Sustaining Rice Production through Biofertilization with N2-Fixing Cyanobacteria
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites and Isolation of Cyanobacteria
2.2. DNA Extraction and PCR Amplification
2.3. Bioinformatic Analyses and Phylogenetic Tree Construction
2.4. Light Microscopy
2.5. Batch Cultivation of Cyanobacteria for Field Trials
3. Results and Discussion
3.1. Isolation and Genetic Characterization of N2-Fixing Cyanobacteria from the Rice Paddies of the Andalusian Marshes
3.2. Morphological Description of the Cyanobacterial Isolates
3.3. Plant Growth Promotion in Field Trials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awika, J. Major Cereal Grains Production and Use around the World. In Advances in Cereal Science: Implications to Food Processing and Health Promotion; American Chemical Society: Washington, DC, USA, 2011. [Google Scholar]
- Qi, D.; Wu, Q.; Zhu, J. Nitrogen and phosphorus losses from paddy fields and the yield of rice with different water and nitrogen management practices. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Dhar, D.W.; Tabassum, R. Role of Cyanobacteria in Crop Protection. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2016, 86, 1–8. [Google Scholar] [CrossRef]
- Paredes, I.; Otero, N.; Soler, A.; Green, A.J.; Soto, D.X. Agricultural and urban delivered nitrate pollution input to Mediterranean temporary freshwaters. Agric. Ecosyst. Environ. 2020, 294, 106859. [Google Scholar] [CrossRef]
- Farhad, S.; Gual, M.A.; Ruiz-Ballesteros, E. Linking governance and ecosystem services: The case of Isla Mayor (Andalusia, Spain). Land Use Policy 2015, 46, 91–102. [Google Scholar] [CrossRef]
