1. Introduction
Cryogenic fracturing [
1,
2,
3,
4] is a waterless reservoir stimulation technique which uses cryogenic fluids such as liquid carbon dioxide and liquid nitrogen for fracturing purpose. The technique takes advantage of the effect of thermal shock which presents as a sharp thermal gradient introduced by the cryogen on the rocks. It could enhance the fractures initiation and propagation in the rock stratum, viz. enhancing fracturing. Recently, as the disadvantages of the conventional hydraulic fracturing technique (heavy water consumption, soil pollution, formation damage, etc.) are widely recognized, cryogenic fracturing is gaining an increasing attention in the field of petroleum engineering.
Quite a few researches on cryogenic fracturing [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20] have been carried out by scholars and engineers, in which significant efforts are devoted to investigating the feasibility of cryogenic fracturing for engineering application and figuring out the damage mechanism of cryogenic fracturing. These works are largely comprised of experimental studies in the laboratory. The porous structure of the reservoir stratum makes the fracturing process complicated and it enhances the difficulty of investigation. Nuclear magnetic resonance (NMR) [
7,
8], scanning electron microscope (SEM) [
9,
10], computed tomography (CT) [
11,
12] are widely used to capture the micro-structural changes in the specimens. Fractures are characterized with the aid of acoustic transmission, pressure-decay measurements [
12], which enables further quantitative analysis. A series of cryogenic quenching tests (into liquid nitrogen) of specimens [
13,
14] were conducted and the evolution of permeability (pore distribution) [
15,
16] and mechanical properties (elastic modulus, tensile/compressive strength) [
9,
17,
18] were studied. It was found that liquid nitrogen could induce damage to the specimens more efficiently by comparing the methods such as air cooling and water cooling [
10]. The strength and brittleness of the shale could be reduced through the treatment of liquid nitrogen cooling, and it could help in decreasing the initiation pressure of reservoir stimulation [
19]. It was also revealed that a repeated cooling–heating treatment could be more favorable in fracturing in comparison to a single cooling treatment [
4,
7,
8]. Various factors, such as the cooling rate, initial temperature of specimens, the water saturation of specimens, the period of freeze thaw cycle [
4], are found to affect the fracturing effect. Besides the experimental works, attempts were made to introduce numerical methods into the research on cryogenic fracturing in recent studies. Alqahtani [
21] simulated the cryogenic fracturing process in unconventional reservoirs and evaluated the effect of thermal stresses on fracture development. Yao and co-workers [
20] simulated the cryogenic fracturing process in Niobrara shale sample with the modified TOUGH2-EGS. A numerical scheme was developed by Li and co-workers [
14] which could simulate the temperature history and the rewetting front positions in a laboratory quenching test. Wu and co-workers [
9] numerically analyzed the influences of the cooling rate on rock degradation by conducting a three dimensional simulation using ABAQUS.
Most of the previous researches are focusing on the mechanical changes in the reservoir stratum caused by cryogenic stimulation (initiation and propagation of fracture, rock deformation, evolution of permeability etc.). Nevertheless, only few attentions are aroused to the heat and mass transfer process during cryogenic fracturing. The heat and mass transfer during fracturing would significantly influence the temperature distribution in the reservoir, further affecting the occurrence of thermal shock. Consequently, the heat and mass transfer process would significantly influence the cryogenic fracturing process which should be carefully considered. In this work, displacement flow of the residual water in the stratum by the injected cryogenic fracturing fluid is considered. In this process, the residual water in the reservoir is constantly cooled down by the cryogenic fracturing fluid while flowing in the stratum pore channel, which further leads the residual water to freeze. The newly-formed ice is supposed to occupy the pore channel partly or completely, affecting the subsequent state of flow. In this case, the cold energy transferred in the stratum during fracturing would also be influenced. Additionally, large-scale ice blockage can result in local pressure rise in the reservoir, which could enhance the initiation and propagation of fractures. According to the above analysis, it is noted that the displacement flow of residual water by the cryogenic fracturing fluid would have profound influence on the fracturing process. The process is quite complicated in practice as the following physical processes are involved and worked together including miscible/immiscible displacement flow (depends on the type of fracturing fluid (liquid carbon dioxide, liquid nitrogen)), liquid–gas phase transition (vaporization of the fracturing fluid), liquid–solid phase transition (solidification of residual water), pore-scale flow. It is indicated by the literature survey that nearly no research has focused on such a displacement process by far.
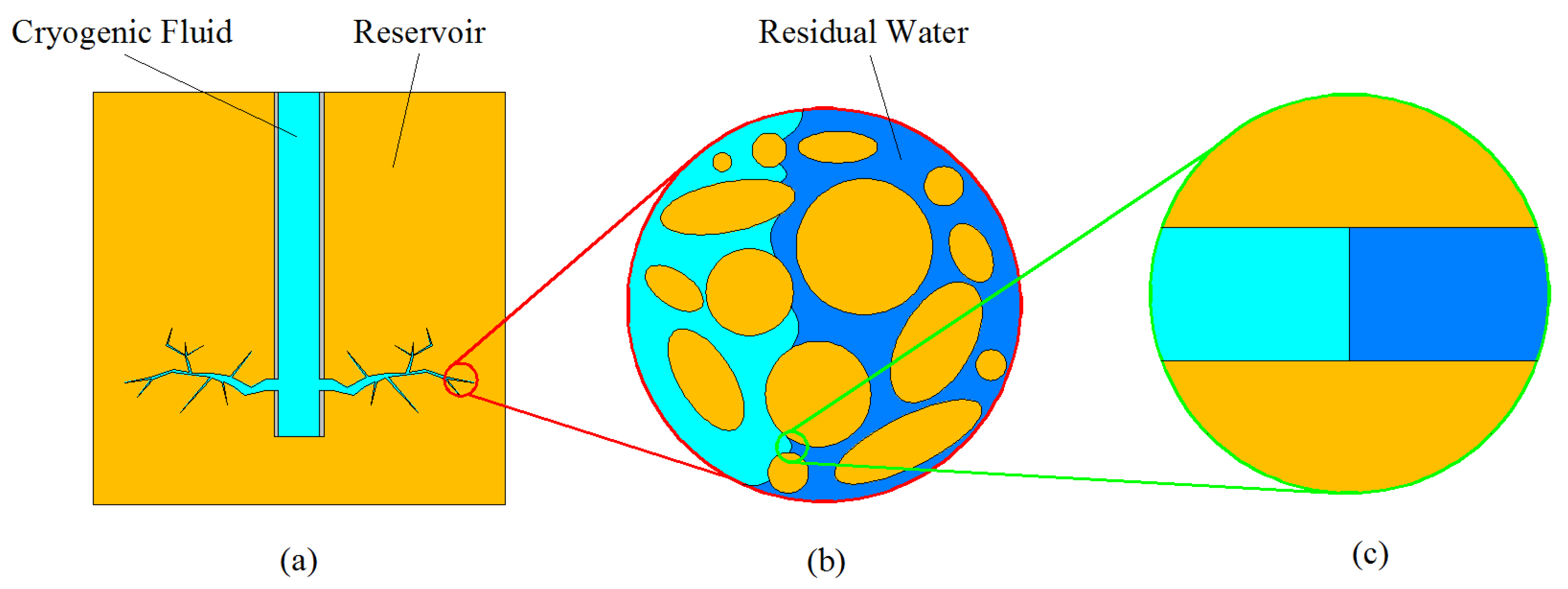
Accordingly, a primary study on this freezing-coupling displacement process is carried out which introduces a newly developed numerical model to simulate the freezing process during the displacement flow. Due to the complexity of the actual process, simplifications as illustrated in
Figure 1 were made as follows.
- (1)
A 2D model is developed. The main purpose of this research is to qualitatively reflect the freezing process during the displacement process and the influence of the formed ice on the flow status in a numerical method. The 2D method is considered sufficient for achieving this goal. Moreover, compared with 3D simulation, 2D simulation could significantly reduce the time cost of calculation.
- (2)
The effect of porous structure is neglected and immiscible displacement flow in a single passage is considered. The “single passage” simplification is made referring to the research works on pure displacement process [
22,
23,
24,
25,
26,
27,
28], in which the essential feature of the displacement process could be reflected. The “immiscible displacement” simplification comes from the fact that as a candidate of the cryogenic fracturing fluid, liquid nitrogen (LN
2) is hardly soluble in water.
