Simulation of Aerosol Evolution within Background Pollution for Nucleated Vehicle Exhaust via TEMOM
Abstract
1. Introduction
2. Governing Equation
2.1. CFD Model
2.2. PBE Solution
2.3. Coagulation Kernel
2.4. Condensation Kernel
2.5. Thermophoresis and Particle Diffusion
2.6. Moment Transport Equation
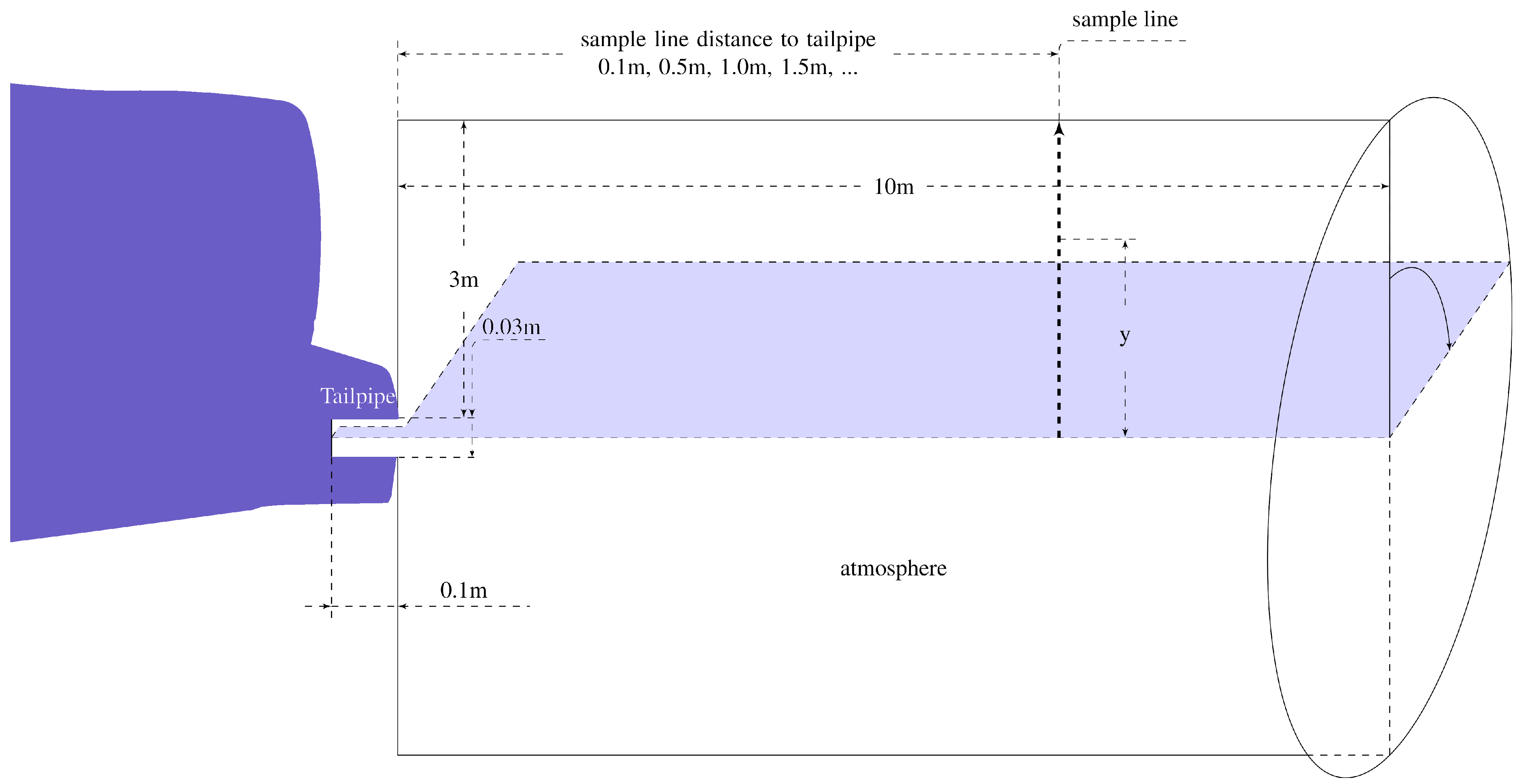
3. Simulation Configuration
3.1. Flow Field
3.2. Initial Conditions Calculation
3.3. Boundary Setting
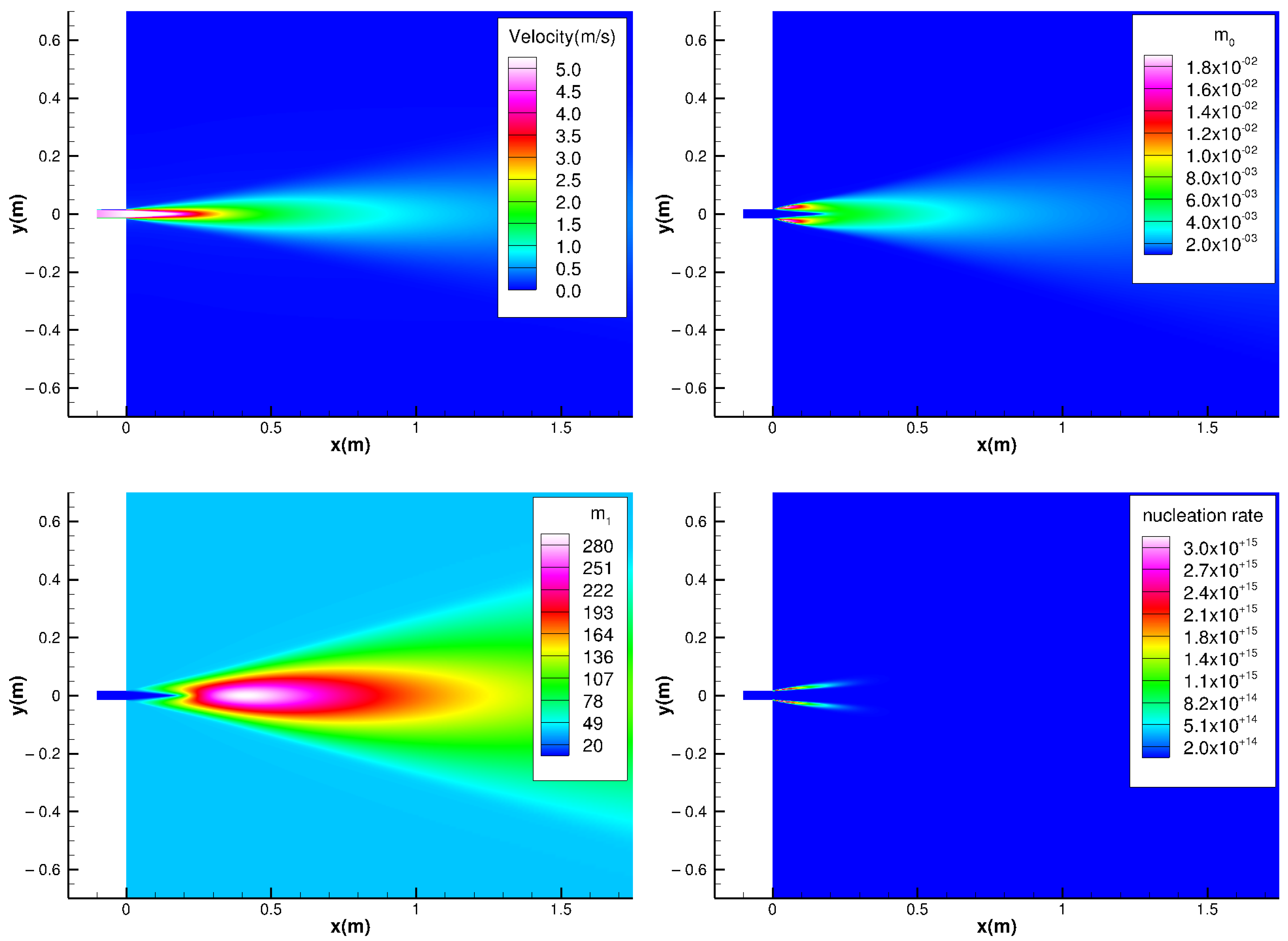
4. Results and Discussion
5. Conclusions
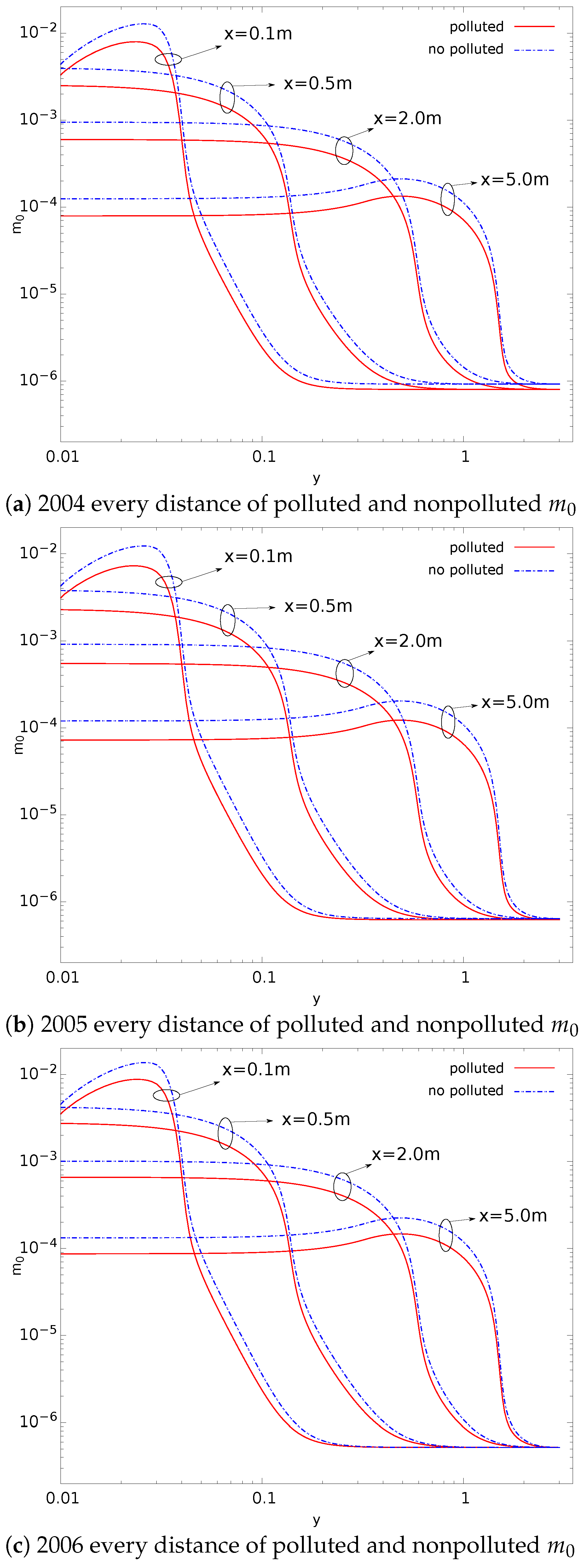
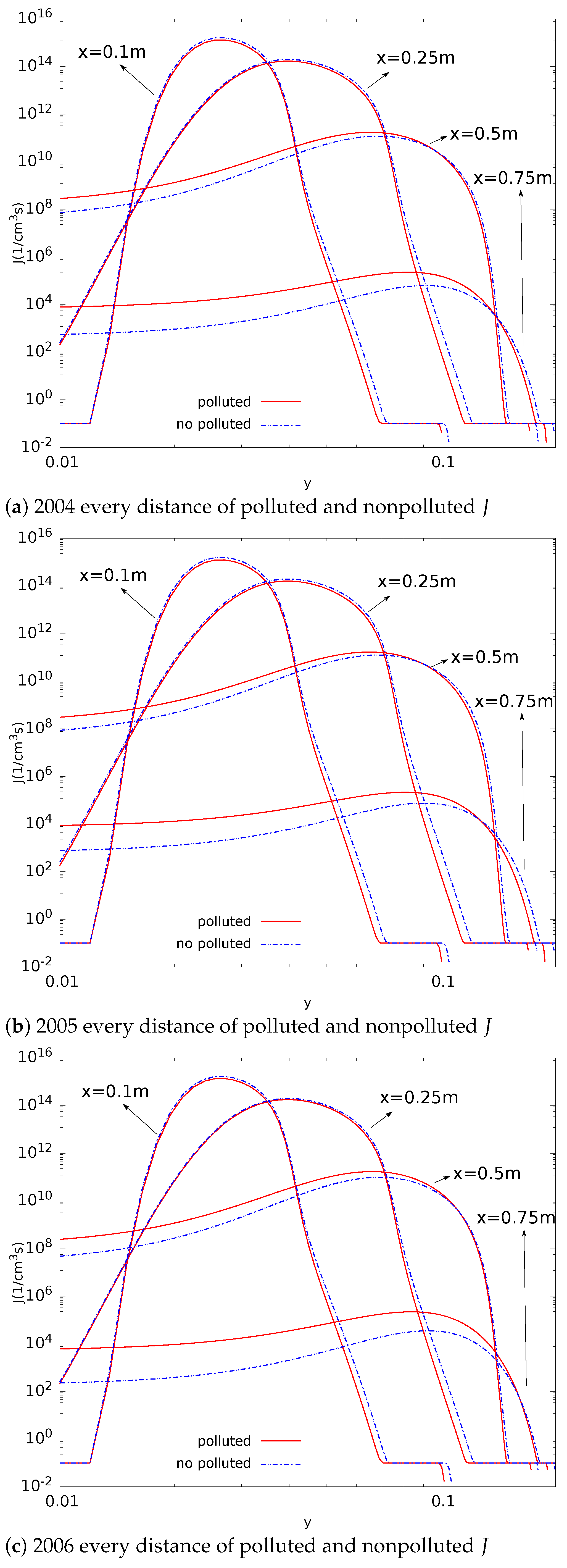
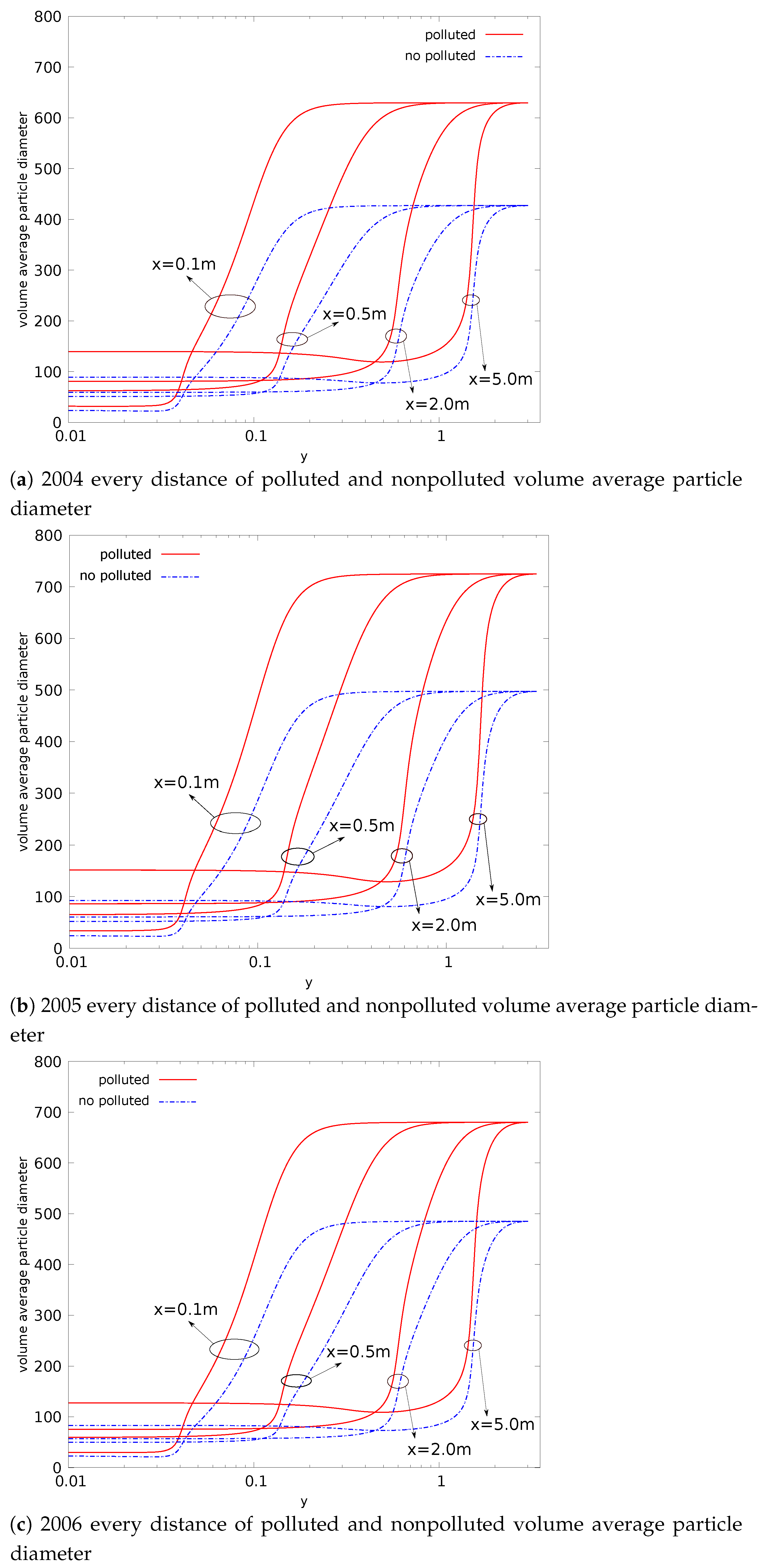
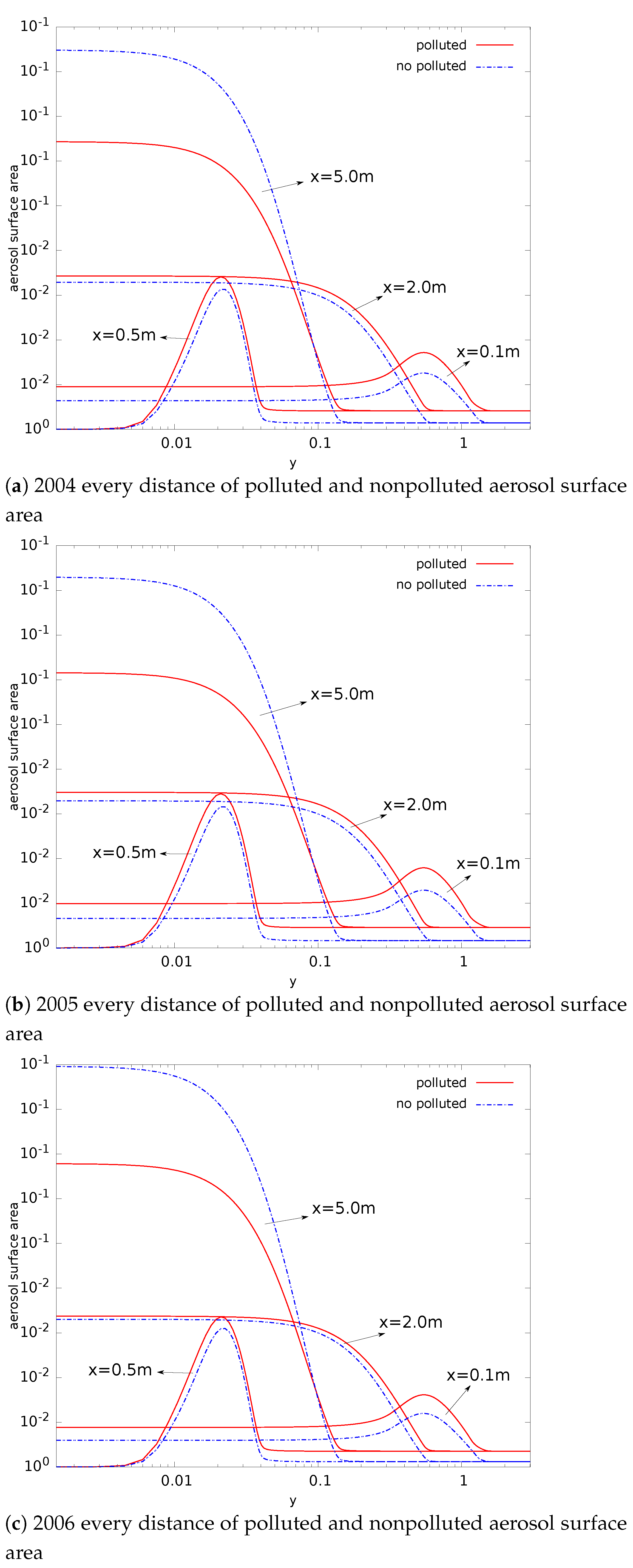
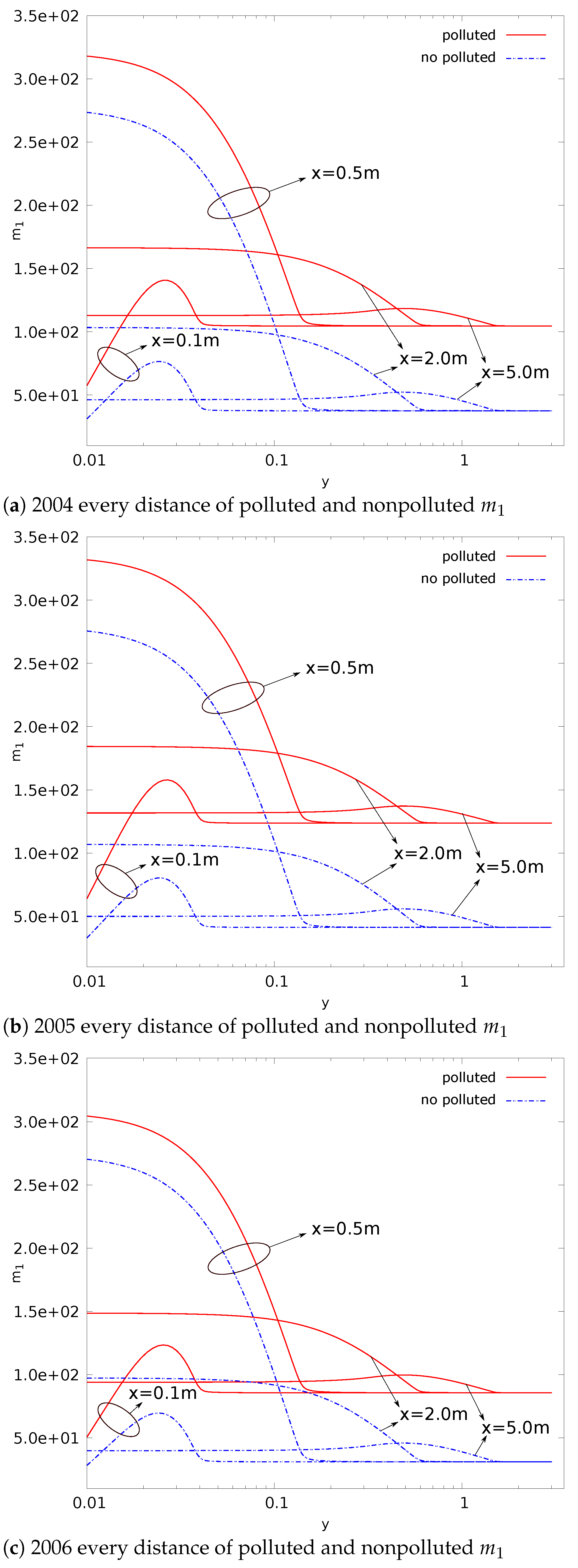
- Compared with nonpolluted conditions (low concentration of background particles), the nucleation rate in polluted conditions will be reduced. In addition, the number concentration of particles will also be reduced. This phenomenon makes number concentration unable to reflect the degree of pollution. The decrease in nucleation rate makes the concentration of critical sulfuric acid molecular clusters higher in the flow field (this is limited to a relatively small area). This is a disadvantage in certain operations that remove contaminants;
- The evolution of aerosols mainly depends on the nucleation rate. In addition, the nucleation rate has a strong regional distribution (that is, the spatial distribution of the nucleation rate is limited to a small range). Therefore, the source of controlling the pollutant is the key. Compared with the core area of nucleation, other regions have little impact on the generation and evolution of particulate matter;
