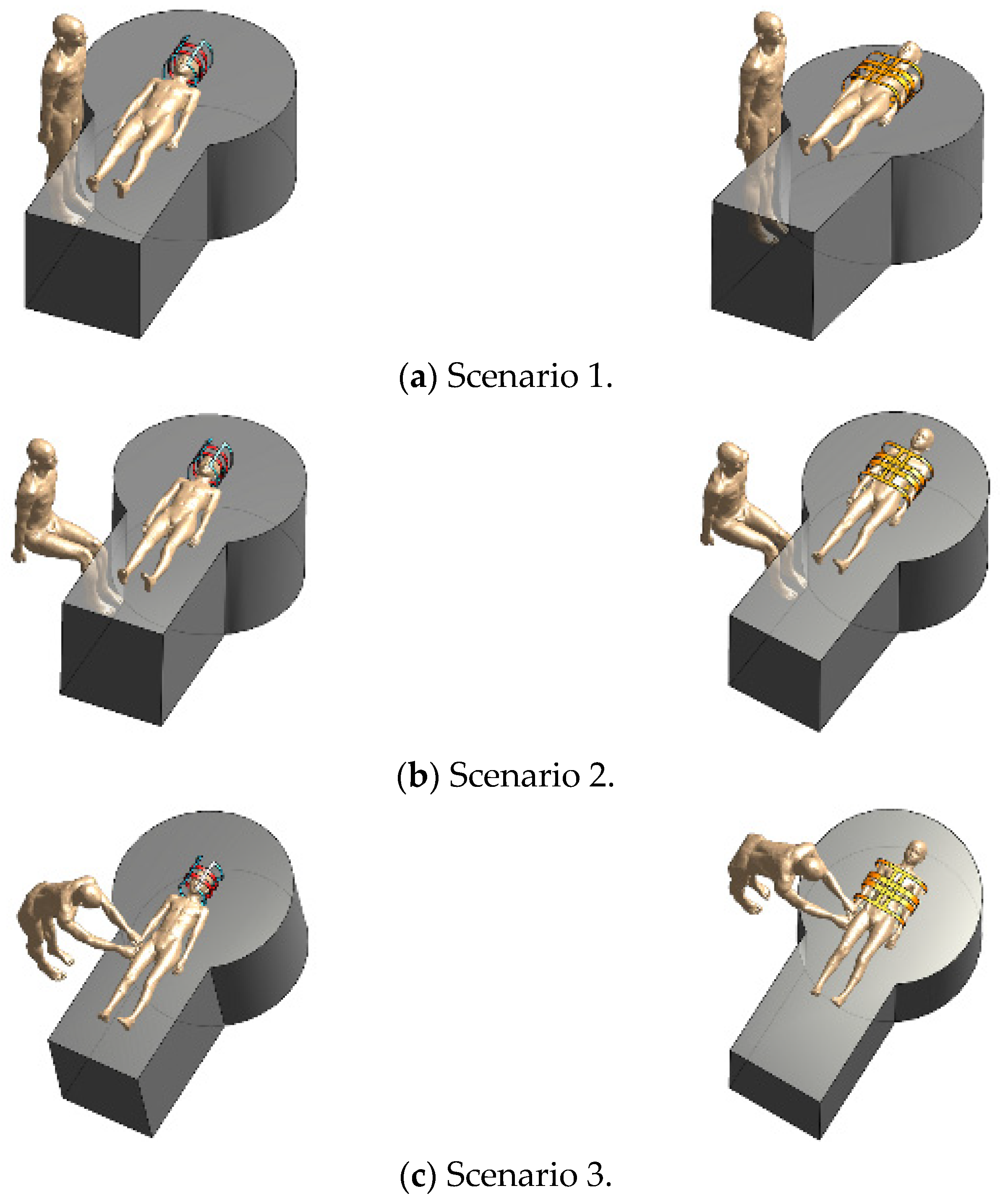
2.3. Assessment Scenarios
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webb, A.; Collins, C.M. Parallel transmit and receive technology in high-field magnetic resonance neuroimaging. Int. J. Imaging. Syst. Technol. 2010, 20, 2–13. [Google Scholar] [CrossRef]
- ICNIRP. 1998 Guidelines for limiting exposure to time-varying electric, magnetic and electromagnetic fields (up to 300 GHz). Health Phys. 1998, 74, 494–522. [Google Scholar]
- ICNIRP. 2010 Guidelines for limiting exposure to time-varying electric, magnetic and electromagnetic fields (1 Hz to 100 kHz). Health Phys. 2010, 99, 816–836. [Google Scholar]
- ICNIRP. 2020 Guidelines for limiting exposure to time-varying electric, magnetic and electromagnetic fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar]
- ICNIRP. 2009 Statement Amendment to the ICNIRP 2009 statement on medical magnetic resonance (MR) procedures: Pro-tection of patients. Health Phys. 2009, 97, 259–261. [Google Scholar]
- IEC. IEC 60601-2-33: Medical Electrical Equipment-Part 2-33: Practical Requirements for the Safety of Magnetic Resonance Equipment for Medical Diagnosis; IEC: Geneva, Switzerland, 2015; Chapter 201.12. [Google Scholar]
- Rianne, S.; Sachiko, Y.-S. Occupational exposure to electromagnetic fields from medical sources. Ind. Health 2018, 56, 96–105. [Google Scholar]
- Rathebe, P.; Weyers, C.; Raphela, F. A health and safety model for occupational exposure to radiofrequency fields and static magnetic fields from 1.5 and 3 T MRI scanners. Health Technol. 2020, 10, 39–50. [Google Scholar] [CrossRef]
- Huss, A.; Özdemir, E.; Schaap, K.; Kromhout, H. Occupational exposure to MRI-related magnetic stray fields and sleep quality among MRI–Technicians–A cross-sectional study in the Netherlands. Int. J. Hyg. Environ. Health 2021, 231, 113636. [Google Scholar] [CrossRef]
