1. Introduction
S100A9 is a member of the calcium-binding S100 protein family, which participates in cytoskeleton rearrangement and arachidonic acid metabolism [
1]. As S100A9 is secreted at high concentrations from immigrated and activated phagocytic cells during inflammation [
2], it plays a critical role in modulating the inflammatory response by stimulating leukocyte recruitment or inducing cytokine secretion [
1]. S100A9 mediates the inflammatory process in myeloid cells and is involved throughout the initial development of cancer cells and metastatic disease progression [
3,
4]. Moreover, S100A9 is involved in the formation of amyloid aggregates, integrating the amyloid-neuroinflammatory cascade in Alzheimer’s disease, and enhancing the neurodegenerative effects of Parkinson’s disease [
5,
6,
7,
8]. Elevated levels of S100A9 have also been noted in chronic bronchitis, cystic fibrosis, systemic lupus erythematosus, rheumatoid arthritis, colitis ulcerosa, Crohn’s disease, inflammatory bowel disease, colorectal cancer, multiple sclerosis, and human immunodeficiency virus infection [
9,
10,
11,
12]. Detection strategies are needed if S100A9 is to be considered a biomarker of these diseases.
Antibody assays are the method of choice for disease diagnosis and treatment because they strongly bind to the target antigen, even in complex blood or tissue samples. Antibodies are classified as polyclonal or monoclonal, and hybridoma technology is a traditional and robust method for generating antibodies. Antibody detection tests are cost effective and are neither labor intensive nor technically demanding compared with the more expensive and complex phage display technology. Polyclonal antibodies are purified from the serum of an immunized animal, and monoclonal antibodies are secreted by immortalized B cells from the spleen of an immunized animal. In order to generate a unique monoclonal antibody of the desired specificity, the splenic B cells are fused with myeloma cells, resulting in immortal hybridomas [
13]. Although polyclonal antibodies can be generated more rapidly, at less expense, and with less technical skill than is required to generate hybridomas and, subsequently, monoclonal antibodies, the principal advantages of monoclonal antibodies are their homogeneity and consistency, whereas polyclonal antibodies are heterogeneous. Specifically, as a monoclonal antibody recognizes a single epitope of an antigen, its affinity and selectivity to the target antigen are greater than those of a polyclonal antibody. Another advantage of monoclonal antibodies is that once the desired hybridoma has been generated, it becomes a constant and renewable resource. In contrast, polyclonal antibodies generated to the same antigen using multiple animals will differ between individuals, and their avidity may change as they are harvested over time. Therefore, the concentration and purity levels of specific antibodies are higher in monoclonal than in polyclonal antibodies. A few commercially available purified anti-S100A9 monoclonal antibodies have been developed. However, to the best of our knowledge, no study has been published on the production and sequencing of monoclonal anti-S100A9 antibodies.
We aimed to generate and sequence a monoclonal antibody against S100A9. The sequence information can be used for the development of recombinant antibodies or antibody fragments against S100A9. Additionally, the detailed methods for antigen production, antibody generation, and sequencing will support other efforts to produce monoclonal anti-S100A9 antibodies for use as a biological reagent to bind S100A9 in diagnostic and therapeutic approaches.
2. Materials and Methods
2.1. Construction of Human S100A9 Expressing Gene
A gene encoding S100A9 was PCR amplified using the primers 5′-atatggatccgatgacttgcaaaatgtcg-3′ and 5′-aattaagcttagggggtgccctccc-3′ and Herculase II fusion DNA polymerase to add a His-tag at the N- or C-terminus of the expressed protein. The product was digested with restriction enzymes, BamHI and HindIII, and the product was inserted into BamHI- and HindIII-digested pET-Duet1 (Addgene) using a ligation high, resulting in pET-Duet (S100A9). The obtained plasmid was prepared using the plasmid miniprep system (Genesgen, Pusan, Korea), and the entire coding-region sequence was confirmed by sequencing.
