Solid-State Lithium Battery Cycle Life Prediction Using Machine Learning
Abstract
1. Introduction
2. Materials and Methods
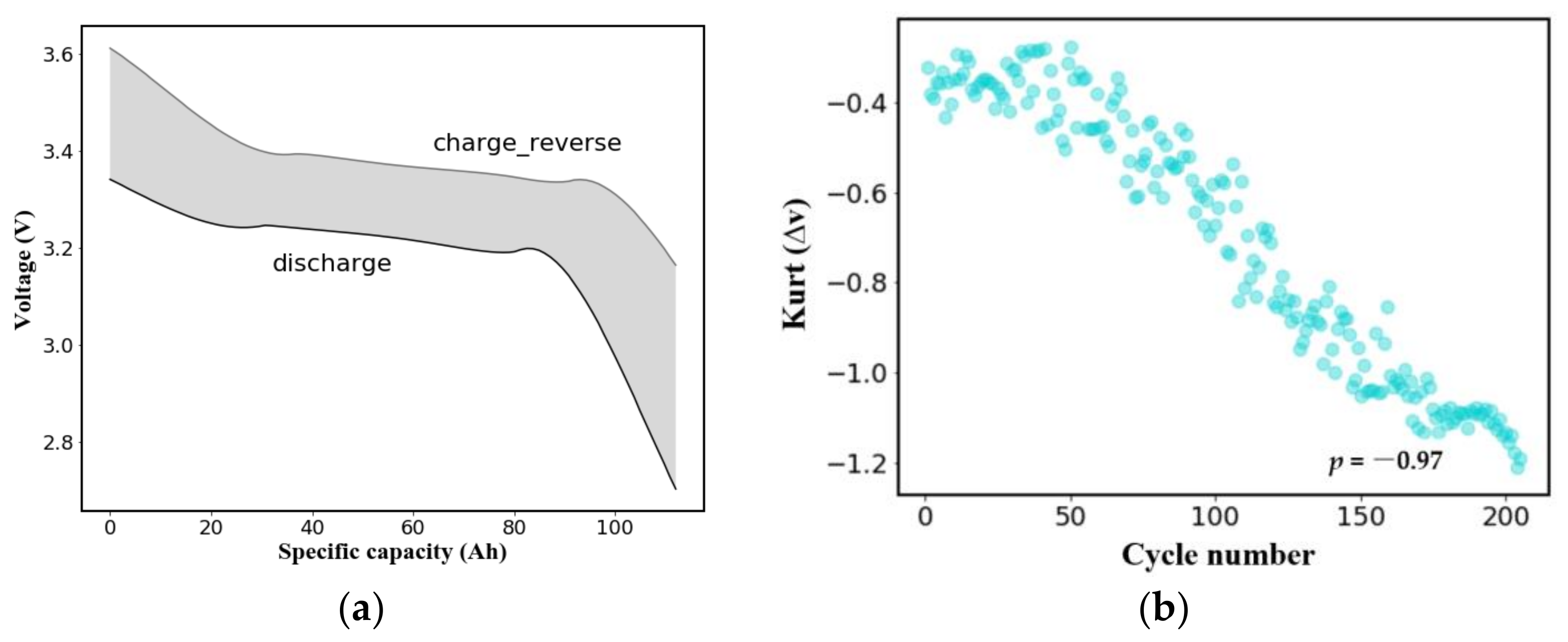
2.1. Battery Cycle Data Generation: Dataset Description
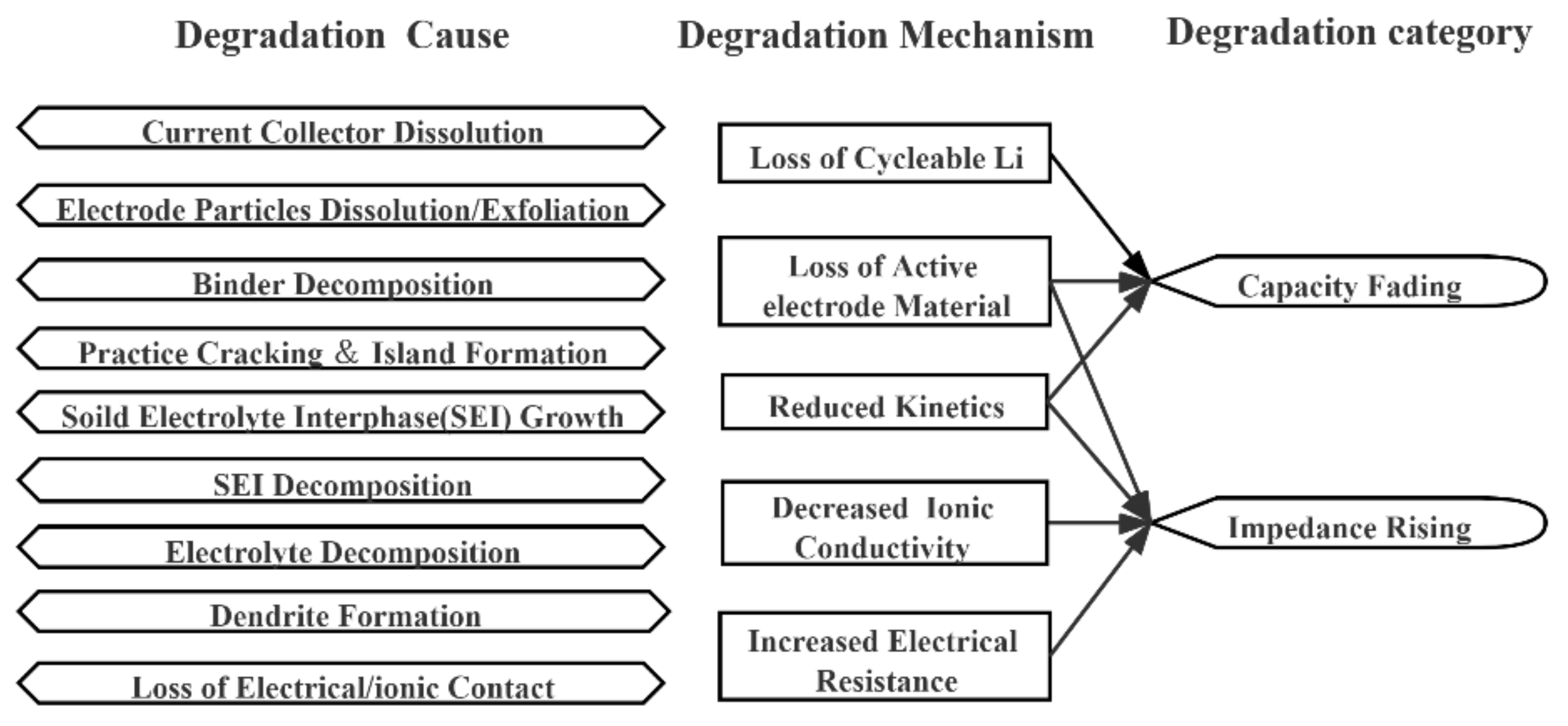
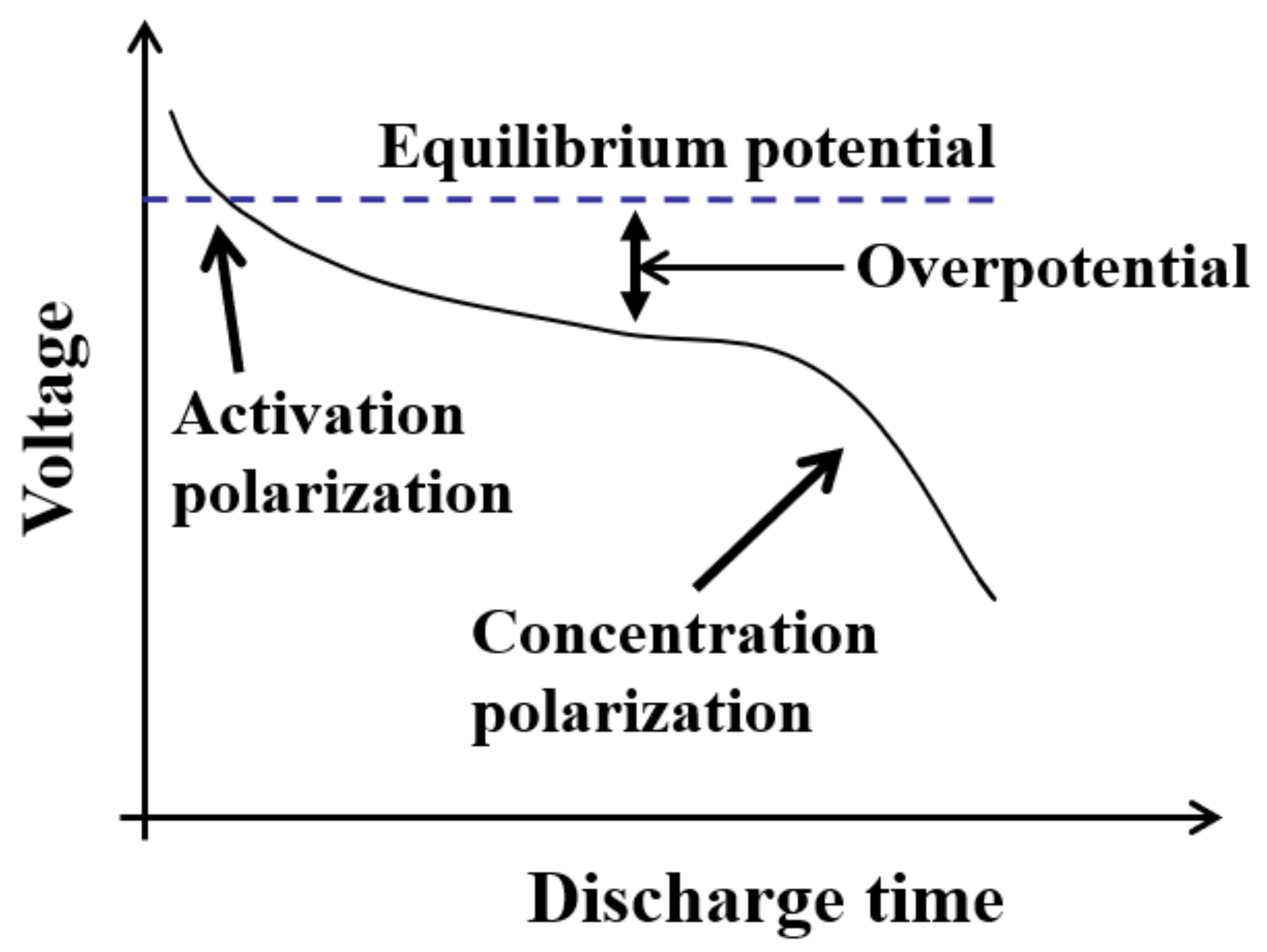
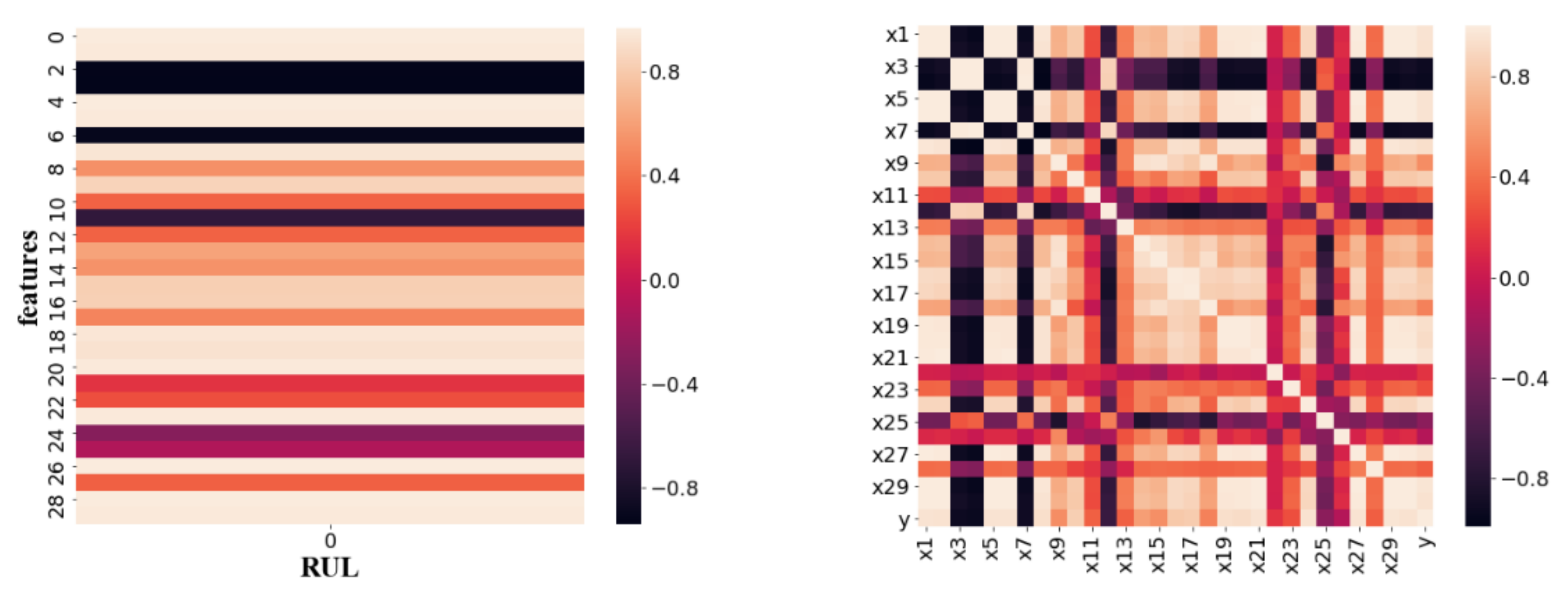
2.2. Feature Selection
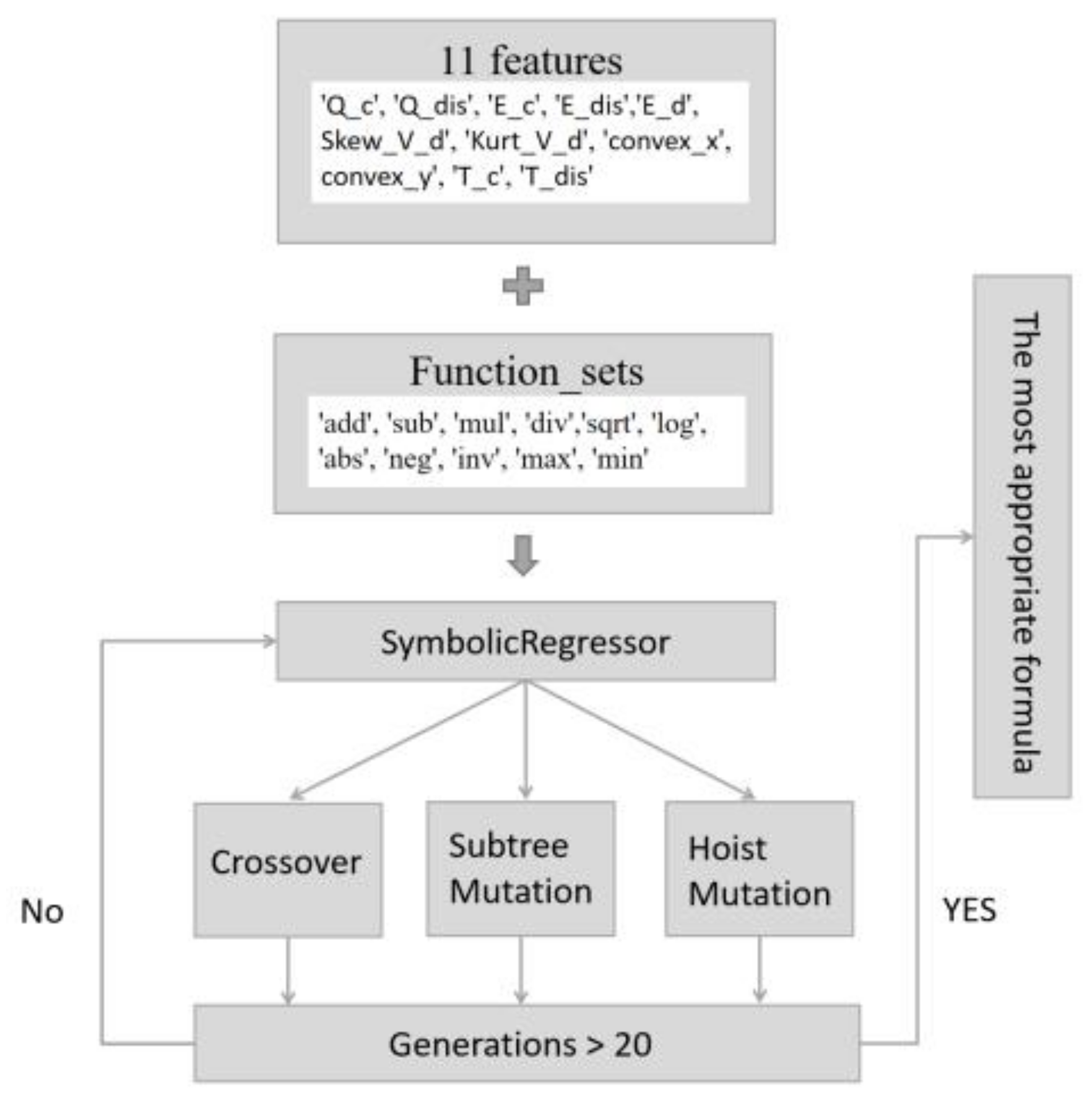
2.3. Algorithm Selection
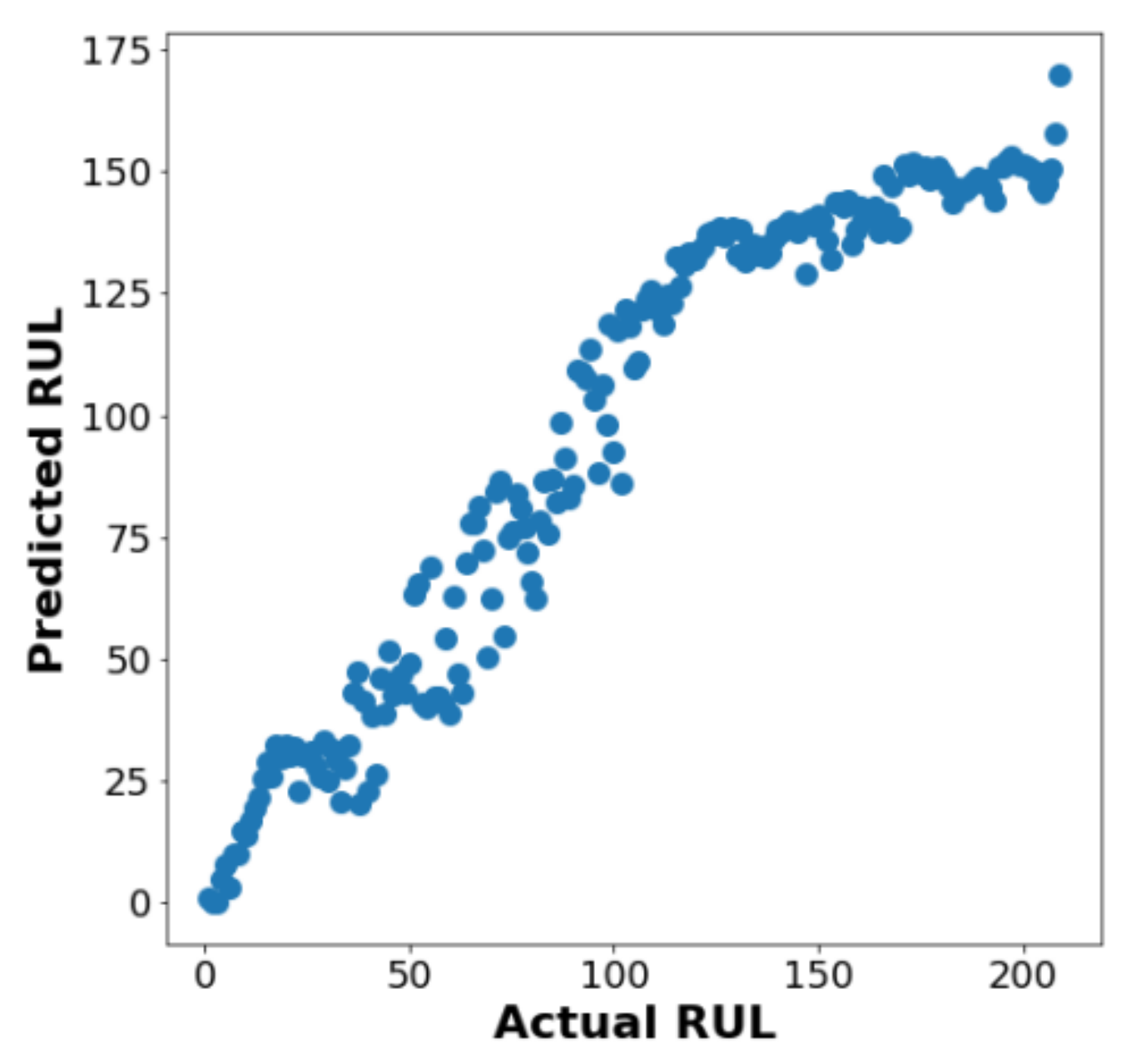
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Nykvist, B.; Nilsson, M. Rapidly falling costs of battery packs for electric vehicles. Nat. Clim. Chang. 2015, 5, 329–332. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Severson, K.A.; Attia, P.M.; Jin, N.; Perkins, N.; Jiang, B.; Yang, Z.; Chen, M.H.; Aykol, M.; Herring, P.K.; Fraggedakis, D.; et al. Data-driven prediction of battery cycle life before capacity degradation. Nat. Energy 2019, 4, 383–391. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Xu, L.; Tang, S.; Cheng, Y.; Wang, K.; Liang, J.; Liu, C.; Cao, Y.-C.; Wei, F.; Mai, L. Interfaces in Solid-State Lithium Batteries. Joule 2018, 2, 1991–2015. [Google Scholar] [CrossRef]
- Tang, X.; Zou, C.; Wik, T.; Yao, K.; Gao, F. Run-to-Run control for active balancing of lithium iron phosphate battery packs. IEEE Trans. Power Electron 2020, 2, 1499–1512. [Google Scholar] [CrossRef]
