Estimation of Dose Enhancement for Inhomogeneous Distribution of Nanoparticles: A Monte Carlo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Simulation Toolkit
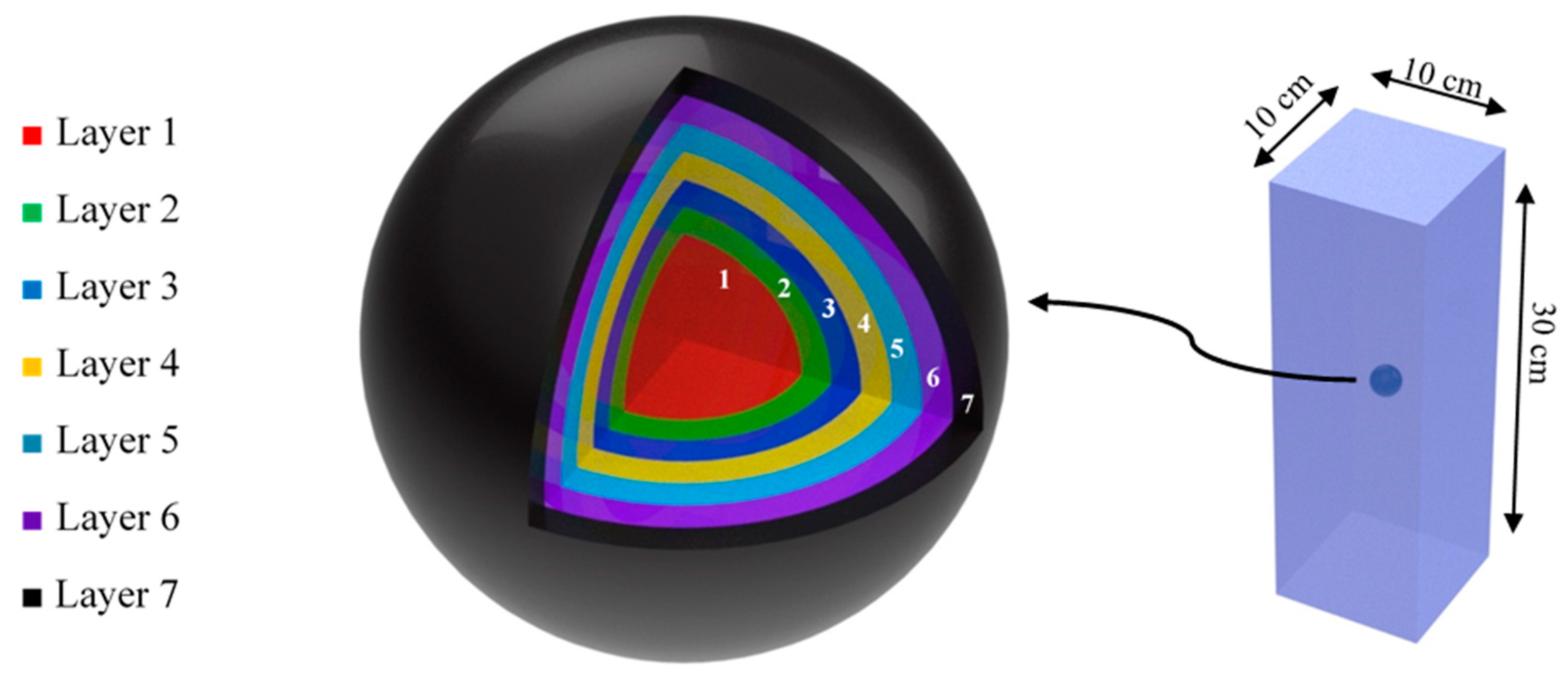
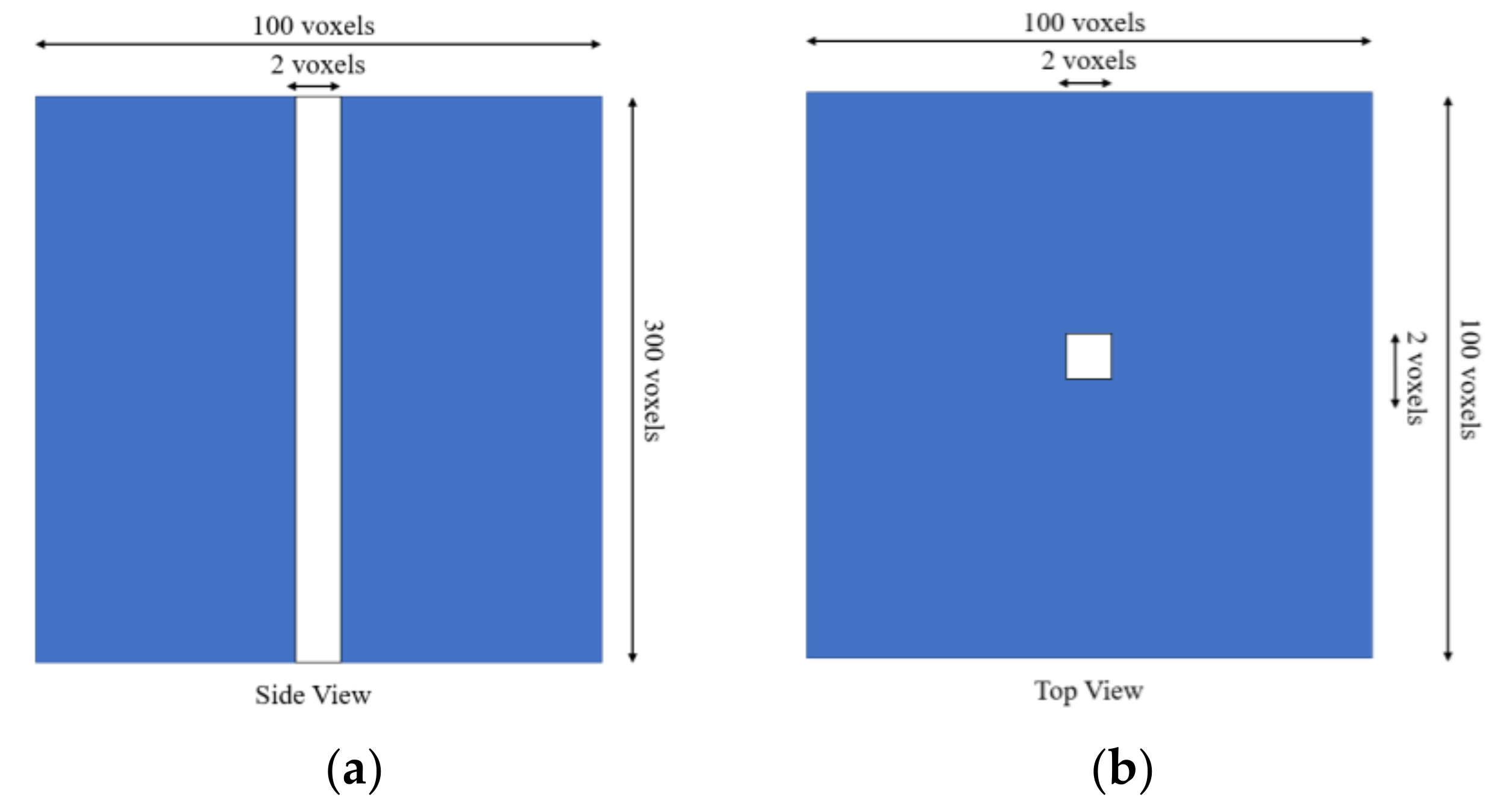
2.2. Phantom
2.3. Nanoparticles Modeling
2.4. Beam and Physics Modeling
2.5. Simulation Output
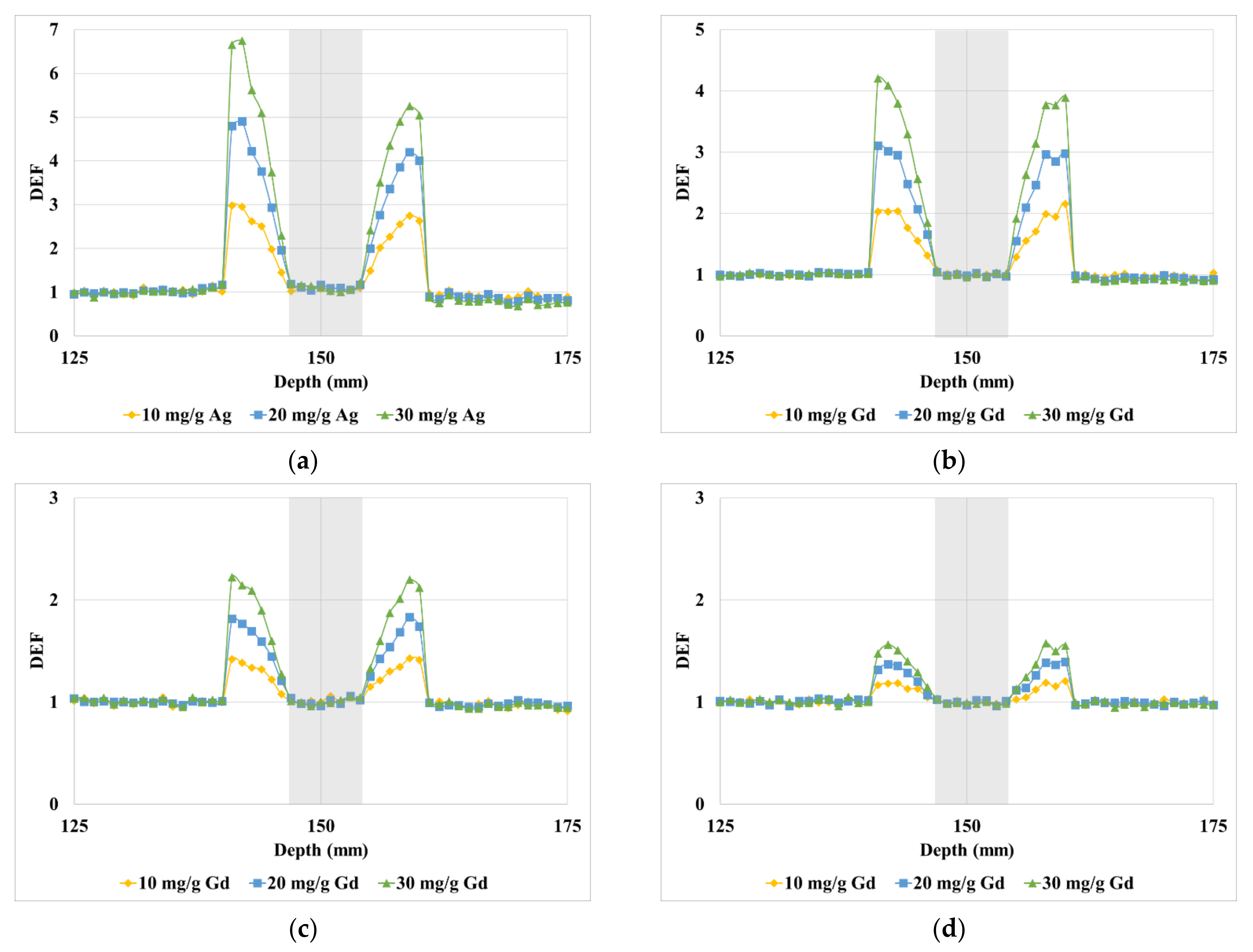
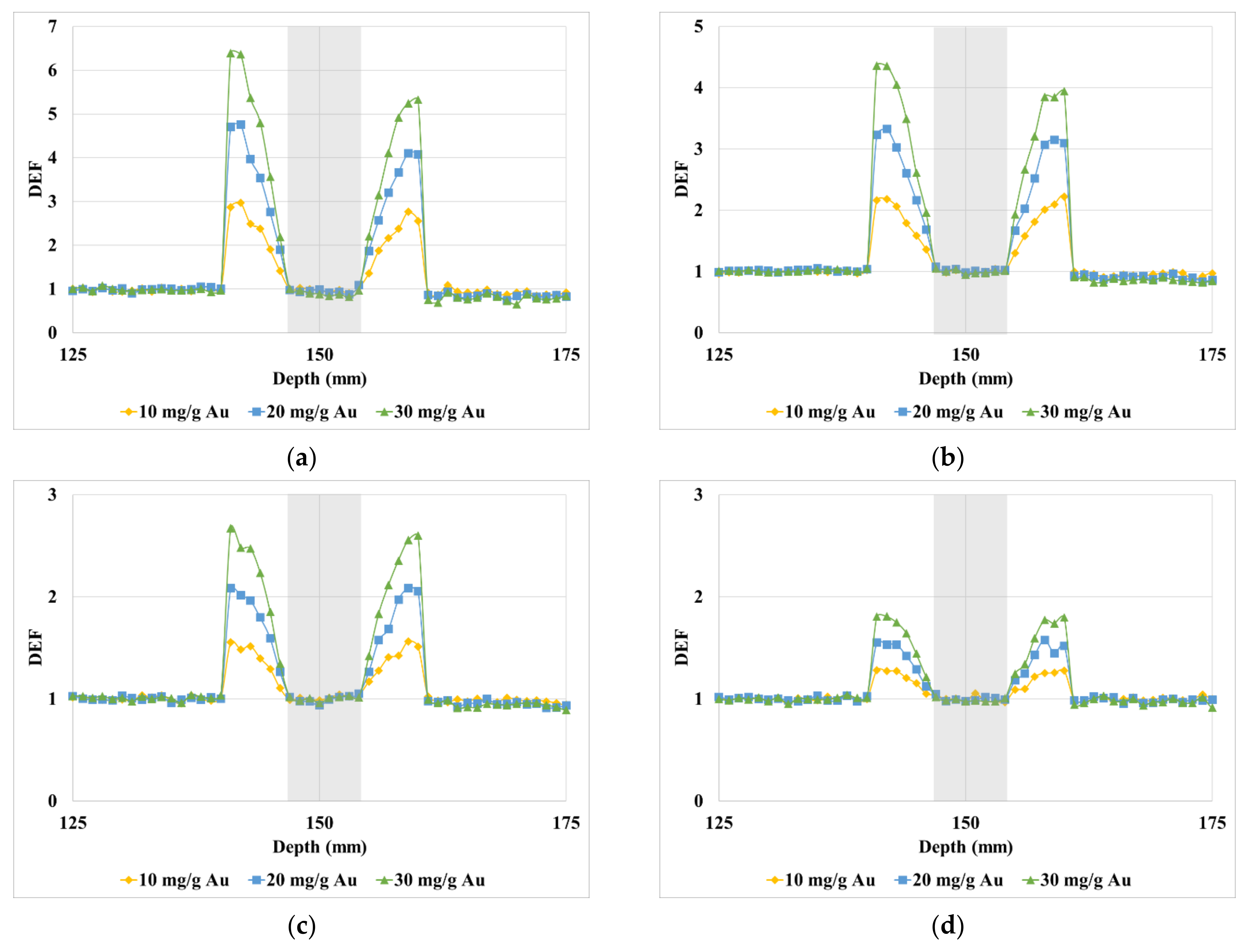
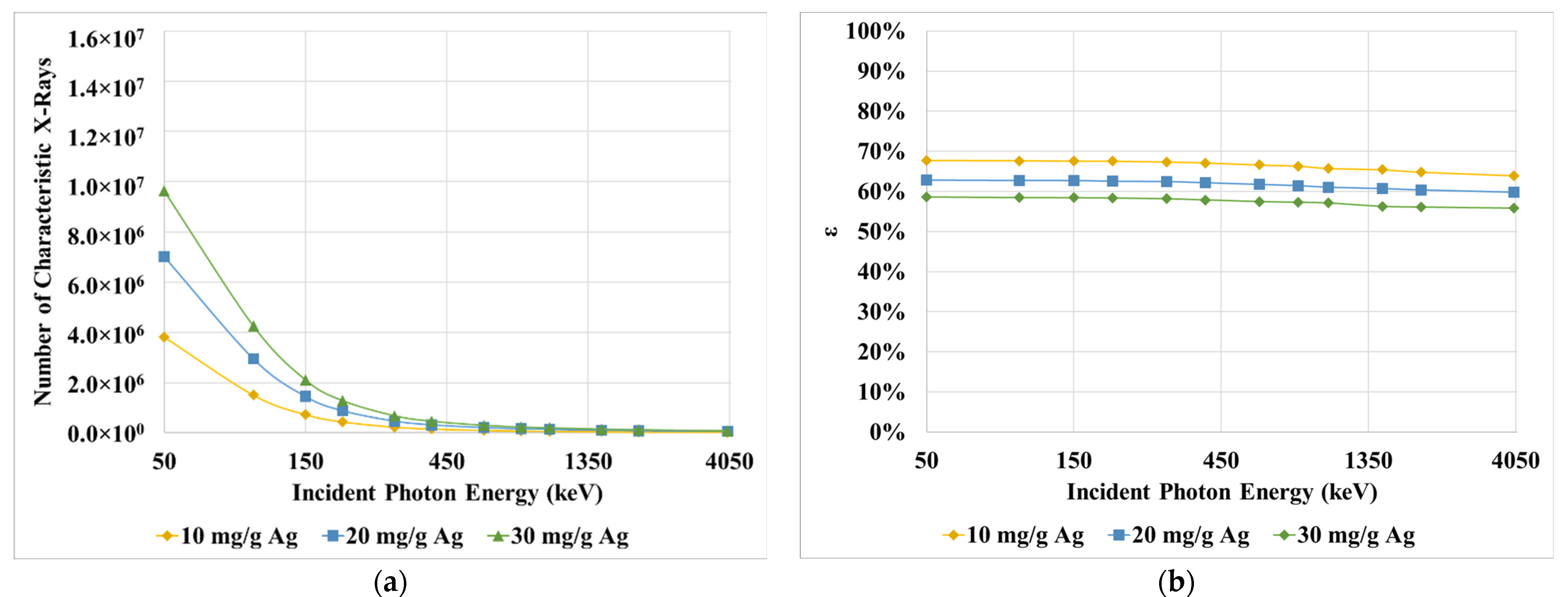
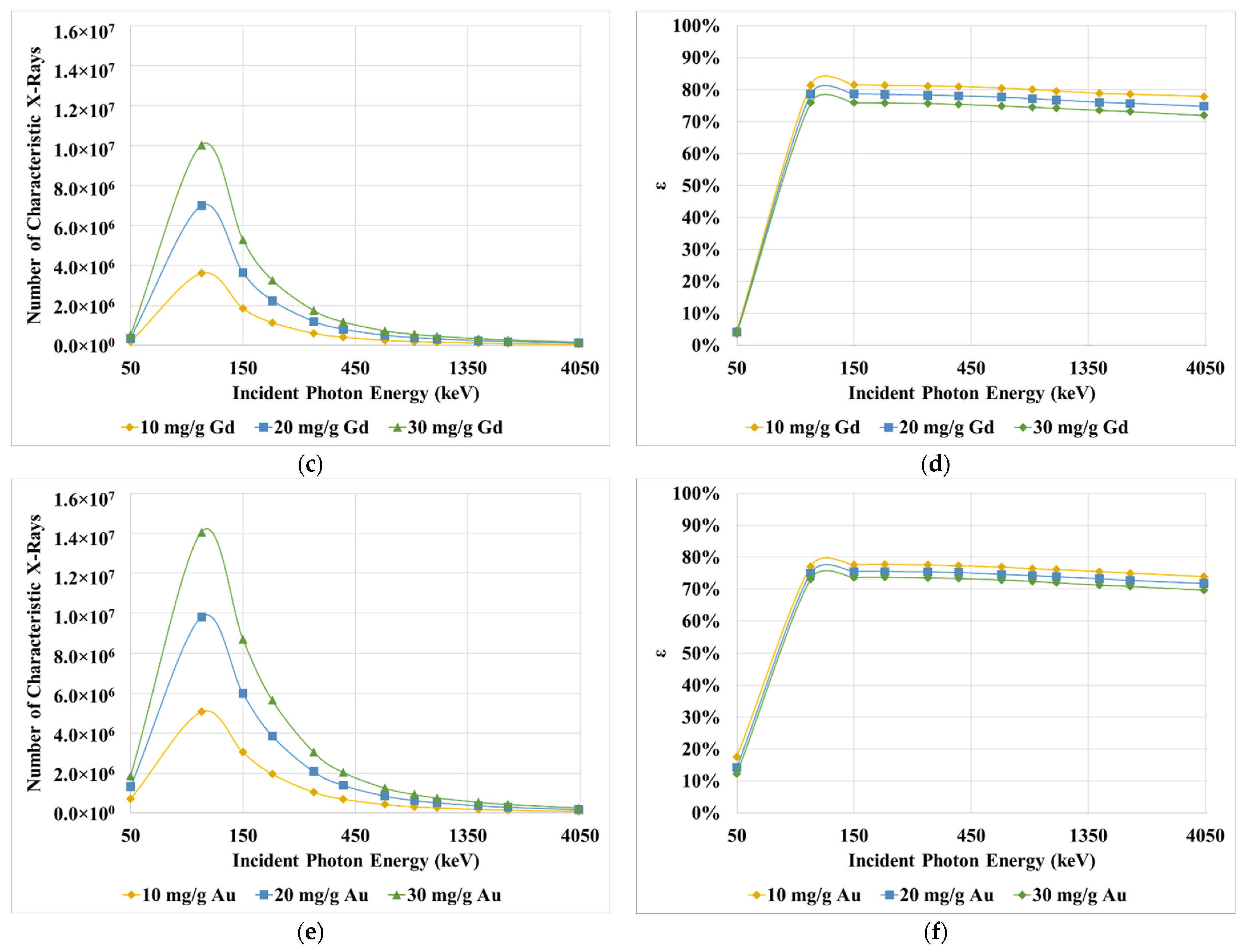
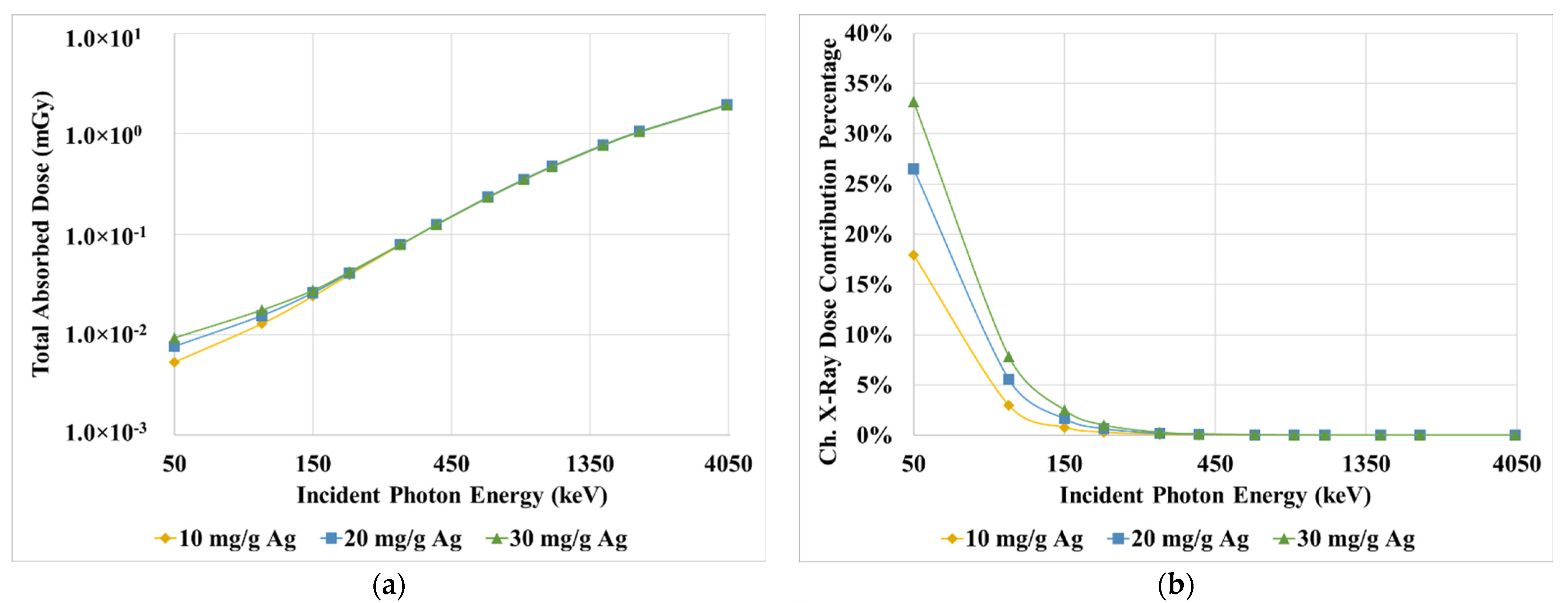
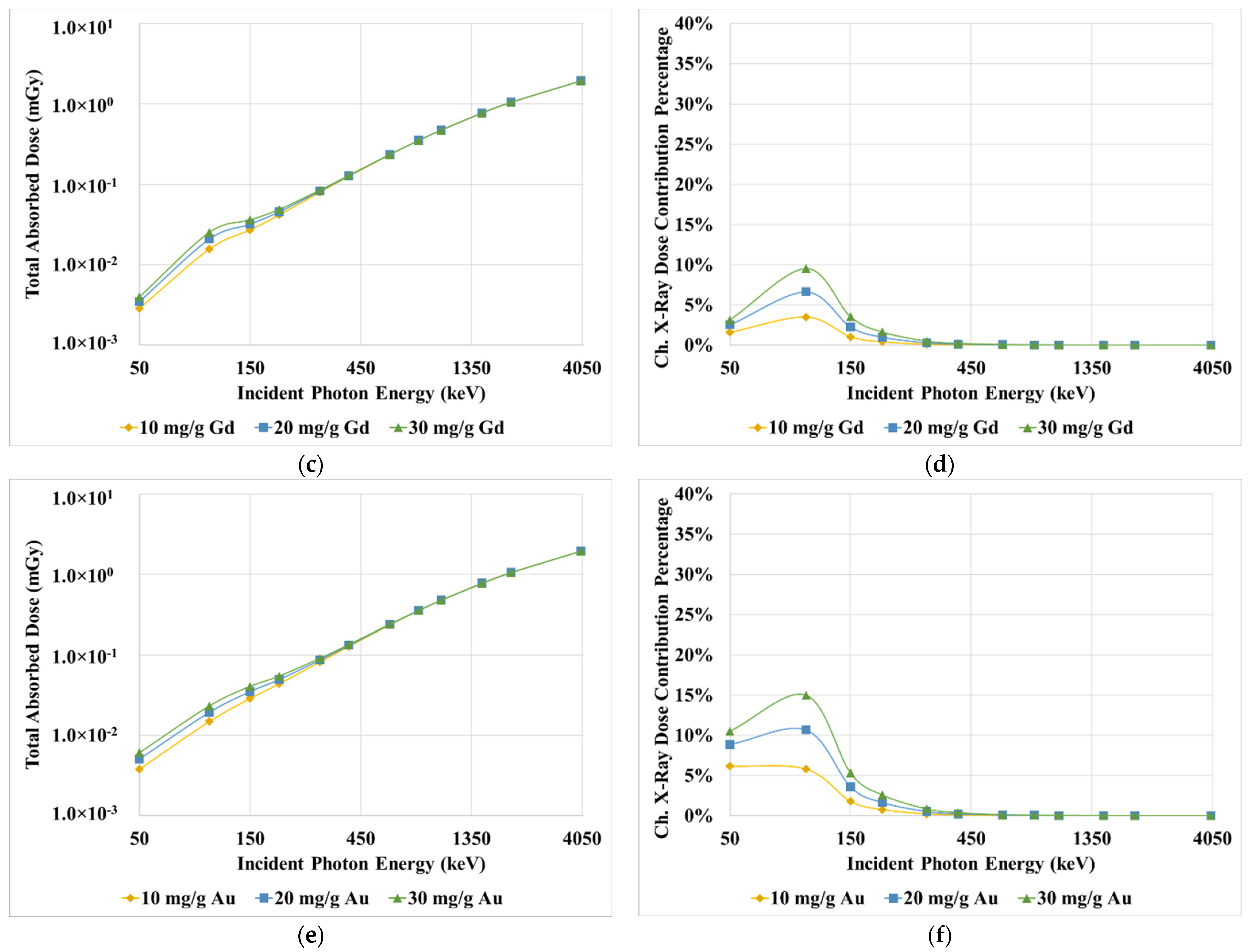
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsujimoto, Y. Apoptosis and necrosis: Intracellular ATP level as a determinant for cell death modes. Cell Death Differ. 1997, 4, 429–434. [Google Scholar] [CrossRef] [Green Version]
- Zong, W.-X.; Thompson, C.B. Necrotic death as a cell fate. Genes Dev. 2006, 20, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Kupelian, P.A.; Ramsey, C.; Meeks, S.L.; Willoughby, T.R.; Forbes, A.; Wagner, T.H.; Langen, K.M. Serial megavoltage CT imaging during external beam radiotherapy for non-small-cell lung cancer: Observations on tumor regression during treatment. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 1024–1028. [Google Scholar] [CrossRef]
- Shirato, H.; Le, Q.T.; Kobashi, K.; Prayongrat, A.; Takao, S.; Shimizu, S.; Giaccia, A.; Xing, L.; Umegaki, K. Selection of external beam radiotherapy approaches for precise and accurate cancer treatment. J. Radiat. Res. 2018, 59, i2–i10. [Google Scholar] [CrossRef] [Green Version]
- Moding, E.J.; Kastan, M.B.; Kirsch, D.G. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat. Rev. Drug Discov. 2013, 12, 526–542. [Google Scholar] [CrossRef] [Green Version]