- Murbach, M.; Neufeld, E.; Kainz, W.; Pruessmann, K.P.; Kuster, N. Whole-body and local RF absorption in human modles as a function of anatomy and position within 1.5T body coil. Magn. Reson. Med. 2013, 71, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Noh, Y.; Kim, K.-N.; Ryu, Y.C. Evaluation of the specific absorption rate for simultaneous multi frequency RF ex-citation in 7-T magnetic resonance imaging. J. Magn. 2017, 637–641. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Zhang, P.; Li, C.; Sun, J.; Chen, Z.; Xing, S.; Liang, P.; Wu, T. Statistical evaluation of radiofrequency ex-posure during magnetic resonant imaging: Application of whole-body individual human model and body motion in the coil. Int. J. Environ. Res. Public Health 2019, 16, 1069. [Google Scholar] [CrossRef] [Green Version]
- Fujimot, K.; Angelone, L.M.; Lucano, E.; Rajan, S.S.; Lacono, M.I. Radio-frequency safety assessment of stents in Blood Ves-sels During Magnetic Resonance Imaging. Front. Physiol. 2018, 9, 1439. [Google Scholar] [CrossRef] [Green Version]
- Golestanirad, L.; Rahsepar, A.A.; Kirsch, J.E.; Suwa, K.; Collins, J.C.; Angelone, L.M.; Keil, B.; Passman, R.S.; Bonmassar, G.; Serano, P.; et al. Changes in the specific absorption rate (SAR) of ra-diofrequency energy in patients with retained cardiac leads during MRI at 1.5 T and 3 T. Magn. Reson. Med. 2019, 81, 653–669. [Google Scholar] [CrossRef]
- Rahsepar, A.A.; Zimmerman, S.L.; Hansford, R.; Guttman, M.A.; Castro, V.; McVeigh, D.; Kirsch, J.E.; Halperin, H.R.; Nazarian, S. The Relationship between MRI Radiofrequency Energy and Function of Nonconditional Implanted Cardiac Devices: A Prospective Evaluation. Radiology 2020, 295, 307–313. [Google Scholar] [CrossRef]
- Seo, Y.; Wang, Z.J. Measurement and evaluation of specific absorption rate and temperature elevation caused by an artificial hip joint during MRI scanning. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Malik, S.J.; Beqiri, A.; Price, A.N.; Teixeira, J.N.; Hand, J.W.; Hajnal, J.V. Specific absorption rate in neonates undergoing magnetic resonance procedures at 1.5 T and 3 T. NMR Biomed. 2015, 28, 344–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murbach, M.; Neufeld, E.; Samaras, T.; Córcoles, J.; Robb, F.J.; Kainz, W.; Kuster, N. Pregnant women models analyzed for RF exposure and temperature increase in 3T RF shimmed birdcages. Magn. Reson. Med. 2017, 77, 2048–2056. [Google Scholar] [CrossRef] [PubMed]