- Schuster, S.F.; Bach, T.; Fleder, E.; Müller, J.; Brand, M.; Sextl, G.; Jossen, A.J. New charging method to avoid nonlinear aging of Lithium-Ion Batteries. Energy Storage 2015, 1, 44–53. [Google Scholar] [CrossRef]
- Schuster, S.F.; Brand, M.J.; Berg, P.; Gleissenberger, M.; Jossen, A.J. Lithium-ion cell-to-cell variation during battery electric vehicle operation. Power Sources 2015, 297, 242–251. [Google Scholar] [CrossRef]
- Harris, S.J.; Harris, D.J.; Li, C.J. Failure statistics for commercial lithium ion batteries: A study of 24 pouch cells. Power Sources 2017, 342, 589–597. [Google Scholar] [CrossRef]
- Butler, K.T.; Davies, D.W.; Cartwright, H.; Isayev, O.; Walsh, A. Machine learning for molecular and materials science. Nature 2018, 559, 547–555. [Google Scholar] [CrossRef]
- Granda, J.M.; Donina, L.; Dragone, V.; Long, D.L.; Cronin, L. Controlling an organic synthesis robot with machine learning to search for new reactivity. Nature 2018, 559, 377–381. [Google Scholar] [CrossRef]
- Raccuglia, P.; Elbert, K.C.; Adler, P.D.; Falk, C.; Wenny, M.B.; Mollo, A.; Zeller, M.; Friedler, S.A.; Schrier, J.; Norquist, A.J. Machine-learning-assisted materials discovery using failed experiments. Nature 2016, 533, 73–76. [Google Scholar] [CrossRef]
- Segler, M.H.S.; Preuss, M.; Waller, M.P. Planning chemical syntheses with deep neural networks and symbolic AI. Nature 2018, 555, 604–610. [Google Scholar] [CrossRef]