- Veldeman, L.; Madani, I.; Hulstaert, F.; De Meerleer, G.; Mareel, M.; De Neve, W. Evidence behind use of intensity-modulated radiotherapy: A systematic review of comparative clinical studies. Lancet Oncol. 2008, 9, 367–375. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Dilmanian, F.A.; Slatkin, D.N.; Smilowitz, H.M. Radiotherapy enhancement with gold nanoparticles. J. Pharm. Pharmacol. 2008, 60, 977–985. [Google Scholar] [CrossRef] [Green Version]
- Banoqitah, E.; Djouider, F. Dose Distribution and Dose Enhancement by Using Gadolinium Nanoparticles Implant in Brain Tumor in Stereotactic Brachytherapy. Radiat. Phys. Chem. 2016, 127, 68–71. [Google Scholar] [CrossRef]
- Taha, E.; Djouider, F.; Banoqitah, E. Monte Carlo simulations for dose enhancement in cancer treatment using bismuth oxide nanoparticles implanted in brain soft tissue. Australas. Phys. Eng. Sci. Med. 2018, 41, 363–370. [Google Scholar] [CrossRef]
- Taha, E.; Djouider, F.; Banoqitah, E. Monte Carlo simulation of dose enhancement due to silver nanoparticles implantation in brain tumor brachytherapy using a digital phantom. Radiat. Phys. Chem. 2019, 156, 15–21. [Google Scholar] [CrossRef]
- Podgorsak, E.B. Radiation Physics for Medical Physicists; Biological and Medical Physics, Biomedical Engineering; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-642-00874-0. [Google Scholar]
- Su, X.Y.; Liu, P.D.; Wu, H.; Gu, N. Enhancement of radiosensitization by metal-based nanoparticles in cancer radiation therapy. Cancer Biol. Med. 2014, 11, 86–91. [Google Scholar]
- Butterworth, K.T.; Coulter, J.A.; Jain, S.; Forker, J.; McMahon, S.J.; Schettino, G.; Prise, K.M.; Currell, F.J.; Hirst, D.G. Evaluation of cytotoxicity and radiation enhancement using 1.9 nm gold particles: Potential application for cancer therapy. Nanotechnology 2010, 21, 295101. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Coulter, J.A.; Butterworth, K.T.; Hounsell, A.R.; McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Dickson, G.R.; Prise, K.M.; Currell, F.J.; et al. Gold nanoparticle cellular uptake, toxicity and radiosensitisation in hypoxic conditions. Radiother. Oncol. 2014, 110, 342–347. [Google Scholar] [CrossRef]