- Barrera, C.A.; Francavilla, M.L.; Serai, S.D.; Edgar, J.C.; Jaimes, C.; Gee, M.S.; Roberts, T.P.L.; Otero, H.J.; Adzick, N.S.; Victoria, T. Specific Absorption Rate and Specific Energy Dose: Comparison of 1.5-T versus 3.0-T Fetal MRI. Radiology 2020, 295, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.P.; Simonis, F.F.; Webb, A.G. Low-field MRI: An MR physics perspective. J. Magn. Reson. Imaging 2019, 49, 1528–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannetti, G.; Frijia, F.; Flori, A.; Montanaro, D. Design and simulation of a Helmholtz coil for magnetic resonance imag-ing and spectroscopy experiments with a 3T MR clinical scanner. Appl. Magn. Reson. 2019, 50, 1083–1097. [Google Scholar] [CrossRef]
- Tomanek, B.; Volotovskyy, V.; Tyson, R.; Yin, D.; Sharp, J.; Blasiak, B. A quadrature volum RF coil for vertical B0 field open MRI systems. Concepts. Magn. Reson. Part B 2016, 46B, 118–122. [Google Scholar]
- Christ, A.; Kainz, W.; Hahn, E.G.; Honegger, K.; Zefferer, M.; Neufeld, E.; Rascher, R.W.; Janka, W.; Bautz, J.; Chen, B.; et al. The virtual fami-ly-development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys. Med. Biol. 2010, 55, N23–N38. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, M.-C.; Neufeld, E.; Moser, H.; Huber, E.; Farcito, S.; Gerber, L.; Jedensjö, M.; Hilber, I.; Gennaro, F.D.; Lloyd, B.; et al. Development of a new generation of high-resolution anatomical models for medical device evaluation: The Virtual Population 3. Phys. Med. Biol. 2014, 59, 5287–5303. [Google Scholar] [CrossRef] [PubMed]
- Hasgall, P.; Di Gennaro, F.; Baumgartner, C.; Neufeld, E.; Lloyd, B.; Gosselin, M.C.; Payne, D.; Klingenböck, A.; Kuster, N. IT’IS Database for Thermal and Electromagnetic Parameters of Biological Tissues, 4th ed. Available online: https://itis.swiss/virtual-population/tissue-properties/downloads/ (accessed on 28 May 2021).