- The presence of background particulate matter will slow down the nucleation process of aerosol. The mass concentration of particulate matter in the polluted condition is higher than that in the nonpolluted condition. Therefore, the slowing effect of particulate matter on the nucleation process of aerosol will be more obvious in pollution conditions;
- In the process of aerosol evolution, the independence of mass concentration and number concentration has guiding significance in various occasions involving the control of aerosol concentration. In this paper, the evolution mechanism behind this phenomenon is obtained by the simulation method. This is conducive to the use of this phenomenon. For example, it is necessary to confirm which factors effectively control the pollution situation; filtering to reduce the mass concentration of pollutants in order to reduce the number concentration is invalid;
- The significant change regions of nucleation, condensation, and coagulation are different. The nucleation process is spatially closer to the source of pollution. The nucleation process also provides more particles for the condensation and coagulation processes. Condensation comes next, and coagulation comes last.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brunekreef, B.; Holgate, S.T. Air Pollution and Health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, S.; van Holten, J.W.; Pal, S. Generalized Particle Dynamics: Modifying the Motion of Particles and Branes. J. High Energy Phys. 2009, 2009, 115. [Google Scholar] [CrossRef]
- Kumar, P.; Robins, A.; Vardoulakis, S.; Britter, R. A Review of the Characteristics of Nanoparticles in the Urban Atmosphere and the Prospects for Developing Regulatory Controls. Atmos. Environ. 2010, 44, 5035–5052. [Google Scholar] [CrossRef]
- Zhang, R.; Khalizov, A.; Wang, L.; Hu, M.; Xu, W. Nucleation and Growth of Nanoparticles in the Atmosphere. Chem. Rev. 2012, 112, 1957–2011. [Google Scholar] [CrossRef]
- Silva, L.; Lage, P. Development and Implementation of a Polydispersed Multiphase Flow Model in OpenFOAM. Comput. Chem. Eng. 2011, 35, 2653–2666. [Google Scholar] [CrossRef]
- Yu, M.; Lin, J.; Chan, T. Numerical Simulation for Nucleated Vehicle Exhaust Particulate Matters Via the Temom/Les Method. Int. J. Mod. Phys. C 2009, 20, 399–421. [Google Scholar] [CrossRef]
- Brines, M.; Dall’Osto, M.; Beddows, D.C.S.; Harrison, R.M.; Gómez-Moreno, F.; Núñez, L.; Artinano, B.; Costabile, F.; Gobbi, G.P.; Salimi, F. Traffic and Nucleation Events as Main Sources of Ultrafine Particles in High-Insolation Developed World Cities. Atmos. Chem. Phys. 2015, 15, 5929–5945. [Google Scholar] [CrossRef]