- Toral, G.M.; Aragonés, D.; Bustamante, J.; Figuerola, J. Using Landsat images to map habitat availability for waterbirds in rice fields. IBIS 2011, 153, 684–694. [Google Scholar] [CrossRef]
- López-Rodas, V.; Maneiro, E.; Lanzarot, M.P.; Perdigones, N.; Costas, E. Mass wildlife mortality due to cyanobacteria in the Doñana National Park, Spain. Veter.-Rec. 2008, 162, 317–318. [Google Scholar] [CrossRef]
- Fernández-Delgado, C. Doñana Natural Space: The Uncertain Future of a Crown Jewel in Europe’s Protected Areas. Case Stud. Environ. 2017, 1, 1–12. [Google Scholar] [CrossRef]
- Erisman, J.W.; Van Grinsven, H.; Grizzetti, B.; Bouraoui, F.; Powlson, D.; Sutton, M.A.; Bleeker, A.; Reis, S. The European nitrogen problem in a global perspective. In The European Nitrogen Assessment; Cambridge University Press (CUP): Cambridge, UK, 2011; pp. 9–31. [Google Scholar]
- Selman, M.; Sugg, Z.; Greenhalgh, S.; Diaz, R. Eutrophication and Hypoxia in Coastal Areas. Water quality: Eutrophication and hypoxia. WRI Policy Note 2008, 1, 1–6. [Google Scholar]
- Prasanna, R.; Jaiswal, P.; Nayak, S.; Sood, A.; Kaushik, B.D. Cyanobacterial diversity in the rhizosphere of rice and its ecological significance. Indian J. Microbiol. 2009, 49, 89–97. [Google Scholar] [CrossRef]
- Álvarez, C.; Navarro, J.A.; Molina-Heredia, F.P.; Mariscal, V. Endophytic Colonization of Rice (Oryza sativa L.) by the Symbiotic Strain Nostoc punctiforme PCC. Mol. Plant-Microbe Interact. 2020, 33, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, R.; Nain, L.; Pandey, A.K.; Saxena, A.K. Microbial diversity and multidimensional interactions in the rice ecosystem. Arch. Agron. Soil Sci. 2011, 58, 723–744. [Google Scholar] [CrossRef]