- Sha, W.; Guo, Y.; Yuan, Q.; Tang, S.; Zhang, X.; Lu, S.; Guo, X.; Cao, Y.; Cheng, S. Artificial Intelligence to Power the Future of Materials Science and Engineering. Adv. Intell. Syst. 2020, 2, 2070320. [Google Scholar] [CrossRef]
- Sidorov, D.; Liu, F.; Sun, Y. Machine learning for energy systems. Energies 2020, 13, 4708. [Google Scholar] [CrossRef]
- Phattara, K.; Yodo, N. A data-driven predictive prognostic model for lithium-ion batteries based on a deep learning algorithm. Energies 2019, 12, 660. [Google Scholar]
- Chandran, V.; Patil, C.; Karthick, A.; Ganeshaperumal, D.; Rahim, R.; Ghosh, A. State of charge estimation of lithium-ion battery for electric vehicles using machine learning algorithms. World Electr. Veh. J. 2021, 12, 38. [Google Scholar] [CrossRef]
- Sheikh, S.; Anjum, M.; Khan, M.; Hassan, S.; Khalid, H.; Gastli, A.; Lazhar, B. A battery health monitoring method using machine learning: A data-driven approach. Energies 2020, 13, 3658. [Google Scholar] [CrossRef]
- Attia, P.M.; Grover, A.; Jin, N.; Severson, K.A.; Markov, T.M.; Liao, Y.H.; Chen, M.H.; Cheong, B.; Perkins, N.; Yang, Z.; et al. Closed-loop optimization of fast-charging protocols for batteries with machine learning. Nature 2020, 578, 397–402. [Google Scholar] [CrossRef]