- Vaattovaara, P.; Petäjä, T.; Joutsensaari, J.; Miettinen, P.; Zaprudin, B.; Kortelainen, A.; Heijari, J.; Yli-Pirilä, P.; Aalto, P.; Worsnop, D.R. The Evolution of Nucleation-and Aitken-Mode Particle Compositions in a Boreal Forest Environment during Clean and Pollution-Affected New-Particle Formation Events. Boreal Environ. Res. 2009, 14, 662–682. [Google Scholar]
- Rodríguez, S.; Van Dingenen, R.; Putaud, J.P.; Martins-Dos Santos, S.; Roselli, D. Nucleation and Growth of New Particles in the Rural Atmosphere of Northern Italy—Relationship to Air Quality Monitoring. Atmos. Environ. 2005, 39, 6734–6746. [Google Scholar] [CrossRef]
- Kulmala, M.; Laaksonen, A.; Pirjola, L. Parameterizations for Sulfuric Acid/Water Nucleation Rates. J. Geophys. Res. Atmos. 1998, 103, 8301–8307. [Google Scholar] [CrossRef]
- Vehkamäki, H.; Kulmala, M.; Lehtinen, K.E.J.; Noppel, M. Modelling Binary Homogeneous Nucleation of Water-Sulfuric Acid Vapours: Parameterisation for High Temperature Emissions. Environ. Sci. Technol. 2003, 37, 3392–3398. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Gautam, M.; Gera, D. Modeling Nucleation and Coagulation Modes in the Formation of Particulate Matter inside a Turbulent Exhaust Plume of a Diesel Engine. J. Colloid Interface Sci. 2002, 249, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Gautam, M.; Gera, D. Parametric Studies on the Formation of Diesel Particulate Matter via Nucleation and Coagulation Modes. J. Aerosol Sci. 2002, 33, 1609–1621. [Google Scholar] [CrossRef]
- Yang, H.; Lin, J.; Chan, T. Effect of Fluctuating Aerosol Concentrations on the Aerosol Distributions in a Turbulent Jet. Aerosol Air Qual. Res. 2020, 20, 1629–1639. [Google Scholar] [CrossRef]