- Adams, D.G.; Bergman, B.; Nierzwicki-Bauer, S.A.; Duggan, P.; Rai, A.N.; Schußler, A. Cyanobactrial-plant symbioses. In The Prokaryotes–Prokaryotic Biology and Symbiotic Associations; Rosenberg, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 360–381. [Google Scholar]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assigments, strain stories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Prasanna, R.; Nayak, S. Influence of diverse rice soil ecologies on cyanobacterial diversity and abundance. Wetl. Ecol. Manag. 2006, 15, 127–134. [Google Scholar] [CrossRef]
- Prasanna, R.; Jaiswal, P.; Kaushik, B.D. Cyanobacteria as potential options for environmental sustainability—Promises and challenges. Indian J. Microbiol. 2008, 48, 89–94. [Google Scholar] [CrossRef]
- Rippka, R.; Waterbury, J.B.; Stanier, R.Y. Isolation and Purification of Cyanobacteria: Some General Principles. In The Prokaryotes; Starr, M.P., Stolp, H., Trüper, H.G., Balows, A., Schlegel, H.G., Eds.; Springer: Berlin/Heidelberg, Germany, 1981. [Google Scholar]
- Hasan, M. Investigation on the Nitrogen Fixing Cyanobacteria (BGA) in Rice Fields of North-West Region of Bangladesh. III: Filamentous (Heterocystous). J. Environ. Sci. Nat. Resour. 2015, 6, 253–259. [Google Scholar] [CrossRef]
- Vijayan, D.; Ray, J.G. Ecology and Diversity of Cyanobacteria in Kuttanadu Paddy Wetlands, Kerala, India. Am. J. Plant Sci. 2015, 6, 2924–2938. [Google Scholar] [CrossRef]
- Pham, H.T.; Nguyen, L.T.; Duong, T.A.; Bui, D.T.; Doan, Q.T.; Nguyen, H.T.; Mundt, S. Diversity and bioactivities of nostocacean cyanobacteria isolated from paddy soil in Vietnam. Syst. Appl. Microbiol. 2017, 40, 470–481. [Google Scholar] [CrossRef]