- Dai, H.; Zhao, G.; Lin, M.; Wu, J.; Zheng, G. A novel estimation method for the state of health of lithium-ion battery using prior knowledge-based neural network and Markov chain. IEEE Trans. Ind. Electron 2019, 66, 7706–7716. [Google Scholar] [CrossRef]
- Liu, D.; Song, Y.; Li, L.; Liao, H.; Peng, Y. On-line life cycle health assessment for lithium-ion battery in electric vehicles. J. Clean. Prod. 2018, 199, 1050–1065. [Google Scholar] [CrossRef]
- Zhou, D.; Li, Z.; Zhu, J.; Zhang, H.; Hou, L. State of health monitoring and remaining useful life prediction of lithium-ion batteries based on temporal convolutional network. IEEE Access 2020, 8, 53307–53320. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Q.; Zhang, Y.; Wang, J.; Stimming, U.; Lee, A.A. Identifying degradation patterns of lithium-ion batteries from impedance spectroscopy using machine learning. Nat. Commun. 2020, 11, 1706. [Google Scholar] [CrossRef]
- Ramakumar, C.; Deviannapoorani, L.; Dhivya, L.; Shankar, S.; Murugan, R. Lithium garnets: Synthesis, structure, Li+ conductivity, Li+ dynamics and applications. Prog. Mater. Sci. 2017, 88, 325–411. [Google Scholar] [CrossRef]
- Christensen, J.; Newman, J. Erratum: A mathematical model for the lithium-ion negative electrode solid electrolyte interphase. J. Electrochem. Soc. 2004, 151, A1977. [Google Scholar] [CrossRef]