- Cheng, C.H.; Cheung, C.S.; Chan, T.L.; Lee, S.C.; Yao, C.D.; Tsang, K.S. Comparison of Emissions of a Direct Injection Diesel Engine Operating on Biodiesel with Emulsified and Fumigated Methanol. Fuel 2008, 87, 1870–1879. [Google Scholar] [CrossRef]
- Ma, H.Y.; Yu, M.Z.; Jin, H.G. A Study of the Evolution of Nanoparticle Dynamics in a Homogeneous Isotropic Turbulence Flow via a DNS-TEMOM Method. J. Hydrodyn. 2020. [Google Scholar] [CrossRef]
- Ning, Z.; Cheung, C.; Lu, Y.; Liu, M.; Hung, W. Experimental and Numerical Study of the Dispersion of Motor Vehicle Pollutants under Idle Condition. Atmos. Environ. 2005, 39, 7880–7893. [Google Scholar] [CrossRef]
- Seipenbusch, M.; Kasper, M.; Yu, M. Interdependence of Particle Number Concentration and PM 2.5 in Highly Polluted Urban Atmospheres; Karlsruhe Institute of Technology: Karlsruhe, Germany, 2003; p. 14. [Google Scholar]
- Von Smoluchowski, M. Mathematical Theory of the Kinetics of the Coagulation of Colloidal Solutions. Z. Phys. Chem. 1917, 92, 129–168. [Google Scholar]
- Müller, H. Zur allgemeinen Theorie der raschen Koagulation. Kolloidchem. Beih. 1928, 27, 223–250. [Google Scholar] [CrossRef]
- Pratsinis, S.E. Simultaneous Nucleation, Condensation, and Coagulation in Aerosol Reactors. J. Colloid Interface Sci. 1988, 124, 416–427. [Google Scholar] [CrossRef]
- Gelbard, F.; Tambour, Y.; Seinfeld, J.H. Sectional Representations for Simulating Aerosol Dynamics. J. Colloid Interface Sci. 1980, 76, 541–556. [Google Scholar] [CrossRef]