- Song, T.; Mårtensson, L.; Eriksson, T.; Zheng, W.; Rasmussen, U. Biodiversity and seasonal variation of the cyanobacterial assemblage in a rice paddy field in Fujian, China. FEMS Microbiol. Ecol. 2005, 54, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Rani, A.; Metwally, A.; McGee, H.S.; Perkins, D.L. Analysis of the microbiome: Advantages of whole genome shotgun versus 16S amplicon sequencing. Biochem. Biophys. Res. Commun. 2016, 469, 967–977. [Google Scholar] [CrossRef]
- Mariscal, V.; Herrero, A.; Nenninger, A.; Mullineaux, C.W.; Flores, E. Functional dissection of the three-domain SepJ protein joining the cells in cyanobacterial trichomes. Mol. Microbiol. 2010, 79, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Komárek, J. A polyphasic approach for the taxonomy of cyanobacteria: Principles and applications. Eur. J. Phycol. 2016, 51, 346–353. [Google Scholar] [CrossRef]
- Meeks, J.C.; Elhai, J. Regulation of Cellular Differentiation in Filamentous Cyanobacteria in Free-Living and Plant-Associated Symbiotic Growth States. Microbiol. Mol. Biol. Rev. 2002, 66, 94–121. [Google Scholar] [CrossRef]
- Kozlíková-Zapomělová, E.; Chatchawan, T.; Kaštovský, J.; Komárek, J. Phylogenetic and taxonomic position of the genus Wollea with the description of Wollea salina sp. nov. (Cyanobacteria, Nostocales). Fottea 2016, 16, 43–55. [Google Scholar] [CrossRef]
- Qin, H.; Li, Y.; Huang, R. Advances and Challenges in the Breeding of Salt-Tolerant Rice. Int. J. Mol. Sci. 2020, 21, 8385. [Google Scholar] [CrossRef]