- Pinson, M.B.; Bazant, M.Z. Theory of sei formation in rechargeable batteries: Capacity fade, accelerated aging and lifetime prediction. J. Electrochem. Soc. 2012, 160, A243–A250. [Google Scholar] [CrossRef]
- Yang, X.-G.; Leng, Y.; Zhang, G.; Ge, S.; Wang, C.-Y. Computational design and refinement of self-heating lithium-ion batteries. J. Power Sources 2017, 360, 28–40. [Google Scholar] [CrossRef]
- Christensen, J.; Newman, J. Cyclable lithium and capacity loss in li-ion cells. J. Electrochem. Soc. 2005, 152, A818–A829. [Google Scholar] [CrossRef]
- Zhang, Q.; White, R.E. Capacity fade analysis of a lithium ion cell. J. Power Sources 2008, 179, 793–798. [Google Scholar] [CrossRef]
- Koerver, R.; Dursun, I.; Leichtwei, T.; Dietrich, C.; Zhang, W.; Binder, J.; Hartmann, P.; Zeier, W.; Janek, J. Capacity fade in solid-state batteries: Interphase formation and chemomechanical processes in nickel-rich layered oxide cathodes and lithium thiophosphate solid electrolytes. Chem. Mater. 2017, 29, 5574–5582. [Google Scholar] [CrossRef]
- Kaneko, F.; Wada, S.; Nakayama, M.; Wakihara, M.; Koki, J.; Kuroki, S. Capacity fading mechanism in all solid-state lithium polymer secondary batteries using peg-borate/aluminate ester as plasticizer for polymer electrolytes. Adv. Funct. Mater. 2009, 19, 918–925. [Google Scholar] [CrossRef]