- Balthasar, M.; Kraft, M. A Stochastic Approach to Calculate the Particle Size Distribution Function of Soot Particles in Laminar Premixed Flames. Combust. Flame 2003, 133, 289–298. [Google Scholar] [CrossRef]
- Yu, M.; Lin, J.; Chan, T. A New Moment Method for Solving the Coagulation Equation for Particles in Brownian Motion. Aerosol Sci. Technol. 2008, 42, 705–713. [Google Scholar] [CrossRef]
- Kittelson, D.B.; Arnold, M.; Watts, W.F. Review of Diesel Particulate Matter Sampling Methods: Final Report; University of Minnesota: Minneapolis, MN, USA, 1999; Volume 63. [Google Scholar]
- Friedlander, S.K.; Marlow, W.H. Smoke, Dust and Haze: Fundamentals of Aerosol Behavior. New York 1977, 30, 58–59. [Google Scholar] [CrossRef]
- Kulmala, M.; Laaksonen, A. Binary Nucleation of Water–Sulfuric Acid System: Comparison of Classical Theories with Different H 2 SO 4 Saturation Vapor Pressures. J. Chem. Phys. 1990, 93, 696–701. [Google Scholar] [CrossRef]
- Friedlander, S.K. Smoke, Dust, and Haze; Oxford University Press: New York, NY, USA, 2000; Volume 198. [Google Scholar]
- Miller, S.E.; Garrick, S.C. Nanoparticle Coagulation in a Planar Jet. Aerosol Sci. Technol. 2004, 38, 79–89. [Google Scholar] [CrossRef]
- Cunningham, E. On the Velocity of Steady Fall of Spherical Particles through Fluid Medium. Proc. R. Soc. London. Ser. Contain. Pap. Math. Phys. Character 1910, 83, 357–365. [Google Scholar]