- Xue, C.; Wang, L.; Wu, T.; Zhang, S.; Tang, T.; Wang, L.; Zhao, Q.; Sun, Y. Characterization of Co-Cultivation of Cyanobacteria on Growth, Productions of Polysaccharides and Extracellular Proteins, Nitrogenase Activity, and Photosynthetic Activity. Appl. Biochem. Biotechnol. 2017, 181, 340–349. [Google Scholar] [CrossRef]
- Priya, H.; Prasanna, R.; Ramakrishnan, B.; Bidyarani, N.; Babu, S.; Thapa, S.; Renuka, N. Influence of cyano-bacterial inoculation on the culturable microbiome and growth of rice. Microbiol. Res. 2014, 171, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Won, S. Evaluation of 16S rRNA Databases for Taxonomic Assignments Using Mock Community. Genom. Inform. 2018, 16, e24. [Google Scholar] [CrossRef] [PubMed]
- Chandler, D.P.; Brockman, F.J.; Fredrickson, J.K. Use of 16S rDNA clone libraries to study changes in a microbial community resulting from ex situ perturbation of a subsurface sediment. FEMS Microbiol. Rev. 1997, 20, 217–230. [Google Scholar] [CrossRef]
- Huang, R.; McGrath, S.P.; Hirsch, P.R.; Clark, I.M.; Storkey, J.; Wu, L.; Zhou, J.; Liang, Y. Plant-microbe net-works in soil are weakened by century-long use of inorganic fertilizers. Microb. Biotechnol. 2019, 12, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Nosheen, S.; Ajmal, I.; Song, Y. Microbes as Biofertilizers, a Potential Approach for Sustainable Crop Production. Sustainability 2021, 13, 1868. [Google Scholar] [CrossRef]


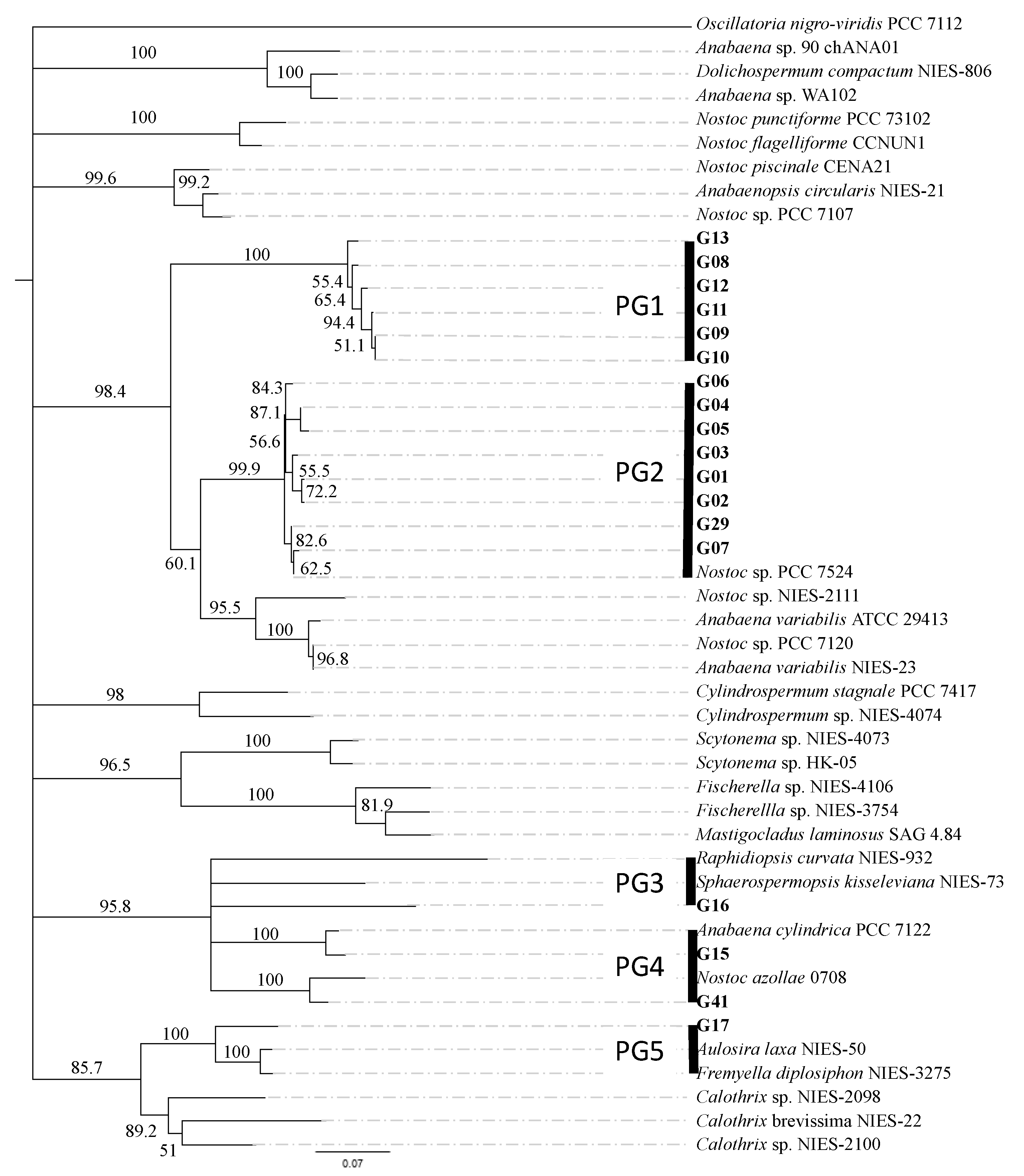
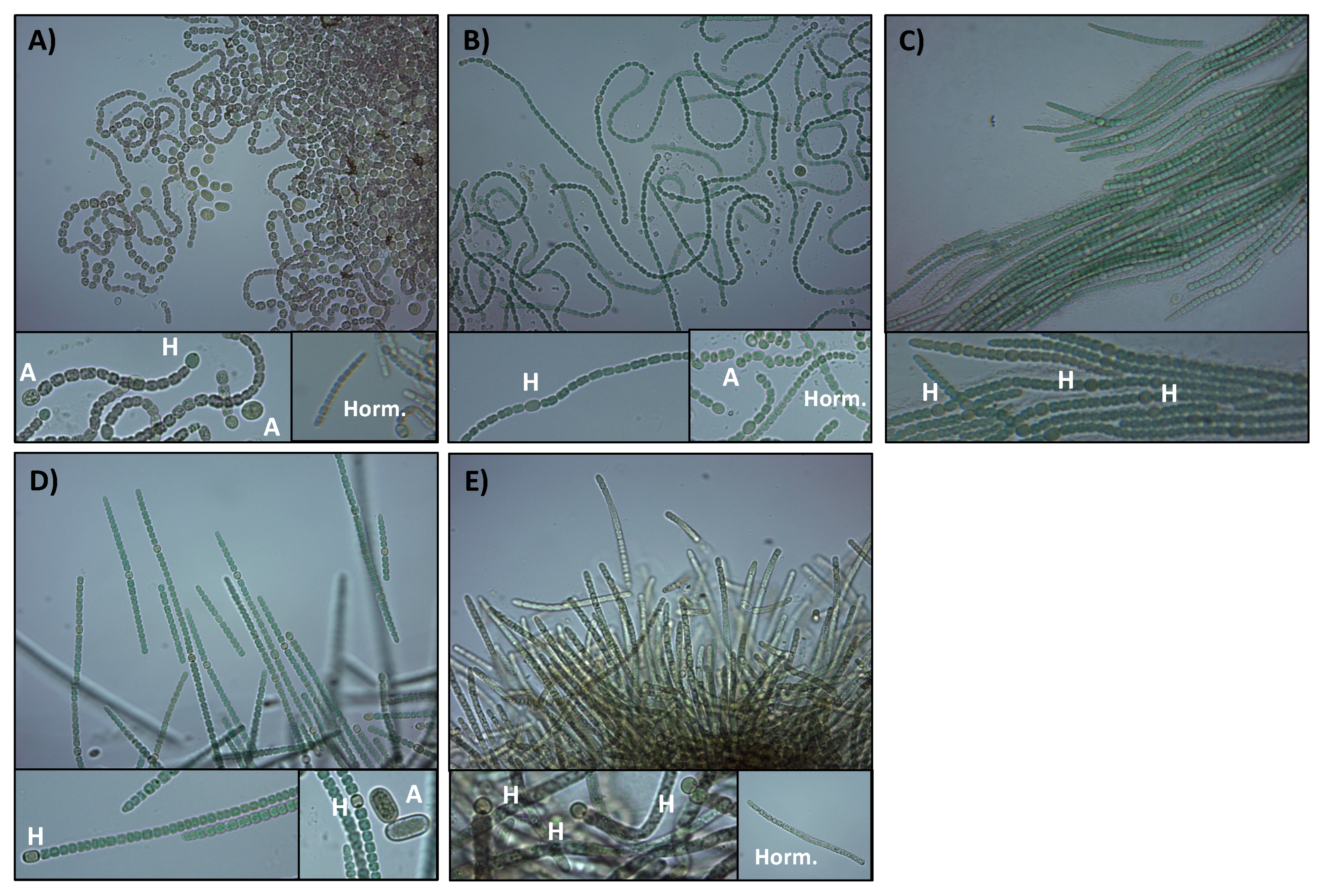
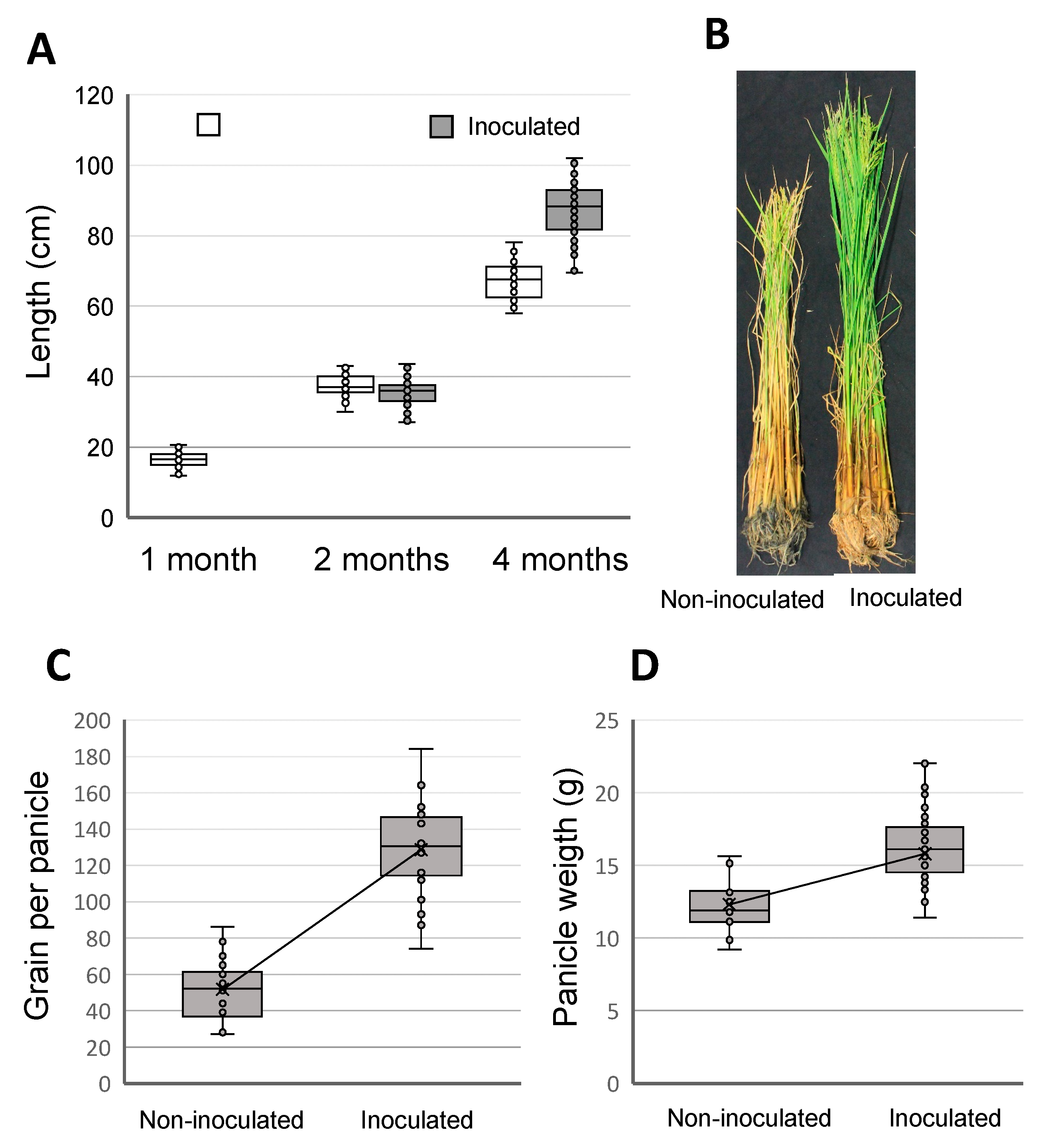
| Phylogenetic Group (Strain) | Culture Color | Cells Per Trichome | % Heterocysts | Akinetes | Hormogonia | Closest Related |
|---|---|---|---|---|---|---|