- Du, J.; Gu, Z.; Yan, L.; Yong, Y.; Yi, X.; Zhang, X.; Liu, J.; Wu, R.; Ge, C.; Chen, C.; et al. Poly(Vinylpyrollidone)- and Selenocysteine-Modified Bi2Se3 Nanoparticles Enhance Radiotherapy Efficacy in Tumors and Promote Radioprotection in Normal Tissues. Adv. Mater. 2017, 29, 1701268. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309–N315. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Smilowitz, H.M.; O’Connor, M.J.; Dilmanian, F.A.; Slatkin, D.N. Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomedicine 2013, 8, 1601–1609. [Google Scholar] [CrossRef] [Green Version]
- Hainfeld, J.F.; Ridwan, S.M.; Stanishevskiy, Y.; Panchal, R.; Slatkin, D.N.; Smilowitz, H.M. Iodine nanoparticles enhance radiotherapy of intracerebral human glioma in mice and increase efficacy of chemotherapy. Sci. Rep. 2019, 9, 4505. [Google Scholar] [CrossRef] [Green Version]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Ricketts, K.; Ahmad, R.; Beaton, L.; Cousins, B.; Critchley, K.; Davies, M.; Evans, S.; Fenuyi, I.; Gavriilidis, A.; Harmer, Q.J.; et al. Recommendations for clinical translation of nanoparticle-enhanced radiotherapy. Br. J. Radiol. 2018, 91, 20180325. [Google Scholar] [CrossRef]
- Bonvalot, S.; Rutkowski, P.L.; Thariat, J.; Carrère, S.; Ducassou, A.; Sunyach, M.-P.P.; Agoston, P.; Hong, A.; Mervoyer, A.; Rastrelli, M.; et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): A multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. 2019, 20, 1148–1159. [Google Scholar] [CrossRef]
- Retif, P.; Pinel, S.; Toussaint, M.; Frochot, C.; Chouikrat, R.; Bastogne, T.; Barberi-Heyob, M. Nanoparticles for Radiation Therapy Enhancement: The Key Parameters. Theranostics 2015, 5, 1030–1044. [Google Scholar] [CrossRef] [Green Version]