- Yue, D.; Hu, M.; Wu, Z.; Wang, Z.; Guo, S.; Wehner, B.; Nowak, A.; Achtert, P.; Wiedensohler, A.; Jung, J.; et al. Characteristics of Aerosol Size Distributions and New Particle Formation in the Summer in Beijing. J. Geophys. Res. Atmos. 2009, 114, D00G12. [Google Scholar] [CrossRef]
- Binder, K.; Stauffer, D. Statistical Theory of Nucleation, Condensation and Coagulation. Adv. Phys. 1976, 25, 343–396. [Google Scholar] [CrossRef]
| Mode | Nucleation | Aitken | Accumulation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ni | GMD | δ | Ni | GMD | δ | Ni | GMD | δ | |
| 2004 nonpolluted | 16 | 15.5 | 1.80 | 27 | 60.4 | 1.87 | 3 | 200 | 1.70 |
| 2004 polluted | 14 | 17.0 | 1.90 | 19 | 80.9 | 1.93 | 7 | 245 | 1.58 |
| 2005 nonpolluted | 7 | 14.4 | 2.00 | 20 | 58.9 | 2.00 | 5 | 174 | 1.71 |
| 2005 polluted | 6 | 18.0 | 2.00 | 17 | 75.8 | 1.99 | 8 | 251 | 1.59 |
| 2006 nonpolluted | 9 | 14.3 | 1.92 | 15 | 61.8 | 2.00 | 2 | 225 | 1.64 |
| 2006 polluted | 5 | 18.7 | 1.89 | 15 | 84.6 | 1.96 | 6 | 246 | 1.54 |
| Condition | Nucleation | Aitken | Accumulation | Sum | Non-Dimensional | |
|---|---|---|---|---|---|---|
| Nonpolluted 2004 | ||||||
| Polluted 2004 | ||||||
| Nonpolluted 2005 | ||||||
| Polluted 2005 | ||||||
| Nonpolluted 2006 | ||||||
| Polluted 2006 | ||||||
| Nonpolluted 2004 | ||||||
| Polluted 2004 | ||||||
| Nonpolluted 2005 | ||||||
| Polluted 2005 | ||||||
| Nonpolluted 2006 | ||||||
| Polluted 2006 | ||||||
| Nonpolluted 2004 | ||||||
| Polluted 2004 | ||||||
| Nonpolluted 2005 | ||||||
| Polluted 2005 | ||||||
| Nonpolluted 2006 | ||||||