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271–2293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Lin, J.; Mao, W.; Liu, M.; Smith, M.B.; Collins, C.M. SAR and temperature: Simulations and comparison to regula-tory limits for MRI. J. Magn. Reson. Imaging. 2007, 26, 437–441. [Google Scholar] [CrossRef] [Green Version]
- Collins, C.M.; Liu, W.; Wang, J.; Gruetter, R.; Vaughan, J.T.; Ugurbil, K.; Smith, M.B. Temperature and SAR calculations for a human head within volume and surface coils at 64 and 300 MHz. J. Magn. Reson. Imaging 2004, 19, 650–656. [Google Scholar] [CrossRef]
- Vaughan, J.T.; Snyder, C.J.; DelaBarre, L.J.; Bolan, P.J.; Tian, J.; Bolinger, L.; Adriany, G.; Andersen, P.; Strupp, J.; Ugurbil, K. Whole-body imaging at 7T: Preliminary results. Magn. Reson. Med. 2009, 61, 244–248. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Mao, W.; Qiu, M.; Smith, M.B.; Constable, R.T. Factors influencing flip angle mapping in MRI: RF pulse shape, slice-select gradients, off-resonance excitation, and B0 inhomogeneities. Magn. Reson. Med. 2006, 56, 463–468. [Google Scholar] [CrossRef]
- Chou, C.K.; Bassen, H.; Osepchuk, J.; Balzano, Q.; Peterson, R.; Meltz, M.; Cleveland, R.; Lin, J.C.; Heynick, L. Radio frequency electromagnetic exposure: Tutorial review on experimental dosimetry. Bioelectromagnetics 1996, 17, 195–208. [Google Scholar] [CrossRef]
- Collins, C.M.; Wang, Z. Calculation of radiofrequency electromagnetic fields and their effects in MRI of human subjects. Magn. Reson. Med. 2011, 65, 1470–1482. [Google Scholar] [CrossRef] [PubMed]
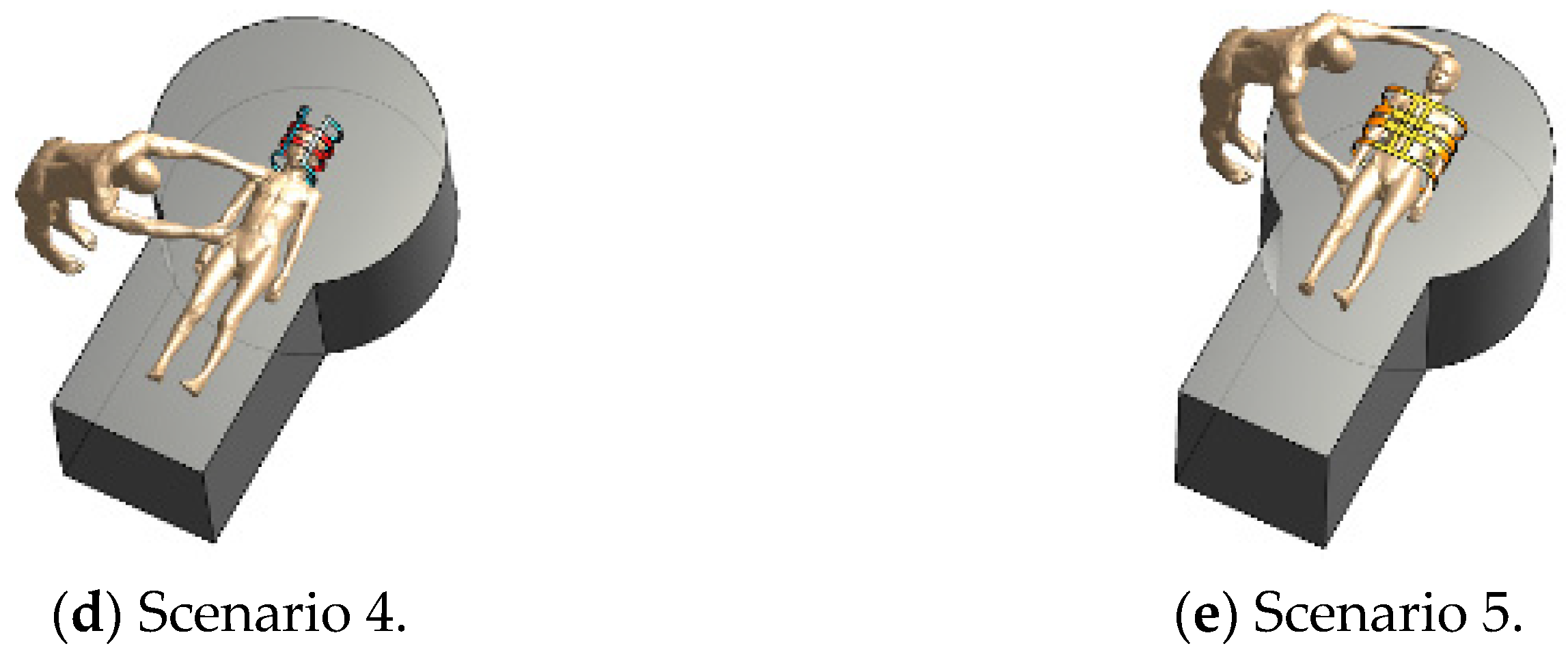

| Name | Age | Sex | Height (cm) | Mass (kg) | BMI (kg/m2) | Tissues (No.) | |
|---|---|---|---|---|---|---|---|
| Patient assistant | Duke | 34 y | M | 174 | 70 | 23.1 | 77 |
| Patient | Billie | 11 y | F | 146 | 36 | 16.7 | 75 |
| Patient | Thelonious | 6 y | M | 117 | 20 | 14.2 | 76 |
| Scenario | Patient (Billie) | Patient assistant (Duke) | ||
|---|---|---|---|---|
| 1 g | 10 g | 1 g | 10 g | |