- (3)
The influences due to vaporizing of the fracturing fluid and pore scale are neglected. This simplification is made in consideration of the complexity of the vaporization process and the capillary effect. Research works on freezing soil [
29] were also referred to, where the influence of vapor component is neglected.
Based on the above simplifications, the major effort is spent on simulating the growth of ice phase and the influence of ice phase on the flow status during the displacement process (
Figure 1c).
Research on this process is supposed to provide a method to predict the ice blockage pattern in the reservoir properly, which is necessary for a further prediction of the fracture development, pressure distribution, permeability evolution, thermal shock position, and other factors during fracturing. An improvement in the fracturing efficiency can then be expected.
2. Mathematical Formulations
During the displacement flow process along with the growth of ice phase as shown in
Figure 1c, the phase interaction can be described as: During the displacement, the cryogenic fluid would push the water towards the forward wall boundaries, which can be implemented by simulating the fluid dynamics based on the N-S governing equations. Furthermore, the interface and the development of each fluid phase can be dealt using the volume of fluid (VOF) method [
30]. Meanwhile, according to the cooling effect induced by the cryogenic fluid, the water near the wall would freeze. The process of cooling and freezing can be simulated by the energy conservation equation and phase-change model [
31,
32,
33,
34,
35]. On the other hand, the newly generated ice phase should attach to the wall and block the development of multiphase fluid. In order to simulate the above process, a transient CFD model for 2D computational domain on the basis of the VOF method is proposed to simulate the process of multiphase flow (water and cryogenic fluid) involving liquid–solid phase transition (water–ice). Three phases including the primary phase (water), the secondary phase (ice), and the third phase (cryogenic fluid) are defined. Therein, the mass transfers among the multiphase through the surface are neglected in this method. The momentum, continuity, energy and VOF equations in the governing equations are solved. This model is also capable of handling the conduction-controlled solidification problem (the classical Stefan problem) by reducing the number of defined phases to 2 (water and ice) and eliminating the momentum equation. In modeling the phase change (forming and growth of ice), the Lee model [
35] is used and the nucleation rate of the classical nucleation theory (CNT) [
36,
37,
38,
39] is innovatively introduced in the calculation of mass transfer rate between water and ice phase. As for the interaction between the fluid and the ice phase [
40,
41,
42], The sponge-layer absorbing technique [
41], by which the ice phase can be handled to be similar to solid and then can perform the blocking effect on the other phases of water and cryogenic fluid, is adopted to reflect the influence of the newly formed ice on the flow status (the fluid variables, such as velocity, pressure).
2.1. Governing Equations
The volume fraction (α) represents the composition of the control cell. Therein, αi = 1 suggests the cell is fully occupied by the i phase, αi = 0 indicates there are no i phase in the cell and 0 < αi < 1 means that only part of the cell contains i phase. The heat transfer process during cryogenic fracturing includes three phases which are the water, ice, and cryogenic fluid, respectively.
For the tracking of interface (water–cryogenic fluid, water–ice), the VOF method in Euler–Euler multiphase system is adopted, in which the interface is tracked by solving volume fraction equations. In the present work, the volume fraction of the
ith phase can be calculated as [
43,
44]:
where
Sαi is the source term of the ice phase, which is induced by the mass transfer during ice formation, and the details would be discussed in the following section. In the computational domain with
n phases, the volume fractions of these phases can be referred to the following constraint:
Therein, the fluid density in the cell can be expressed according to the volume fractions of the
n phases:
The mass conservation equations of each phase are solved, and the continuity equation of the
ith phase could be written as:
where
and
represent the fluid density and the fluid velocity of the
ith phase, respectively. Furthermore,
Smi represents the mass source which equals to zero at the flow interface (water–cryogenic fluid) and equals to mass transfer rate at the water–ice interface. The formulation of the mass source is presented in
Section 2.2.
In this case, the momentum equations are applied to model the flow behavior of various phases, which can be written as following for the
ith phase.
where
p is pressure,
Kji is interfacial drag coefficient,
Sui is the source term of the momentum induced by the mass transfer which would be given in the next section in details. Furthermore,
is the tensor of the stress-strain and it is described as:
where
is unit tensor. In all the cases described in this work, Reynolds number is not allowed to exceed the critical value as about 2000, suggesting that a laminar flow model is used for the simulation. The energy conservation equation of the
ith phase is expressed as:
where
H represents specific enthalpy,
k represents the thermal conductivity,
T represents the fluid temperature, and
SHi represents the heat source due to mass transfer which would be described in the next section.
As a result, the governing equations mentioned above constitute a time-dependent and space-dependent model for the physical problems of the temperature distribution, the fluid flow and the phase change of solid–liquid. The source terms in the governing equations are deduced by introducing the nucleation rate of classical nucleation theory (CNT) into the Lee model, which would be presented in detail in the next section.
2.2. Freezing Model
The mass transfer rate in the freezing process is derived as following. Moreover, the sponge-layer absorbing technique, which is used to handle the fluid variables of ice phase, is introduced.
2.2.1. Model of Phase Change
The source terms owing to the phase change in the VOF, mass, momentum, and energy equations are handled using Lee model (Lee, 1979) [
35,
45,
46], which is a physical based model for phase change and it can be employed in conjunction with the VOF multiphase model.
The source term in the mass conservation equation can be defined from Lee’s work [
35]. If
Ts >
Tsat (ice dissolving into water), the rate of mass transfer for ice phase can be expressed as:
For the current solidification process, the process of ice dissolving into water can be neglected. Thus,
can be treated as zero in this work. Furthermore, if
Tl <
Tsat (water freezing into ice), the rate of mass transfer for water phase can be written as:
where
and
are the mass transfer rates from ice to water and from water to ice, respectively, whose units are kg/(m
3s). The
λ is the time relaxation parameter of phase change with the unit of 1/s, which would be presented in detail later,
α represents the volume fraction,
ρ represents the density (kg m
−3), and
T represents the temperature (K). The subscript “
s” and “
l” represent solid (ice) and liquid (water).
Tsat is the freezing point and it is 273.15 K for phase change between water and ice. Therefore, the source terms of the governing Equation (4) are calculated as:
Then, the source term of the volume fraction for each phase is described as:
Furthermore, the momentum source can be written as:
where
and
are the fluid velocities of water and ice. Therein, the velocity of ice phase is treated as zero. Finally, the source term in the energy equation can be calculated as:
where
HL represents the latent heat induced by phase change between water and ice.
2.2.2. Calculation of the Mass Transfer Rate
Nucleation rate, a concept of the classical nucleation theory (CNT) which can reflect the icing capability of water, is introduced into the calculation of mass transfer rate during freezing in the present model. The parameter (
J) provides the number of newly formed nuclei per cubic meter and per second, which is mathematically described as:
where
T represents the water temperature,
h represents Planck constant which equals to 6.626 × 10
−34 J·s,
kB represents Boltzmann constant which equals to 1.380 × 10
−23 J·K
−1, Δ
g represents the activation energy of water molecules passing through water and ice interface which is around 4.000 × 10
−20 J,
nL represents the number of water molecule in a water volume which is about 3.34 × 10
28 m
−3. Furthermore, Δ
G* reflects the variation of Gibbs function for the phase change between ice and water. Gibbs function can be described as [
47,
48]:
where
γiw is the superficial free energy of the water–ice interface,
HLv is latent heat per volume. Moreover,
αey is the shape coefficient of nucleation and can be written as:
where
θ is the contact angle ranging from 0° to 180°. It is shown by Equation (14) to Equation (16) that the influence of the contact angle is considered in calculating the nucleation rate. As the other parameters are constant, the nucleation rate would gradually increase with the contact angle decreasing, which quantitatively reflects the change during nucleation from the uniform state to the inhomogeneous state. To reflect the effects of the contact angle on the variation of the mass transfer rate, a mathematical relationship between the relaxation time parameter
λ in Equation (9) and the nucleation rate
J is established, which is presented as:
where
Vl is the volume of water in the cell. Then, Equation (9) can be rewritten as:
Equation (17) is established on a simple idea that the nucleation rate and the volume of the water in which nucleation occurs would both influence the freezing rate. With the treatment shown by Equation (17), difference between homogeneous nucleation in the fluid domain (without solid surface) and heterogeneous nucleation on the solid wall can be reflected by the mass transfer rate obtained via Equation (18). Furthermore, the influence of the newly formed ice phase (treated as solid surface by specifying contact angle value for it) on the freezing process is also possible to be simulated using the newly proposed model, which is hard to be considered in previous models.