2.2. Expression and Purification of Recombinant Human S100A9
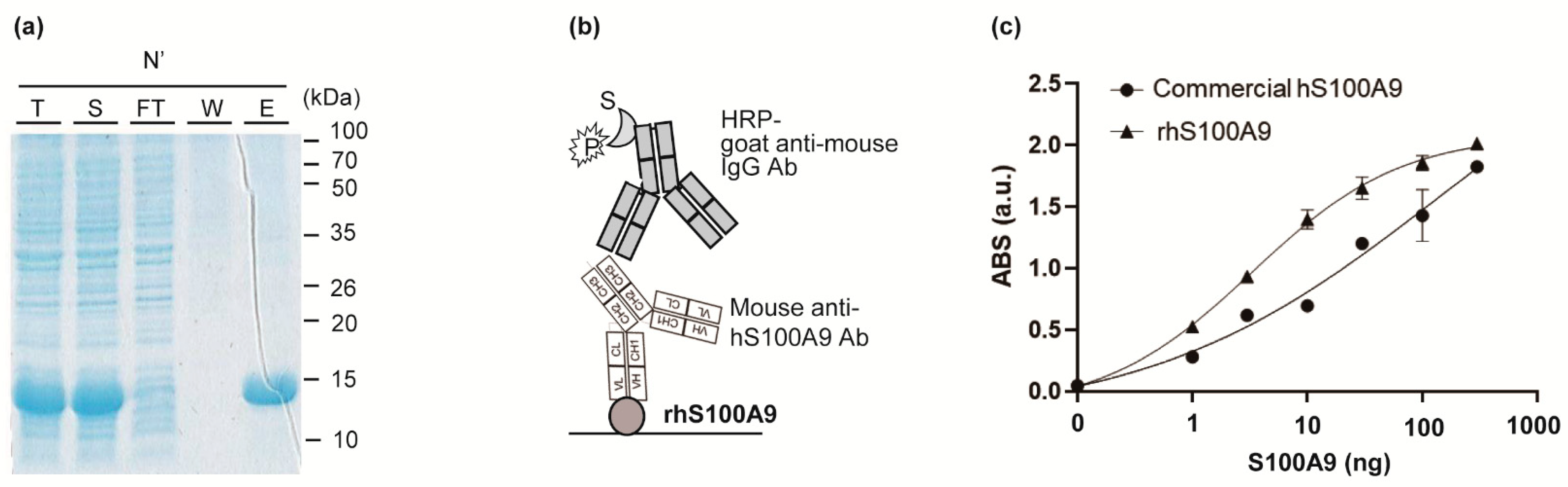
BL21 (DE3) cells were transformed with expression vector pET-Duet (S100A9) and cultured at 37 °C for 16 h in LBA medium (LB medium containing 100 μg/mL ampicillin) and 1.5% agar, and a single colony was picked and grown at 37 °C in 4 mL of LBA medium overnight, from which 1 mL was used to inoculate 50 mL of LBA medium at 37 °C to an OD600 of 0.6. After that, 1 mM isopropylthio-β-galactopyranoside was added and incubated for an additional 20 h at 18 °C for protein induction. After centrifugation at 3500 rpm for 20 min at 4 °C, the pellet was resuspended in 5 mL of purification buffer (50 mM potassium phosphate (pH 7.4), 150 mM sodium chloride (NaCl), 10 mM imidazole, 0.5 mM PMSF), followed by sonication. After centrifugation at 3500 rpm for 20 min at 4 °C, the supernatant was purified using an Ni-NTA His-tag purification kit. The Ni-NTA-bound protein was washed with 20 mL of purification buffer and further washed with 40 mL of the same buffer containing 20 mM imidazole followed by 10 mL of the same buffer containing 50 mM imidazole. The protein was eluted with 3.2 mL of the same buffer containing 200 mM imidazole and collected using a disposable gravity column. The eluent was concentrated with an ultra-filtration device and equilibrated with PBS buffer (10 mM Na2HPO4, 2 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl, pH 7.4) and then concentrated to 2 mg/mL. Protein expression and purification were confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis, and protein concentration was determined using NanoDrop.