| Polluted 2006 | ||||||
| Nonpolluted 2004 | ||||||
| Polluted 2004 | ||||||
| Nonpolluted 2005 | ||||||
| Polluted 2005 | ||||||
| Nonpolluted 2006 | ||||||
| Polluted 2006 |
| Boundary | Temperature (K) | Velocity (m/s) | Boundary Type | Boundary Value | ||||
|---|---|---|---|---|---|---|---|---|
| S.1 | S.2 | S.3 | S.4 | S.5 | ||||
| inlet | 400 | 4.8 | Fixed value | 1 | 1000 | 0 | 0 | 0 |
| inlet wall | 350 | Fixed flux | 0 | 0 | 0 | 0 | 0 | |
| right | 300 | Fixed value | 0.3 | 0 | ||||
| up (down) | 300 | Fixed value | 0.3 | 0 | ||||
| left | 300 | Fixed value | 0.3 | 0 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, C.; Liu, Y.; Wu, T.; Yu, M. Simulation of Aerosol Evolution within Background Pollution for Nucleated Vehicle Exhaust via TEMOM. Appl. Sci. 2021, 11, 4552. https://doi.org/10.3390/app11104552
Tu C, Liu Y, Wu T, Yu M. Simulation of Aerosol Evolution within Background Pollution for Nucleated Vehicle Exhaust via TEMOM. Applied Sciences. 2021; 11(10):4552. https://doi.org/10.3390/app11104552
Chicago/Turabian StyleTu, Can, Yueyan Liu, Taiquan Wu, and Mingzhou Yu. 2021. "Simulation of Aerosol Evolution within Background Pollution for Nucleated Vehicle Exhaust via TEMOM" Applied Sciences 11, no. 10: 4552. https://doi.org/10.3390/app11104552
APA StyleTu, C., Liu, Y., Wu, T., & Yu, M. (2021). Simulation of Aerosol Evolution within Background Pollution for Nucleated Vehicle Exhaust via TEMOM. Applied Sciences, 11(10), 4552. https://doi.org/10.3390/app11104552