- Wakefield, G.; Gardener, M.; Stock, M.; Adair, M. Nanoparticle Augmented Radiotherapy Using Titanium Oxide Nanoparticles. J. Nanomater. Mol. Nanotechnol. 2018, 7, S6-002. [Google Scholar] [CrossRef]
- Çeşmeli, S.; Biray Avci, C. Application of titanium dioxide (TiO2) nanoparticles in cancer therapies. J. Drug Target. 2019, 27, 762–766. [Google Scholar] [CrossRef]
- Rees, P.; Wills, J.W.; Brown, M.R.; Barnes, C.M.; Summers, H.D. The origin of heterogeneous nanoparticle uptake by cells. Nat. Commun. 2019, 10, 2341. [Google Scholar] [CrossRef]
- Koger, B.; Kirkby, C. Optimization of photon beam energies in gold nanoparticle enhanced arc radiation therapy using Monte Carlo methods. Phys. Med. Biol. 2016, 61, 8839–8853. [Google Scholar] [CrossRef]
- Hossain, M.; Su, M. Nanoparticle Location and Material-Dependent Dose Enhancement in X-ray Radiation Therapy. J. Phys. Chem. C 2012, 116, 23047–23052. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.X.; Gao, J.; Buchholz, T.A.; Wang, Z.; Salehpour, M.R.; Drezek, R.A.; Yu, T.-K. Quantifying tumor-selective radiation dose enhancements using gold nanoparticles: A monte carlo simulation study. Biomed. Microdevices 2009, 11, 925–933. [Google Scholar] [CrossRef]
- Cho, S.H.; Jones, B.L.; Krishnan, S. The dosimetric feasibility of gold nanoparticle-aided radiation therapy (GNRT) via brachytherapy using low-energy gamma-/X-ray sources. Phys. Med. Biol. 2009, 54, 4889–4905. [Google Scholar] [CrossRef]
- Cho, S.H. Estimation of tumour dose enhancement due to gold nanoparticles during typical radiation treatments: A preliminary Monte Carlo study. Phys. Med. Biol. 2005, 50, N163–N173. [Google Scholar] [CrossRef]
- Zhan, W.; Gedroyc, W.; Xu, X.Y. Effect of heterogeneous microvasculature distribution on drug delivery to solid tumour. J. Phys. D Appl. Phys. 2014, 47, 475401. [Google Scholar] [CrossRef] [Green Version]