2.2.3. Treatment of Variables in the Cells with Ice Phase
The fluid variables (velocity, pressure) of ice cell are treated based on the theory of the sponge-layer absorbing technique [
41,
49,
50]. For the cell including ice phase, the modified fluid velocities and pressure could be expressed as:
where
uC,
vC, and
pC are the calculated x-velocity, y-velocity and pressure.
uM,
vM, and
pM are the corrected values of x-, y-velocities and pressure. Furthermore,
αs is the volume fraction of ice phase. If α
s equals to 1, it suggests that the cell is fully occupied by the ice phase. In this case, the velocity and pressure would be zero.
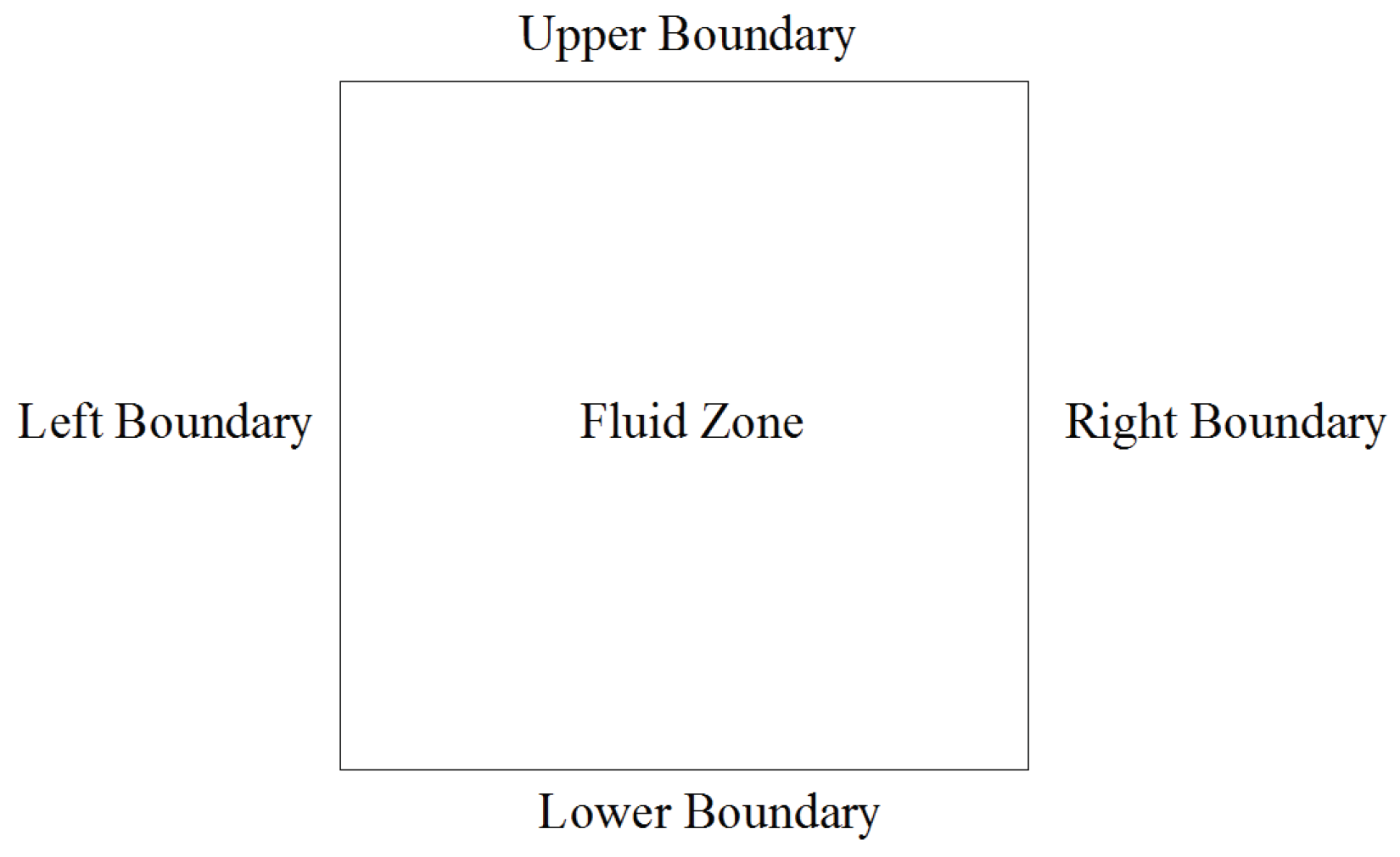
2.3. The Setting of the Boundary Conditions in the Computational Domain
All the numerical studies are carried out in a two dimensional computational domain of 0.01 m × 0.01 m as shown in
Figure 2.
The details of the boundary conditions applied in the case study are as follows:
(1) Velocity-inlet boundary
(2) Outflow boundary
The flux of all the variables at the outflow boundary is set as zero, which means the state in the fluid zone are not affected by the conditions at the outflow boundary
(3) Wall boundary with constant temperature
(4) Adiabatic wall boundary
The heat flux passing through this face is set as zero:
Different combinations of the above four boundary conditions are adopted in different cases, which are noted in the subsequent sections.
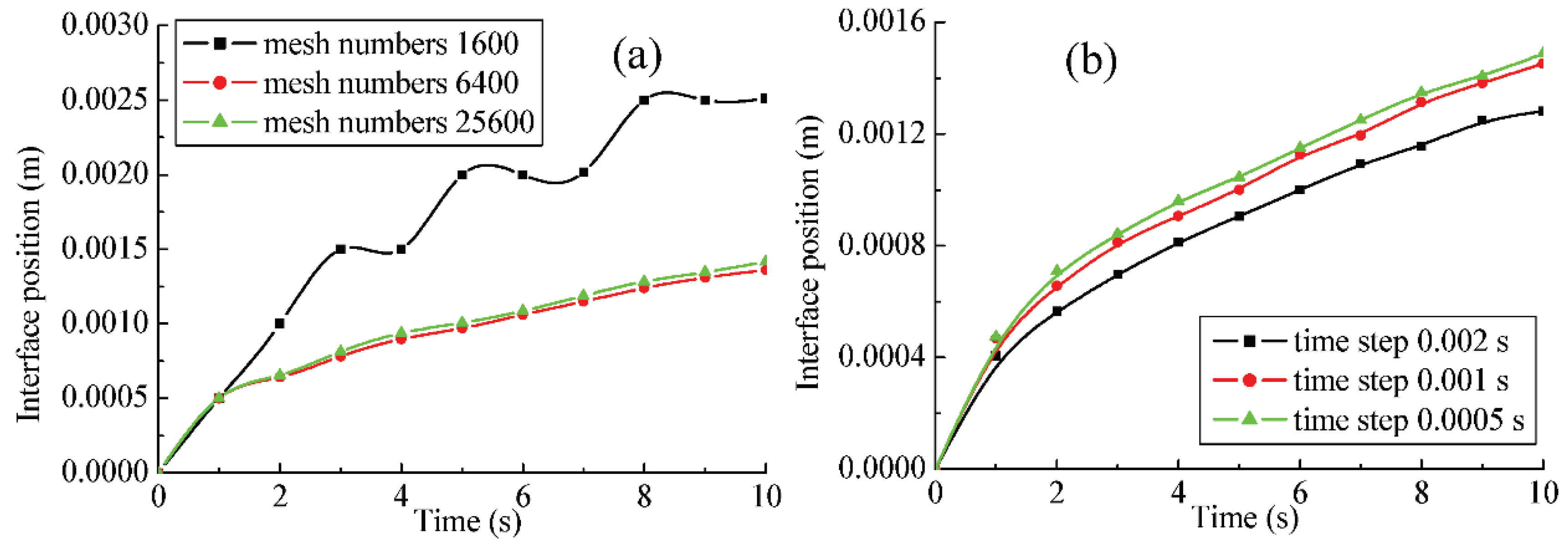
2.4. Solution Scheme of Numerical Model
On the basis of the second order upwind scheme, the governing equations of the mass, momentum, and temperature could be discretized. The SIMPLEC algorithm is selected as the solution method. Furthermore, the first order implicit solver is applied for time advancing in the unsteady simulation. The convergence criterions of all the variables are setting as 10
−6. The above equations are solved in Fluent 6.3.26 CFD software. Finally, the calculation of the source terms in
Section 2.2.1,
Section 2.2.2 and
Section 2.2.3 are carried out by defining the user defined function (UDF) in Fluent.