- Nakayama, M.; Wada, S.; Kuroki, S.; Nogami, M. Factors affecting cyclic durability of all-solid-state lithiumpolymer batteries using poly(ethylene oxide)-based solid polymer electrolytes. Energy Environ. Sci. 2010, 3, 1995–2002. [Google Scholar] [CrossRef]
- Tang, S.; Lan, Q.; Xu, L.; Liang, J.; Lou, P.; Liu, C.; Cao, L.M.Y.; Cheng, S. A novel cross-linked nanocomposite solid-state electrolyte with super flexibility and performance for lithium metal battery. Nano Energy 2020, 71, 104600. [Google Scholar] [CrossRef]
- Koga, C.; Wada, S.; Nakayama, M. All solid-state lithium polymer secondary batteries using spinel Li4/3Ti5/3O4 as an active material. Electrochim. Acta. 2010, 55, 2561–2566. [Google Scholar] [CrossRef]
- Luo, D.; Zeng, S.; Chen, J. A probabilistic linguistic multiple attribute decision making based on a new correlation coefficient method and its application in hospital assessment. Mathematics 2020, 8, 340. [Google Scholar] [CrossRef]
- Ren, L.; Zhao, L.; Hong, S.; Zhao, S.; Wang, H.; Zhang, L. Remaining useful life prediction for lithium-ion battery: A deep learning approach. IEEE Access 2018, 6, 50587–50598. [Google Scholar] [CrossRef]
- Kang, P.; Chen, Z. Aircraft classification method based on the kurtosis. Eng. J. 2019, 21, 7855–7859. [Google Scholar] [CrossRef]
- Sha, W.; Li, Y.; Tang, S.; Tian, J.; Zhao, Y.; Guo, Y.; Zhang, W.; Zhang, X.; Lu, S.; Cao, Y.; et al. Machine learning in polymer informatics. InfoMat 2020, 3, 353–361. [Google Scholar] [CrossRef]