- England, C.G.; Gobin, A.M.; Frieboes, H.B. Evaluation of uptake and distribution of gold nanoparticles in solid tumors. Eur. Phys. J. Plus 2015, 130, 231. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Hoang, B.; Fonge, H.; Reilly, R.M.; Allen, C. In vivo distribution of polymeric nanoparticles at the whole-body, tumor, and cellular levels. Pharm. Res. 2010, 27, 2343–2355. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, S.; Wu, J.; Long, Q. Coupled modelling of tumour angiogenesis, tumour growth and blood perfusion. J. Theor. Biol. 2011, 279, 90–101. [Google Scholar] [CrossRef]
- Grevillot, L.; Boersma, D.J.; Fuchs, H.; Aitkenhead, A.; Elia, A.; Bolsa, M.; Winterhalter, C.; Vidal, M.; Jan, S.; Pietrzyk, U.; et al. Technical Note: GATE-RTion: A GATE/Geant4 release for clinical applications in scanned ion beam therapy. Med. Phys. 2020, 47, 3675–3681. [Google Scholar] [CrossRef]
- Jan, S.; Santin, G.; Strul, D.; Staelens, S.; Assié, K.; Autret, D.; Avner, S.; Barbier, R.; Bardiès, M.; Bloomfield, P.M.; et al. GATE: A simulation toolkit for PET and SPECT. Phys. Med. Biol. 2004, 49, 4543–4561. [Google Scholar] [CrossRef]
- Sarrut, D.; Bardiès, M.; Boussion, N.; Freud, N.; Jan, S.; Létang, J.-M.; Loudos, G.; Maigne, L.; Marcatili, S.; Mauxion, T.; et al. A review of the use and potential of the GATE Monte Carlo simulation code for radiation therapy and dosimetry applications. Med. Phys. 2014, 41, 064301. [Google Scholar] [CrossRef] [Green Version]
- Papadimitroulas, P.; Loudos, G.; Nikiforidis, G.C.; Kagadis, G.C. A dose point kernel database using GATE Monte Carlo simulation toolkit for nuclear medicine applications: Comparison with other Monte Carlo codes. Med. Phys. 2012, 39, 5238–5247. [Google Scholar] [CrossRef]