4. Conclusions
In this paper, a 2D numerical model considering the nucleation characteristics based on VOF method and Lee model is developed to predict the propagation of multiphase flow accompanied by solidification phase change during cryogenic fracturing. The formation and propagation of ice on the wall boundary along with multiphase flow around ice was studied. Conclusions are drawn as follows:
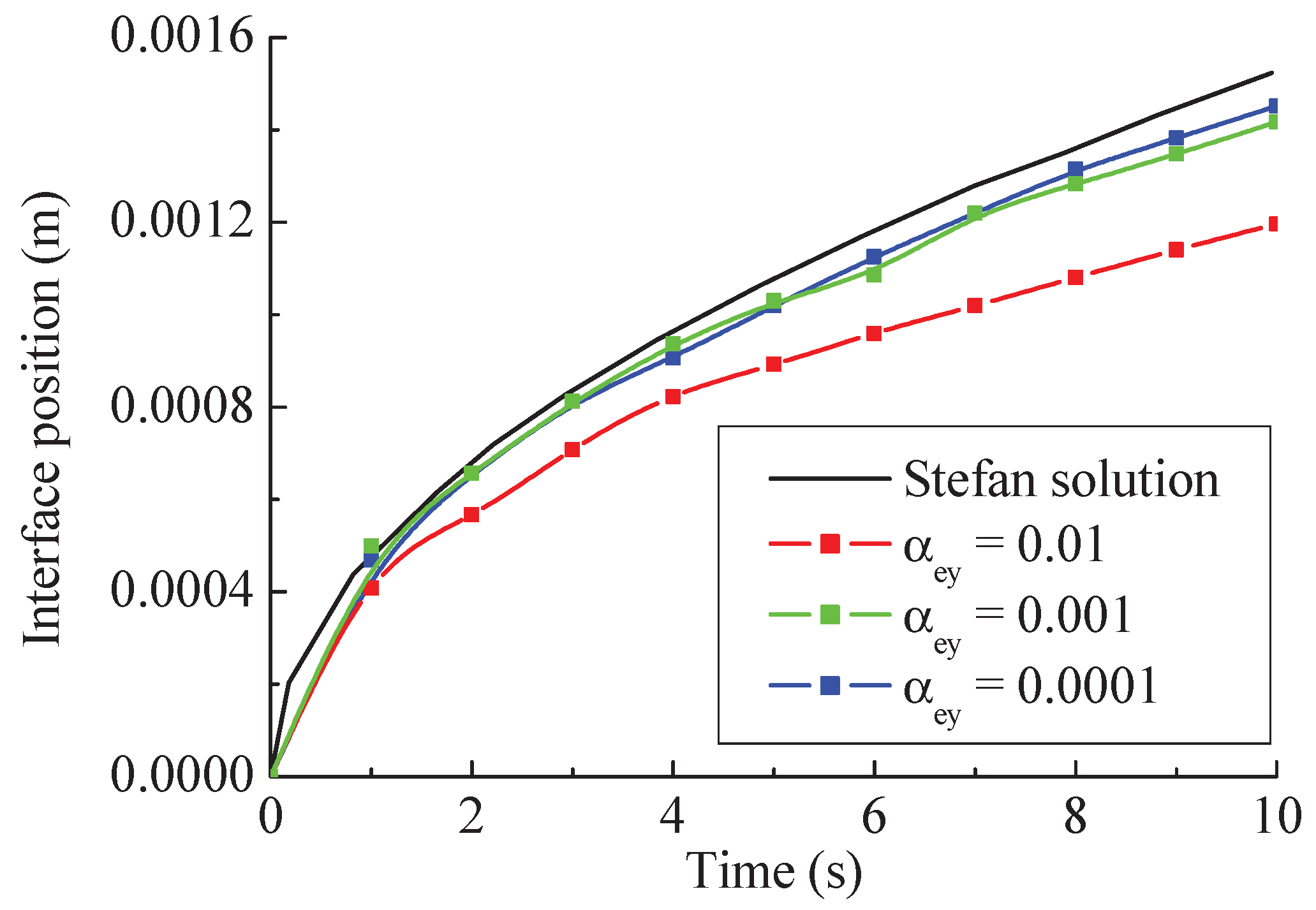
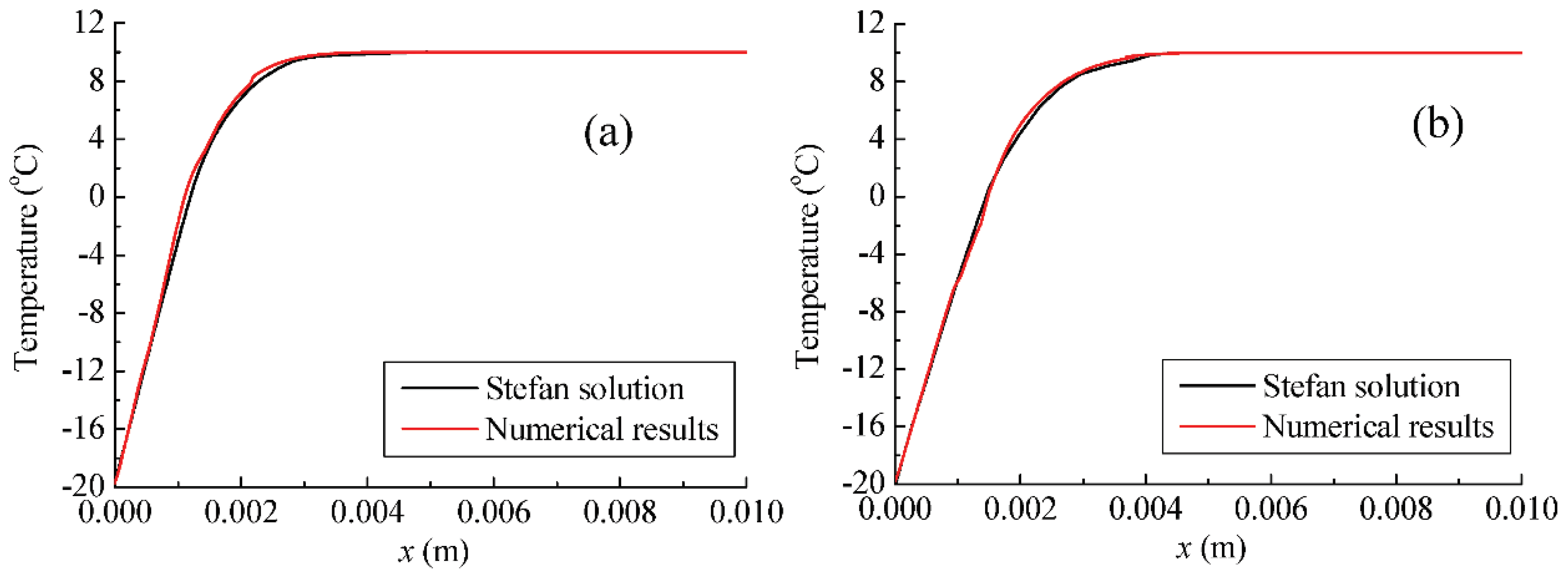
- (1)
For the classical Stefan problem, the max relative difference between the convergent numerical results and the theoretical solutions are about 6.58%, which can validate the feasibility and accuracy of the newly proposed method.