- Zhang, D. A coefficient of determination for generalized linear models. Am. Stat. 2018, 71, 310–316. [Google Scholar] [CrossRef]
- Weng, B.; Song, Z.; Zhu, R.; Yan, Q.; Sun, Q.; Grice, C.; Yan, Y.; Yin, W. Simple catalysis descriptor discovered by symbolic regression. Nat. Commun. 2020, 11, 3513. [Google Scholar] [CrossRef]
- Yuancheng, C. Features values of Solid-State Lithium Battery datasets [Data set]. Zenodo 2021. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| population size | 5000 |
| Generations | 5 |
| stopping criteria | 0.01 |
| p_crossover | 0.7 |
| p_subtree_mutation | 0.1 |
| p_hoist_mutation | 0.05 |
| p_point_mutation | 0.1 |
| function set | ‘add’, ’sub’, ’mul’, ’div’, ’sqrt’, ’log’, ’abs’, ’neg’, ’inv’, ’max’, ’min’ |
| parsimony coefficient | 0.001 |
| Formulas | R2 | Complexity |
|---|---|---|
| X5 | 0.7482 | 0 |
| min(X29, X5) | 0.7730 | 1 |
| 0.820 × X1 | 0.8768 | 1 |
| min(X20, 0.848 × X1) | 0.9093 | 2 |
| max(X1, ) × 24 | 0.7884 | 3 |
| X5 × max(, X6 − (X24/X21)) | 0.9307 | 5 |
| Method | R2 | RMSE | RULError53 (RULTrue = 157) | RULError105 (RULTrue = 105) | RULError179 (RULTrue = 31) |
|---|---|---|---|---|---|
| SVR | 0.81 | 26.50 | 2 | 6 | 27 |
| GPR | 0.83 | 24.64 | 23 | 7 | 13 |
| EN | 0.69 | 33.27 | 30 | 8 | 27 |
| SR | 0.91 | 18.17 | 4 | 4 | 0 |