| PG1 (G10) | Brown | 24.58 ± 2.17 | 7.48 ± 0.40 | Yes | Yes | Nostoc punctiforme |
| PG2 (G04) | Green | 24.31 ± 1.79 | 9.18 ± 0.35 | Yes | Yes | Nostoc sp. |
| PG3 (G16) | Blue-Green | 31.62 ± 1.12 | 5.38 ± 0.25 | No | No | Wollea salina |
| PG4 (G15) | Green | 41.13 ± 1.53 | 4.98 ± 0.26 | Yes | No | Anabaena cylindrica |
| PG5 (G17) | Dark-Green | 12.47 ± 0.59 | 10.51 ± 0.67 | No | Yes | Calothrix membranacea |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iniesta-Pallarés, M.; Álvarez, C.; Gordillo-Cantón, F.M.; Ramírez-Moncayo, C.; Alves-Martínez, P.; Molina-Heredia, F.P.; Mariscal, V. Sustaining Rice Production through Biofertilization with N2-Fixing Cyanobacteria. Appl. Sci. 2021, 11, 4628. https://doi.org/10.3390/app11104628
Iniesta-Pallarés M, Álvarez C, Gordillo-Cantón FM, Ramírez-Moncayo C, Alves-Martínez P, Molina-Heredia FP, Mariscal V. Sustaining Rice Production through Biofertilization with N2-Fixing Cyanobacteria. Applied Sciences. 2021; 11(10):4628. https://doi.org/10.3390/app11104628
Chicago/Turabian StyleIniesta-Pallarés, Macarena, Consolación Álvarez, Francisco M. Gordillo-Cantón, Carmen Ramírez-Moncayo, Pilar Alves-Martínez, Fernando P. Molina-Heredia, and Vicente Mariscal. 2021. "Sustaining Rice Production through Biofertilization with N2-Fixing Cyanobacteria" Applied Sciences 11, no. 10: 4628. https://doi.org/10.3390/app11104628
APA StyleIniesta-Pallarés, M., Álvarez, C., Gordillo-Cantón, F. M., Ramírez-Moncayo, C., Alves-Martínez, P., Molina-Heredia, F. P., & Mariscal, V. (2021). Sustaining Rice Production through Biofertilization with N2-Fixing Cyanobacteria. Applied Sciences, 11(10), 4628. https://doi.org/10.3390/app11104628