- (2)
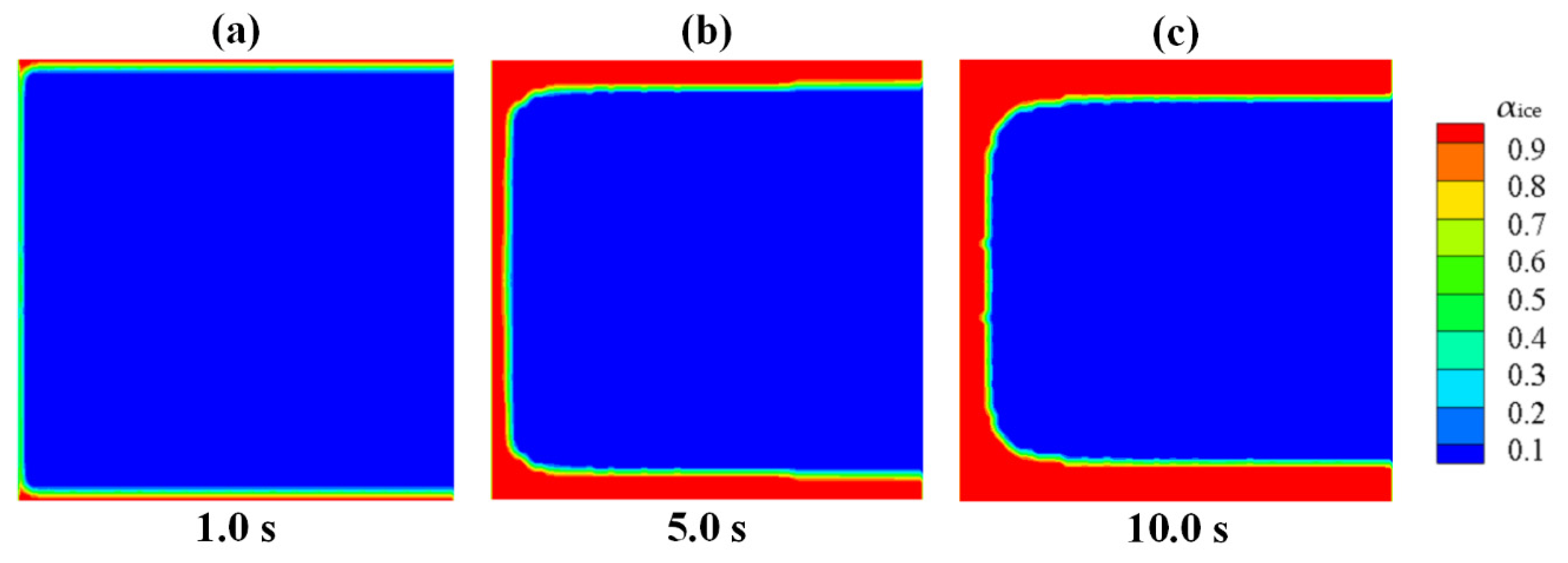
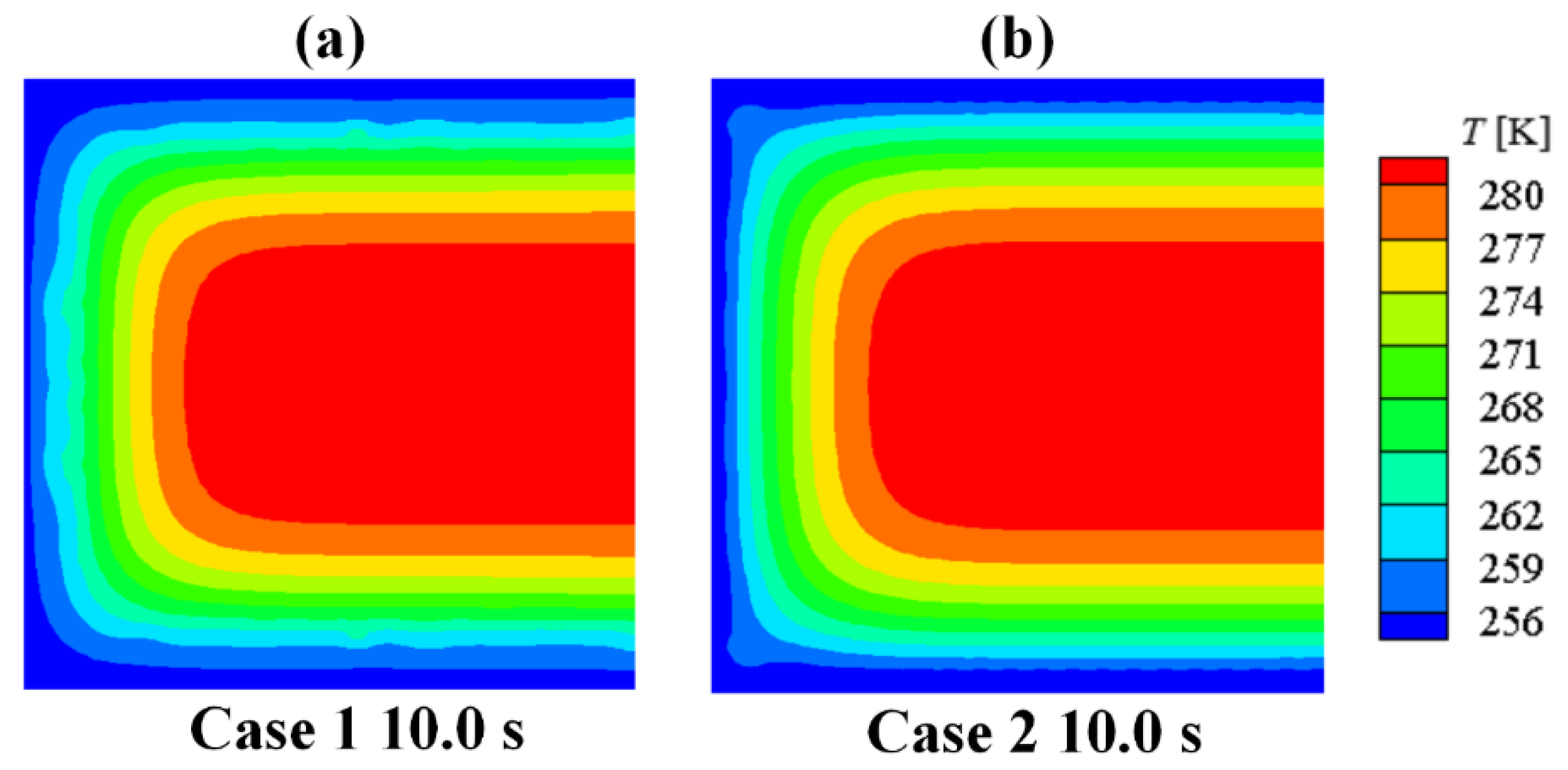
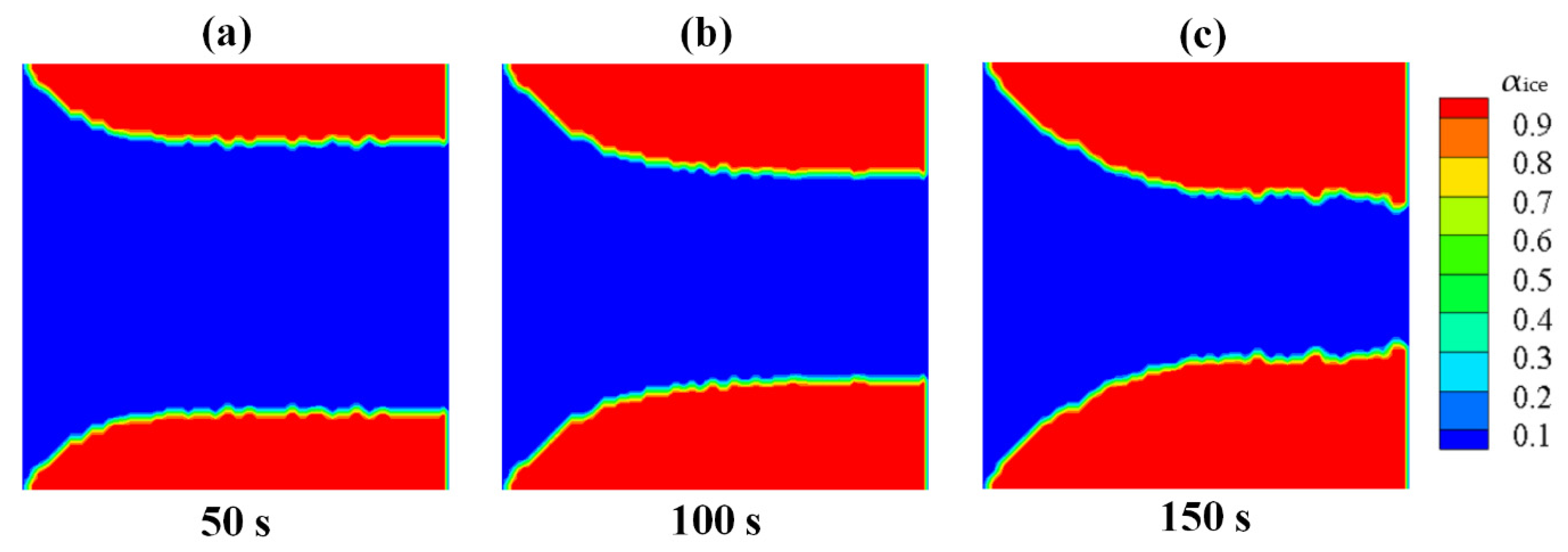
Through the simulation on the process of the ice generation and growth in Case 1, the improved Lee model in the proposed numerical method could consider the effect of low-temperature boundary on the freezing. It also could well reflect the different characteristic of the homogeneous nucleation and the heterogeneous nucleation on various kinds of boundaries.