2.3. Confirmation of the Activity of Recombinant Human S100A9
Indirect ELISA for confirming the activity of purified rhS100A9 was performed as follows: several concentrations of purified rhS100A9 or commercially available purified hS100A9 protein (Sino Biological, Beijing, China, 11145-H08B) in PBS were immobilized on a 96-well plate for 16 h at 4 °C, and the wells were filled with 200 μL of PBST (PBS buffer with 0.05% Tween20) containing 2% BSA for 1 h at 37 °C and washed three times with 200 μL of TBST. Subsequently, 150 ng of anti-hS100A9 antibody (Sino Biological, 11145-MM01) in TBST was added and incubated for 1 h at 25 °C. After washing three times with 200 μL of TBST, the bound protein was probed with 5000-fold diluted HRP-conjugated goat anti-mouse antibody in TBST for 1 h at 25 °C. The wells were washed three times with 200 μL of TBST and developed with 100 μL of tetramethylbenzide (TMB) solution. After incubation for 15 min, the reaction was stopped with 25 μL of 1 N sulphuric acid, and the absorbance was read at 450 nm using a microplate reader Model 680 (Bio-Rad). As a negative control, 200 ng BSA was used instead of antigen, and the titer was used as a baseline. A well coated with 200 ng BSA only was used as a negative control to use its titer as a baseline signal.
2.4. Production of Monoclonal Anti-Human S100A9 Antibodies
Monoclonal antibody was produced at A-Frontier (Seoul, Korea). Female BALB/c mice, 6 weeks old, were immunized by IP injection of 100 μg of purified rhS100A9 in PBS with complete Freund’s adjuvant. After 2 weeks, the mice were tail-bled and the titers of the serially diluted antisera were measured by indirect ELISA, resulting in titers of over 1.5 at the 100- or 1000-fold diluted antisera, which indicated that no additional immunizations would be necessary prior to cell fusion. Three days after the injection, 1–1.5 × 108 spleen cells separated from the immunized mice were washed with culture medium and then fused with SP2/0 murine myeloma cells using polyethylene glycol as a fusing agent for hybridoma production. The fused cells were cultured using HAT medium and culture supernatants were screened by indirect ELISA with the use of 250 ng of rhS100A9 as a seeding antigen, TBST containing 2% skim milk as a blocking buffer, 50 μL of hybridoma supernatant, and 10,000-fold diluted HRP-conjugated mouse anti-IgG antibody as a secondary antibody. Following the above fusion ELISA test, the cells of the 24 positive wells were moved to a 24-well plate and were cultured, and the activity was confirmed by indirect ELISA as above. The cells were pipetted into a 96-well plate using an HT culture medium (HAT without aminopterin) and then cultured for 2 weeks in a CO2 incubator at 37 °C. The single clones with the highest anti-S100A9 antibody titers using indirect ELISA were subcloned and the screening processes were repeated. The dose–response curves were fitted to a four-parameter equation, y = a + (b − a)/(1 + (c/x)^d), using GraphPad Prism software. In the equation, a, b, c, and d indicate ABS in the absence of antigen, ABS in the presence of 300 ng antigen, EC50, and hill slope, respectively. The EC50 values were estimated based on the parameter c in the formula. The LOD was obtained as the estimated antigen concentration that shows the mean blank value +3 standard deviation (SD).