- Grevillot, L.; Bertrand, D.; Dessy, F.; Freud, N.; Sarrut, D. A Monte Carlo pencil beam scanning model for proton treatment plan simulation using GATE/GEANT4. Phys. Med. Biol. 2011, 56, 5203–5219. [Google Scholar] [CrossRef] [Green Version]
- Jan, S.; Benoit, D.; Becheva, E.; Carlier, T.; Cassol, F.; Descourt, P.; Frisson, T.; Grevillot, L.; Guigues, L.; Maigne, L.; et al. GATE V6: A major enhancement of the GATE simulation platform enabling modelling of CT and radiotherapy. Phys. Med. Biol. 2011, 56, 881–901. [Google Scholar] [CrossRef]
- Bouchat, V.; Nuttens, V.E.; Lucas, S.; Michiels, C.; Masereel, B.; Féron, O.; Gallez, B.; Borght, T. Vander Radioimmunotherapy with radioactive nanoparticles: First results of dosimetry for vascularized and necrosed solid tumors. Med. Phys. 2007, 34, 4504–4513. [Google Scholar] [CrossRef]
- Zabihzadeh, M.; Moshirian, T.; Ghorbani, M.; Knaup, C.; Behrooz, M.A. A Monte Carlo Study on Dose Enhancement by Homogeneous and Inhomogeneous Distributions of Gold Nanoparticles in Radiotherapy with Low Energy X-rays. J. Biomed. Phys. Eng. 2018, 8, 13–28. [Google Scholar]
- Brocato, T.A.; Coker, E.N.; Durfee, P.N.; Lin, Y.-S.; Townson, J.; Wyckoff, E.F.; Cristini, V.; Brinker, C.J.; Wang, Z. Understanding the Connection between Nanoparticle Uptake and Cancer Treatment Efficacy using Mathematical Modeling. Sci. Rep. 2018, 8, 7538. [Google Scholar] [CrossRef] [Green Version]
- Humm, J.L.; Cobb, L.M. Nonuniformity of tumor dose in radioimmunotherapy. J. Nucl. Med. 1990, 31, 75–83. [Google Scholar]
- Chou, C.-Y.; Chang, W.-I.; Horng, T.-L.; Lin, W.-L. Numerical modeling of nanodrug distribution in tumors with heterogeneous vasculature. PLoS ONE 2017, 12, e0189802. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release 2011, 153, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Dellian, M.; Fukumura, D.; Leunig, M.; Berk, D.A.; Torchilin, V.P.; Jain, R.K. Vascular permeability in a human tumor xenograft: Molecular size dependence and cutoff size. Cancer Res. 1995, 55, 3752–3756. [Google Scholar]