| Computational Characteristics | Value | |||
|---|---|---|---|---|
| SVR | GPR | EN | SR | |
| Training time | 0.1800 s | 3.1527 s | 0.0021 s | 13.2677 s |
| Inference time | 0.0023 s | 0.0078 s | 0.0013 s | 0.0017 s |
| Total used memory | 0.5975 GB | 0.5999 GB | 0.5963 GB | 0.6346 GB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, D.; Sha, W.; Wang, L.; Tang, S.; Ma, A.; Chen, Y.; Wang, H.; Lou, P.; Lu, S.; Cao, Y.-C. Solid-State Lithium Battery Cycle Life Prediction Using Machine Learning. Appl. Sci. 2021, 11, 4671. https://doi.org/10.3390/app11104671
Cheng D, Sha W, Wang L, Tang S, Ma A, Chen Y, Wang H, Lou P, Lu S, Cao Y-C. Solid-State Lithium Battery Cycle Life Prediction Using Machine Learning. Applied Sciences. 2021; 11(10):4671. https://doi.org/10.3390/app11104671
Chicago/Turabian StyleCheng, Danpeng, Wuxin Sha, Linna Wang, Shun Tang, Aijun Ma, Yongwei Chen, Huawei Wang, Ping Lou, Songfeng Lu, and Yuan-Cheng Cao. 2021. "Solid-State Lithium Battery Cycle Life Prediction Using Machine Learning" Applied Sciences 11, no. 10: 4671. https://doi.org/10.3390/app11104671
APA StyleCheng, D., Sha, W., Wang, L., Tang, S., Ma, A., Chen, Y., Wang, H., Lou, P., Lu, S., & Cao, Y.-C. (2021). Solid-State Lithium Battery Cycle Life Prediction Using Machine Learning. Applied Sciences, 11(10), 4671. https://doi.org/10.3390/app11104671