2.5. Purification of Monoclonal Antibodies
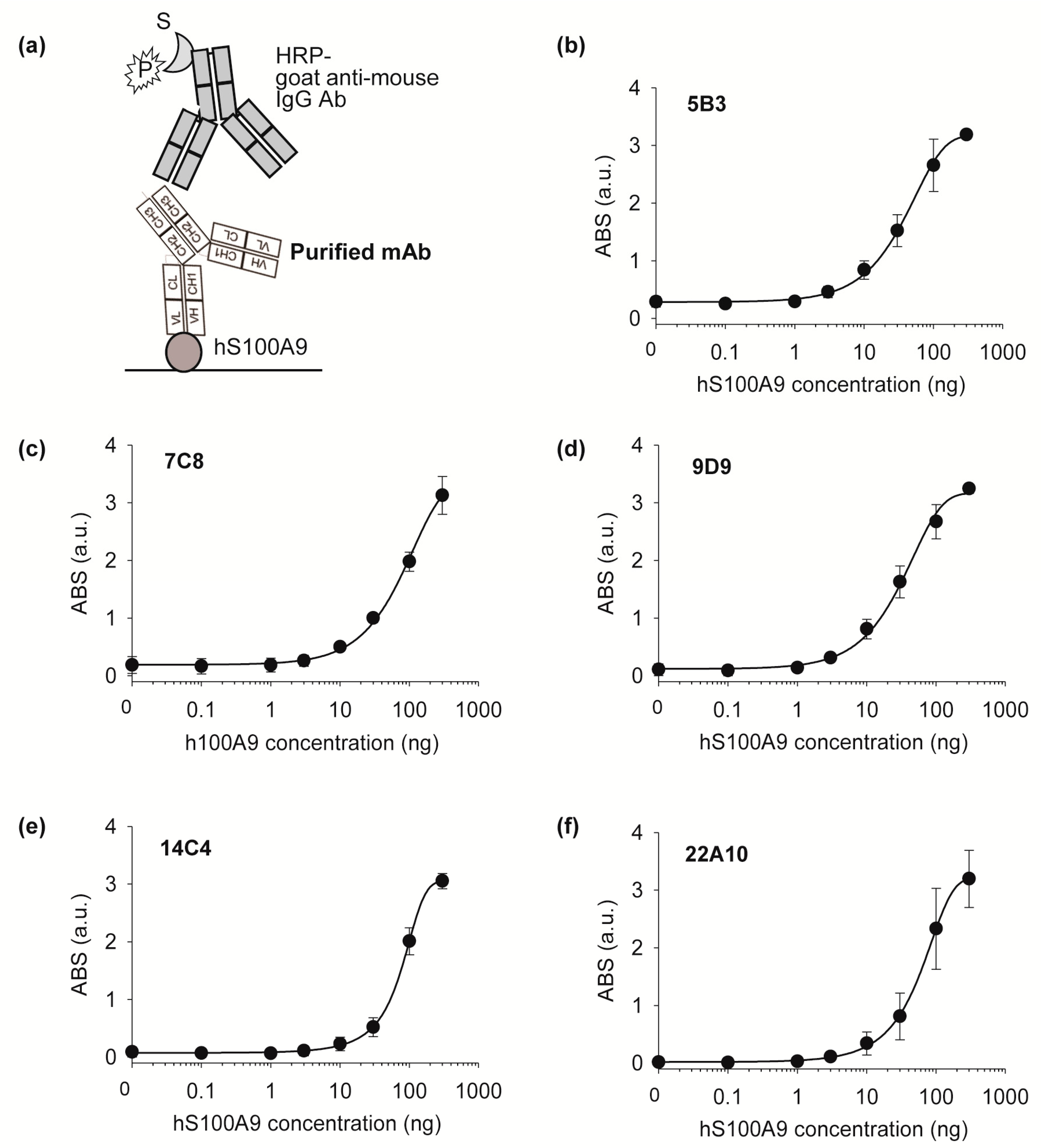
After the final screening, five positive subclones (5B3, 7C8, 9D9, 14E7, and 22A10) were grown in larger volumes, and the antibodies obtained from each cell supernatant were purified using Protein G agarose beads (Pierce) as follows: 10 μL of resin slurry was added to 1 mL of cell supernatant and then incubated at RT for 1 h. After washing using 500 μL of PBS three times, the antibody was eluted using 100 μL of 0.1 M glycine (pH 2.5) and the eluted sample was immediately adjusted to neutral by adding 10 μL of PBS. The antibody concentration was measured from the absorbance at 280 nm using NanoDrop.