- Yildirimer, L.; Thanh, N.T.K.; Loizidou, M.; Seifalian, A.M. Toxicology and clinical potential of nanoparticles. Nano Today 2011, 6, 585–607. [Google Scholar] [CrossRef] [Green Version]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of Nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, J. Clearance Pathways and Tumor Targeting of Imaging Nanoparticles. ACS Nano 2015, 9, 6655–6674. [Google Scholar] [CrossRef] [Green Version]
- Kruszewski, M.; Grądzka, I.; Bartłomiejczyk, T.; Chwastowska, J.; Sommer, S.; Grzelak, A.; Zuberek, M.; Lankoff, A.; Dusinska, M.; Wojewódzka, M. Oxidative DNA damage corresponds to the long term survival of human cells treated with silver nanoparticles. Toxicol. Lett. 2013, 219, 151–159. [Google Scholar] [CrossRef]
- Ailawadi, G.; Stanley, J.C.; Williams, D.M.; Dimick, J.B.; Henke, P.K.; Upchurch, G.R. Gadolinium as a nonnephrotoxic contrast agent for catheter-based arteriographic evaluation of renal arteries in patients with azotemia. J. Vasc. Surg. 2003, 37, 346–352. [Google Scholar] [CrossRef] [Green Version]
- Beaudoux, V.; Blin, G.; Barbrel, B.; Kantor, G.; Zacharatou, C. Geant4 physics list comparison for the simulation of phase-contrast mammography (XPulse project). Phys. Medica 2019, 60, 66–75. [Google Scholar] [CrossRef]
- Apostolakis, J.; Bagulya, A.; Elles, S.; Ivanchenko, V.N.; Jacquemier, J.; Maire, M.; Toshito, T.; Urban, L. Validation and verification of Geant4 standard electromagnetic physics. J. Phys. Conf. Ser. 2010, 219, 032044. [Google Scholar] [CrossRef]
- Berger, M.J.; Hubbell, J.H.; Seltzer, S.M.; Chang, J.; Coursey, J.S.; Sukumar, R.; Zucker, D.S.; Olsen, K. XCOM: Photon Cross Section Database; Version 1.5; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2010. Available online: http://physics.nist.gov/xcom (accessed on 21 February 2021).