- (3)
By comparing the numerical solutions of Case 1 with that of Case 2, the concept of shape coefficient of nucleation in the proposed numerical method has the ability to distinguish the phase-change processes between the ice generation on the wall and the ice growth on the frozen ice, which could restore the practical situation.
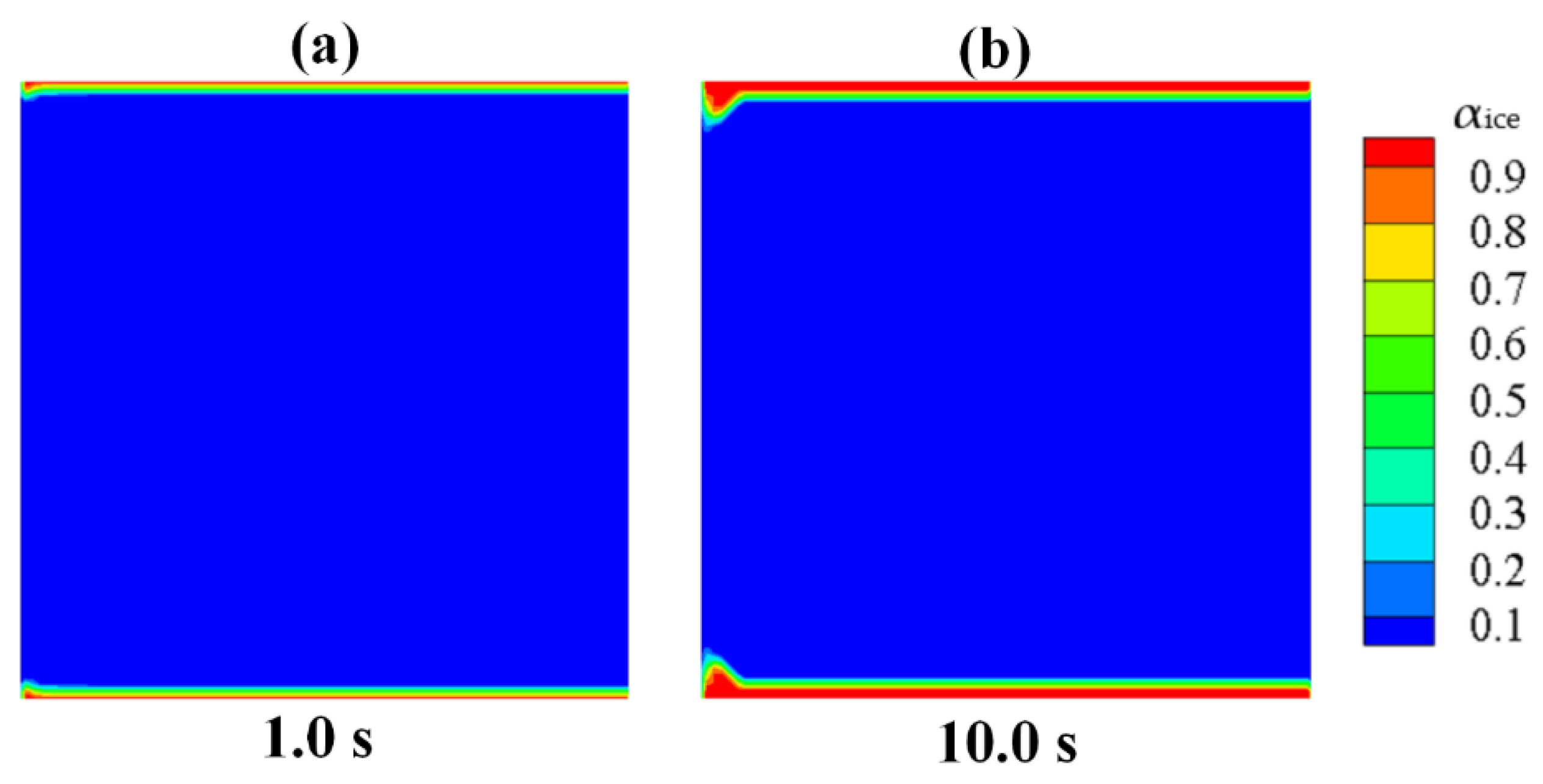
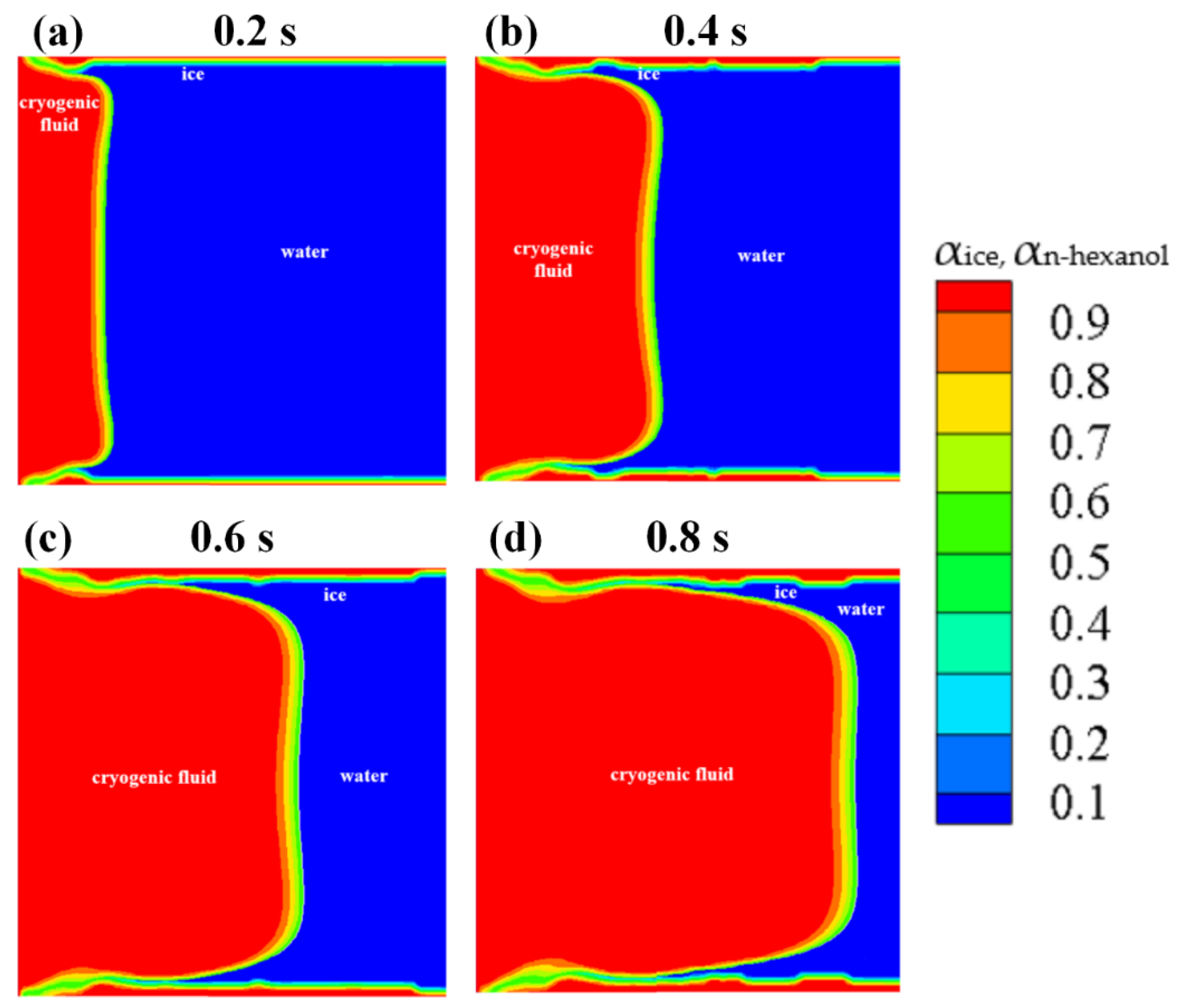
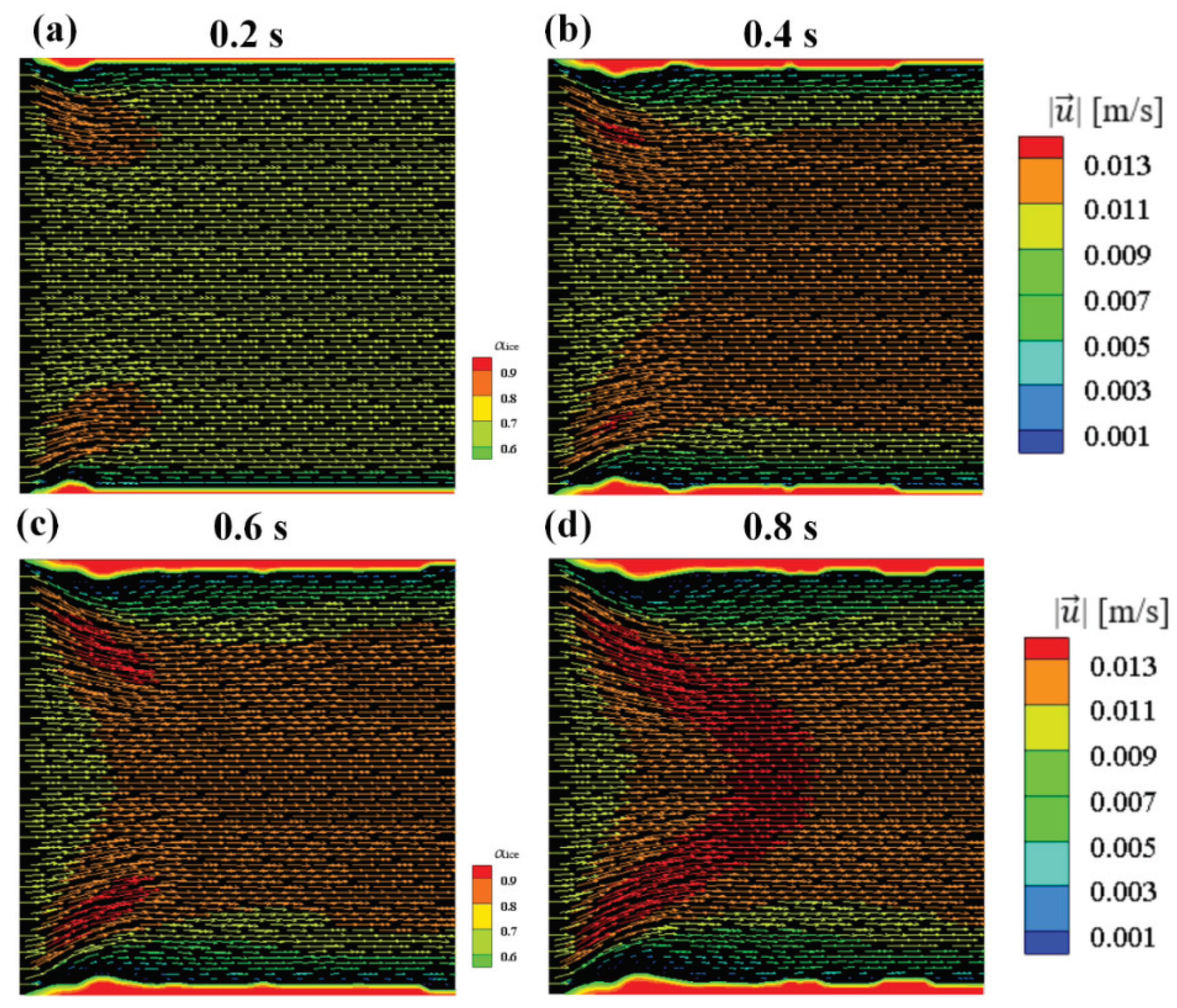
- (4)
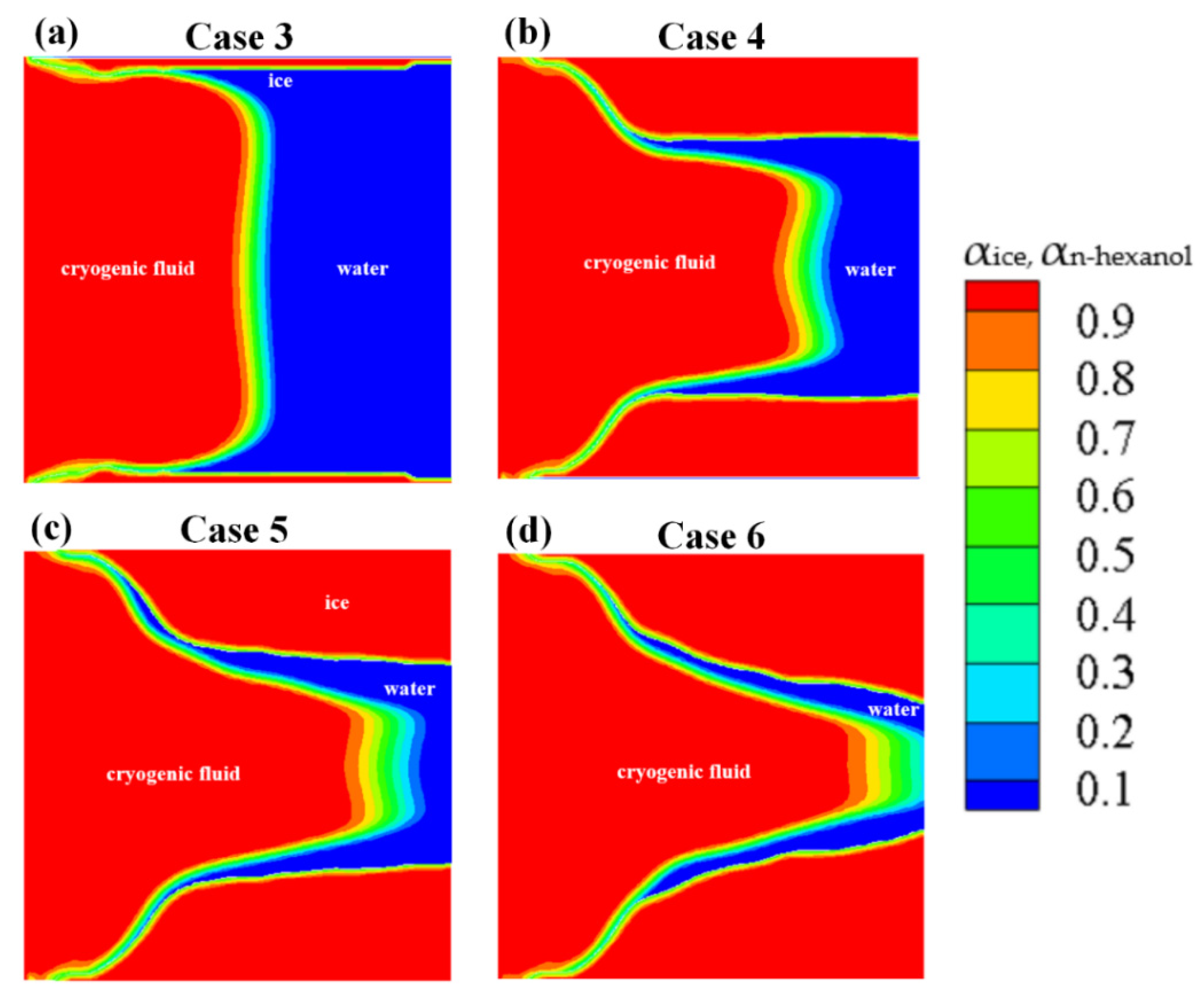