2.6. Confirmation of the Antigen Binding Efficiency of Purified Monoclonal Antibodies
The antigen-binding activities of the five purified monoclonal antibodies were tested using indirect ELISA as follows: 100 μL of 250 ng of commercial hS100A9 (Sino Biological) in PBS was immobilized on a 96-well plate for 37 h at 2 °C. After that, the wells were filled with 200 μL of TBST containing 2% BSA for 1 h at 37 °C and then washed three times with TBST. Subsequently, 150 ng of purified monoclonal antibody in 100 μL of PBS with 0.1% BSA was added and incubated for 1 h at 25 °C. After washing three times with 200 μL of TBST, bound protein was probed with 5000-fold diluted HRP-conjugated goat anti-mouse IgG antibody in PBS with 0.5% BSA for 1 h at 25 °C. The wells were washed three times with 200 μL of TBST and developed with 100 μL of TMB solution. After incubation for 10 min, the reaction was stopped with 50 μL of 1 N sulphuric acid, and the absorbance was read at 450 nm using a microplate reader.
Afterwards, an isotyping test was performed using a Rapid ELISA mouse mAb isotyping kit (Pierce), and the cells were frozen in liquid nitrogen in FBS containing 10% of DMSO. 5B3, 7C8, 9D9: H chain-IgG2b, L chain-kappa; 14C4, 22A10: H chain-IgG1, L chain-kappa.
2.7. Identification of VH and VL Sequences
Total cellular RNA from 5B3, 9D9, and 14C4 hybridoma cells was extracted using an RNeasy kit according to the manufacturer’s protocol. After that, 0.5 μg of RNA was reverse-transcribed by 5 U of Moloney murine leukemia virus (M-MLV) RT using 50 pmol of 5′phosphorylation-modified primer, 5′-tgcaaggcttacaaccacaatcc-3′ for VH or 5′-gatatcttccacttgacattgatg-3′ for VL, in a total volume of 12.5 μL at 42 °C in the presence of 10 U RNasin RNase inhibitor. Reactions were terminated after 30 min by heating to 95 °C for 15 min and subsequently adding 3 volumes of TE buffer supplemented with 4 μg/mL RNase A. The resulting cDNA was circularized by 20 U of T4 RNL1 in the presence of 15% PEG 8000 in a total volume of 50 μL at 37 °C for 1 h. Unreacted residual first-strand primers and cDNA were removed by the addition of 1.5 U of T4 DNA polymerase for 30 min at 37 °C. Next, 2 μL of these intramolecularly joined 5′ cDNAs was amplified by 35 cycles of PCR using inverted primer pairs as follows: IgG-inF, 5′-ccagcgagaccgtcacctg-3′; IgG-inR, 5′-agcccttgaccaggcatcc-3′; Kappa-inF, 5′- gcttcttgaacaacttctacc-3′; and Kappa-inR, 5′-cagttggtgcagcatcagc-3′. Finally, an amplicon of the expected size was observed by TAE agarose gel electrophoresis, purified, and cloned into the T vector. Plasmid DNA minipreps of clones were subjected to Sanger sequencing (Bionics, Seoul, Korea). CDRs of VH and VL were determined via the IgBlast tool.
4. Discussion
S100A9 overexpression is elevated in several inflammatory and oncologic conditions, including ulcerative colitis and colorectal cancer. We generated and sequenced a novel anti-hS100A9 monoclonal antibody with high antigen-binding activity. We produced a large amount of highly pure, soluble rhS100A9 using a standard E. coli expression system and showed its high activity, suggesting that the recombinant antigen maintained its structural integrity. E. coli expressions are known for their simplicity and are highly cost effective for antigen production.
We performed ELISA using a commercial anti-S100A9 antibody and determined its EC50 (135.4 ng) and LOD (0.11 ng). Although the LOD values were lower than those of a commercial antibody, the DNA sequence of the commercial antibody has not been published. We identified the variable domain sequences and the antibody subtypes. The sequence information will be broadly useful for the development of recombinant antibodies or antibody fragments targeting S100A9. The detailed methods for producing antigens, generating antibodies, and identifying the antibody sequence can be used to produce monoclonal antibodies against other antigens of interest and anti-S100A9 monoclonal antibodies.
The high target specificity and selectivity of monoclonal antibodies make them a promising diagnostic option. The anti-hS100A9 antibody produced in this study can be used to specifically detect hS100A9 by conjugating a fluorescent dye to the antibody. The sequence information is a good example of applied research and will be useful for the development of recombinant antibodies or antibody fragments.