- Jiao, D.; Cai, Z.; Choksi, S.; Ma, D.; Choe, M.; Kwon, H.-J.; Baik, J.Y.; Rowan, B.G.; Liu, C.; Liu, Z. Necroptosis of tumor cells leads to tumor necrosis and promotes tumor metastasis. Cell Res. 2018, 28, 868–870. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Jiao, D. Necroptosis, tumor necrosis and tumorigenesis. Cell Stress 2020, 4, 1–8. [Google Scholar] [CrossRef]
- Cho, S.H. TH-C-T-6C-04: Estimation of Tumor Dose Enhancement Due to Gold Nanoparticles during Typical Radiation Treatments: A Preliminary Monte Carlo Study. Med. Phys. 2005, 32, 2162. [Google Scholar] [CrossRef]
- Berbeco, R.I.; Ngwa, W.; Makrigiorgos, G.M. Localized Dose Enhanceent to Tumor Blood Vessel Endothelial Cells via Megavoltage X-rays and Targeted Gold Nanoparticles: New Potential for External Beam Radiotherapy. Int. J. Radiat. Oncol. 2011, 81, 270–276. [Google Scholar] [CrossRef]
- Sheikh-Bagheri, D.; Rogers, D.W.O. Monte Carlo calculation of nine megavoltage photon beam spectra using the BEAM code. Med. Phys. 2002, 29, 391–402. [Google Scholar] [CrossRef]


| Material | Concentration (mg/g) | Energy (keV) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 150 | 200 | 300 | 400 | 600 | 800 | 1000 | 1500 | 2000 | 4000 | ||
| Ag | 10 | 2.98 | 1.66 | 1.27 | 1.12 | 1.06 | 1.12 | 1.05 | 1.04 | 1.03 | 1.04 | 1.02 | 1.02 |
| 20 | 4.90 | 2.30 | 1.46 | 1.20 | 1.11 | 1.07 | 1.05 | 1.05 | 1.03 | 1.05 | 1.03 | 1.02 | |
| 30 | 6.74 | 2.90 | 1.65 | 1.31 | 1.11 | 1.05 | 1.05 | 1.04 | 1.04 | 1.04 | 1.03 | 1.03 | |
| Gd | 10 | 2.05 | 2.16 | 1.43 | 1.21 | 1.10 | 1.09 | 1.05 | 1.04 | 1.03 | 1.04 | 1.02 | 1.02 |
| 20 | 3.03 | 3.12 | 1.83 | 1.39 | 1.17 | 1.08 | 1.07 | 1.05 | 1.04 | 1.04 | 1.03 | 1.03 | |
| 30 | 3.95 | 4.20 | 2.22 | 1.58 | 1.19 | 1.11 | 1.06 | 1.04 | 1.04 | 1.05 | 1.04 | 1.04 | |
| Au | 10 | 2.97 | 2.22 | 1.56 | 1.29 | 1.15 | 1.09 | 1.05 | 1.05 | 1.03 | 1.04 | 1.02 | 1.02 |
| 20 | 4.76 | 3.32 | 2.08 | 1.58 | 1.26 | 1.14 | 1.08 | 1.07 | 1.04 | 1.07 | 1.03 | 1.03 | |
| 30 | 6.40 | 4.36 | 2.67 | 1.81 | 1.33 | 1.19 | 1.08 | 1.05 | 1.04 | 1.07 | 1.04 | 1.05 | |
| Energy (keV) | Material | Mass Absorption Coefficient (cm2/g) | Mass Attenuation Coefficient (cm2/g) |
|---|---|---|---|
| 50 | Ag | 6.06 | 9.44 |
| Gd | 3.24 | 3.85 | |
| Au | 6.12 | 7.25 | |
| 100 | Ag | 1.06 | 1.47 |
| Gd | 1.84 | 3.10 | |
| Au | 2.07 | 5.15 | |
| 150 | Ag | 0.36 | 0.54 |
| Gd | 0.72 | 1.10 | |
| Au | 1.02 | 1.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abolaban, F.; Taha, E.; Alhawsawi, A.; Djouider, F.; Banoqitah, E.; Nisbet, A. Estimation of Dose Enhancement for Inhomogeneous Distribution of Nanoparticles: A Monte Carlo Study. Appl. Sci. 2021, 11, 4900. https://doi.org/10.3390/app11114900
Abolaban F, Taha E, Alhawsawi A, Djouider F, Banoqitah E, Nisbet A. Estimation of Dose Enhancement for Inhomogeneous Distribution of Nanoparticles: A Monte Carlo Study. Applied Sciences. 2021; 11(11):4900. https://doi.org/10.3390/app11114900
Chicago/Turabian StyleAbolaban, Fouad, Eslam Taha, Abdulsalam Alhawsawi, Fathi Djouider, Essam Banoqitah, and Andrew Nisbet. 2021. "Estimation of Dose Enhancement for Inhomogeneous Distribution of Nanoparticles: A Monte Carlo Study" Applied Sciences 11, no. 11: 4900. https://doi.org/10.3390/app11114900
APA StyleAbolaban, F., Taha, E., Alhawsawi, A., Djouider, F., Banoqitah, E., & Nisbet, A. (2021). Estimation of Dose Enhancement for Inhomogeneous Distribution of Nanoparticles: A Monte Carlo Study. Applied Sciences, 11(11), 4900. https://doi.org/10.3390/app11114900