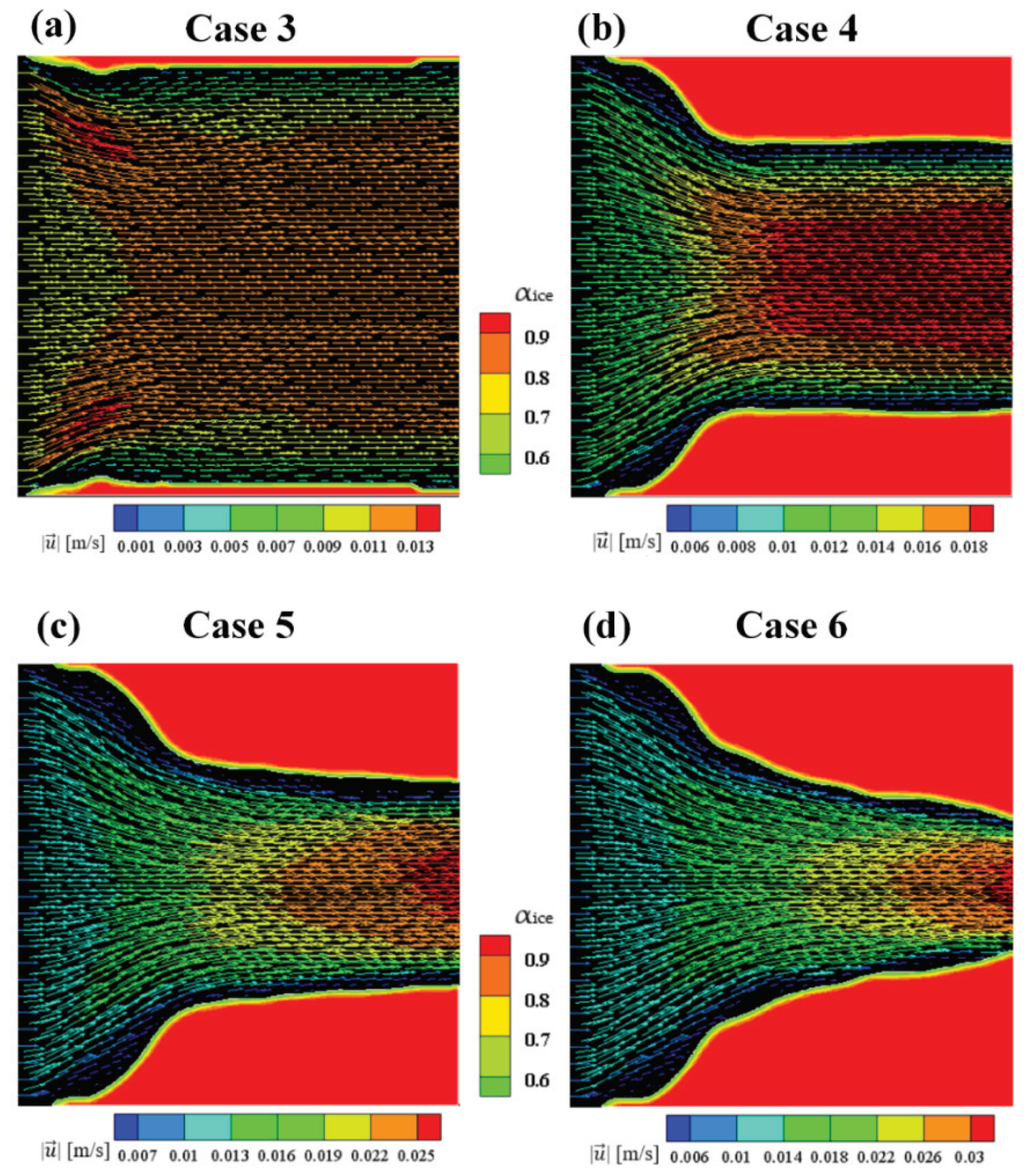
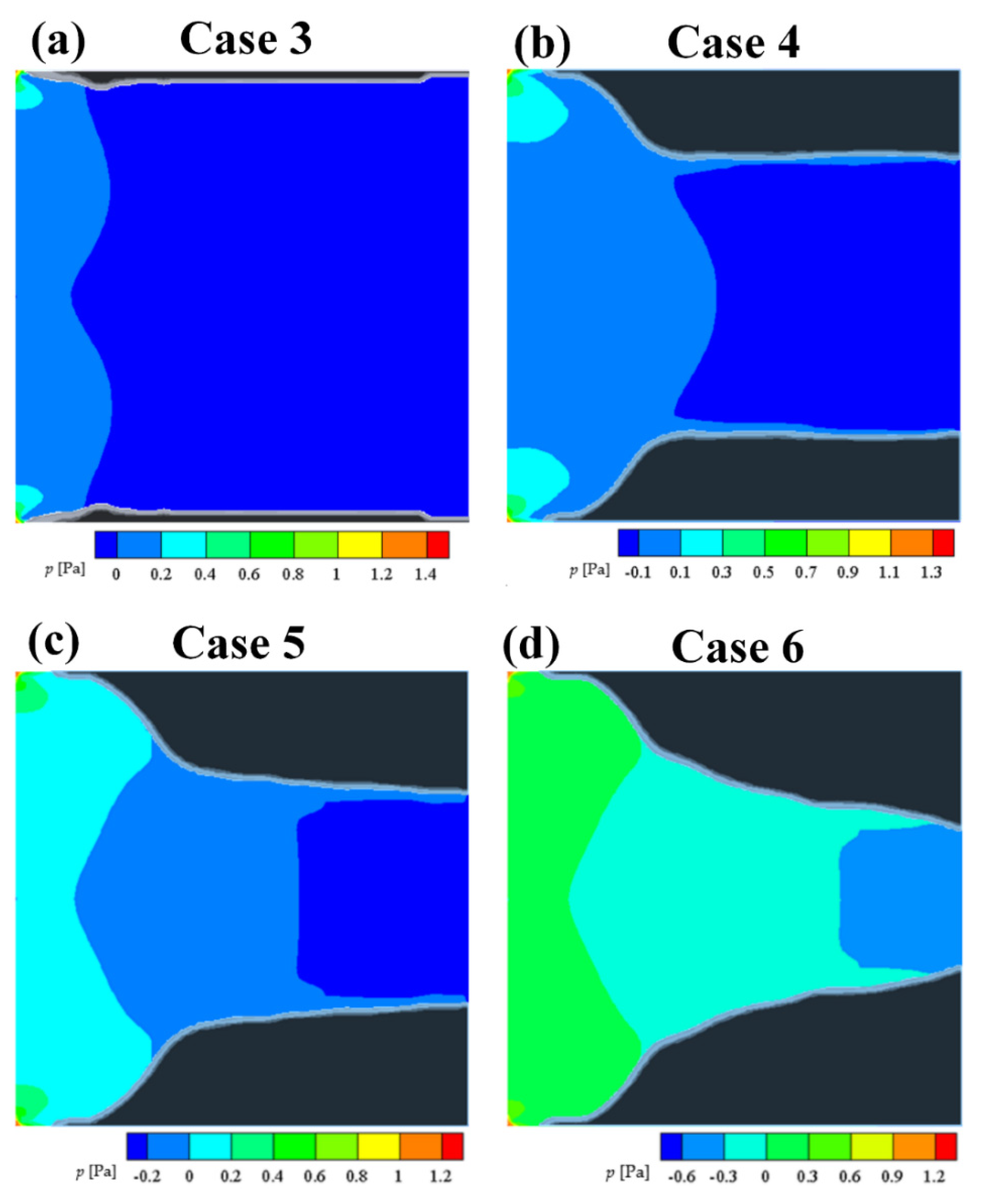
The numerical results of Case 3~6 indicate that the sponge-layer absorbing technique is capable of handling the frozen ice and simulating its blocking effect on fluid flow.
In future researches, the model experiments should be carried out to further validate the newly proposed numerical method. Furthermore, the method can be developed further on the following aspects: Considering the effects of temperature and pressure on the thermo-physical properties (thermal conductivity, density, viscosity, specific heat capacity, etc.) to meet the practical engineering requirements; extending the present method to three dimensions for more extensive application about the practical engineering problems; further performing the complete uncertainty analysis based on the experimental results; and further introducing more interactive effects among the multiphase fluids (such as mass transfer).