| Sab | 1.6670 | 1.0411 | - | - |
| 1 | 1.6960 | 1.0596 | 0.00038 | 0.00019 |
| 2 | 1.6888 | 1.0551 | 0.00016 | 0.00008 |
| 3 | 1.6614 | 1.0380 | 0.00353 | 0.00162 |
| 4 | 1.6583 | 1.0359 | 0.19928 | 0.09240 |
| Scenario | Patient (Thelonious) | Patient Assistant (Duke) | ||
|---|---|---|---|---|
| 1 g | 10 g | 1 g | 10 g | |
| Sab | 1.6500 | 0.8279 | - | - |
| 1 | 1.6663 | 0.8361 | 0.00048 | 0.00024 |
| 2 | 1.6263 | 0.8477 | 0.00019 | 0.00009 |
| 3 | 1.5992 | 0.8336 | 0.00264 | 0.00141 |
| 4 | 1.5811 | 0.8242 | 0.26537 | 0.082449 |
| Scenario | Patient (Billie) | Patient Assistant (Duke) | ||
|---|---|---|---|---|
| 1 g | 10 g | 1 g | 10 g | |
| Sab | 0.8649 | 0.5684 | - | - |
| 1 | 0.8744 | 0.5746 | 0.00017 | 0.00007 |
| 2 | 0.8752 | 0.5752 | 0.00007 | 0.00003 |
| 3 | 0.8713 | 0.5726 | 0.03635 | 0.01294 |
| 5 | 0.8592 | 0.5665 | 0.227301 | 0.15065 |
| Scenario | Patient (Thelonious) | Patient Assistant (Duke) | ||
|---|---|---|---|---|
| 1 g | 10 g | 1 g | 10 g | |
| Sab | 1.2795 | 0.7457 | - | - |
| 1 | 1.1311 | 0.7638 | 0.00029 | 0.00016 |
| 2 | 1.2887 | 0.7564 | 0.00012 | 0.00006 |
| 3 | 1.1000 | 0.6771 | 0.06978 | 0.03815 |
| 5 | 1.0445 | 0.6828 | 0.94568 | 0.41197 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.-E.; Oh, S.; Choi, H.-D. RF Exposure Assessment for Various Poses of Patient Assistant in Open MRI Environment. Appl. Sci. 2021, 11, 4967. https://doi.org/10.3390/app11114967
Hong S-E, Oh S, Choi H-D. RF Exposure Assessment for Various Poses of Patient Assistant in Open MRI Environment. Applied Sciences. 2021; 11(11):4967. https://doi.org/10.3390/app11114967
Chicago/Turabian StyleHong, Seon-Eui, Sukhoon Oh, and Hyung-Do Choi. 2021. "RF Exposure Assessment for Various Poses of Patient Assistant in Open MRI Environment" Applied Sciences 11, no. 11: 4967. https://doi.org/10.3390/app11114967
APA StyleHong, S.-E., Oh, S., & Choi, H.-D. (2021). RF Exposure Assessment for Various Poses of Patient Assistant in Open MRI Environment. Applied Sciences, 11(11), 4967. https://doi.org/10.3390/app11114967







