Prediction of Diabetic Nephropathy from the Relationship between Fatigue, Sleep and Quality of Life
Abstract
1. Introduction
2. Literature Review
2.1. Diabetes-Related Fatigue and Sleep Disturbance
2.2. The Presence of the DM Complication Nephropathy
2.3. Feature Selection
2.3.1. Filter Methods
2.3.2. Wrapper Methods
2.3.3. Embedded Methods
3. Methodology
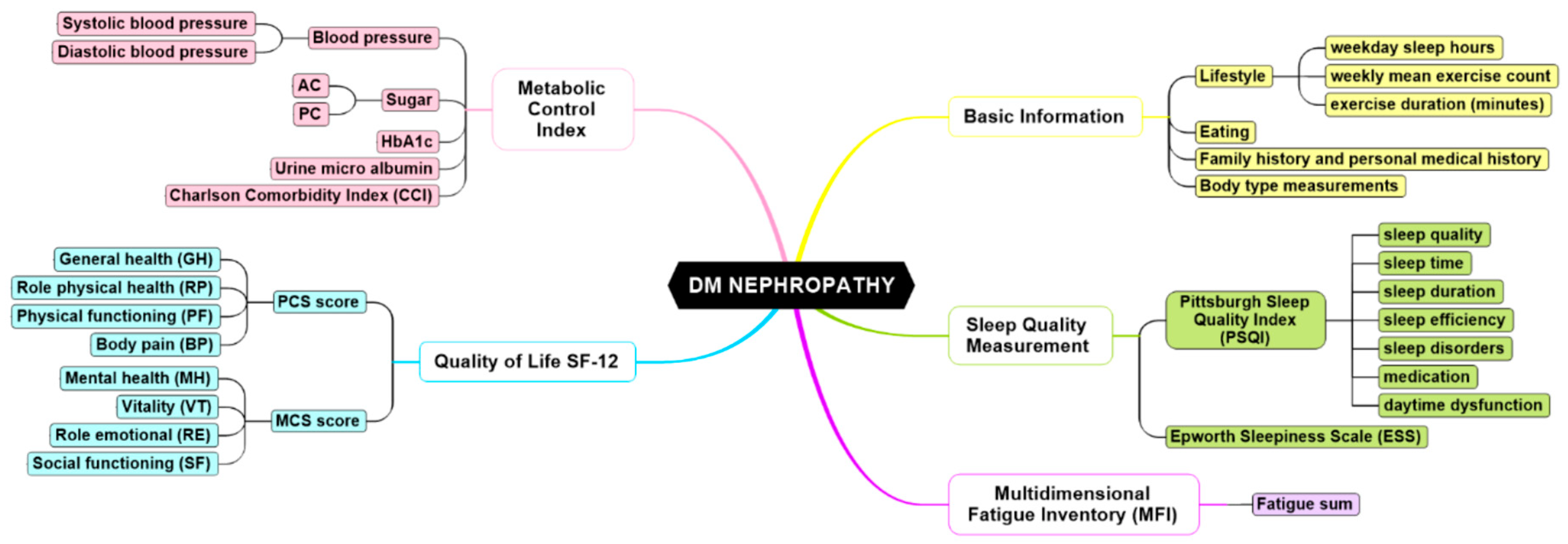
3.1. Data Collection
3.2. Instruments
4. Experiment Results
4.1. Statistical Analysis
4.2. Results of Statistical Analysis
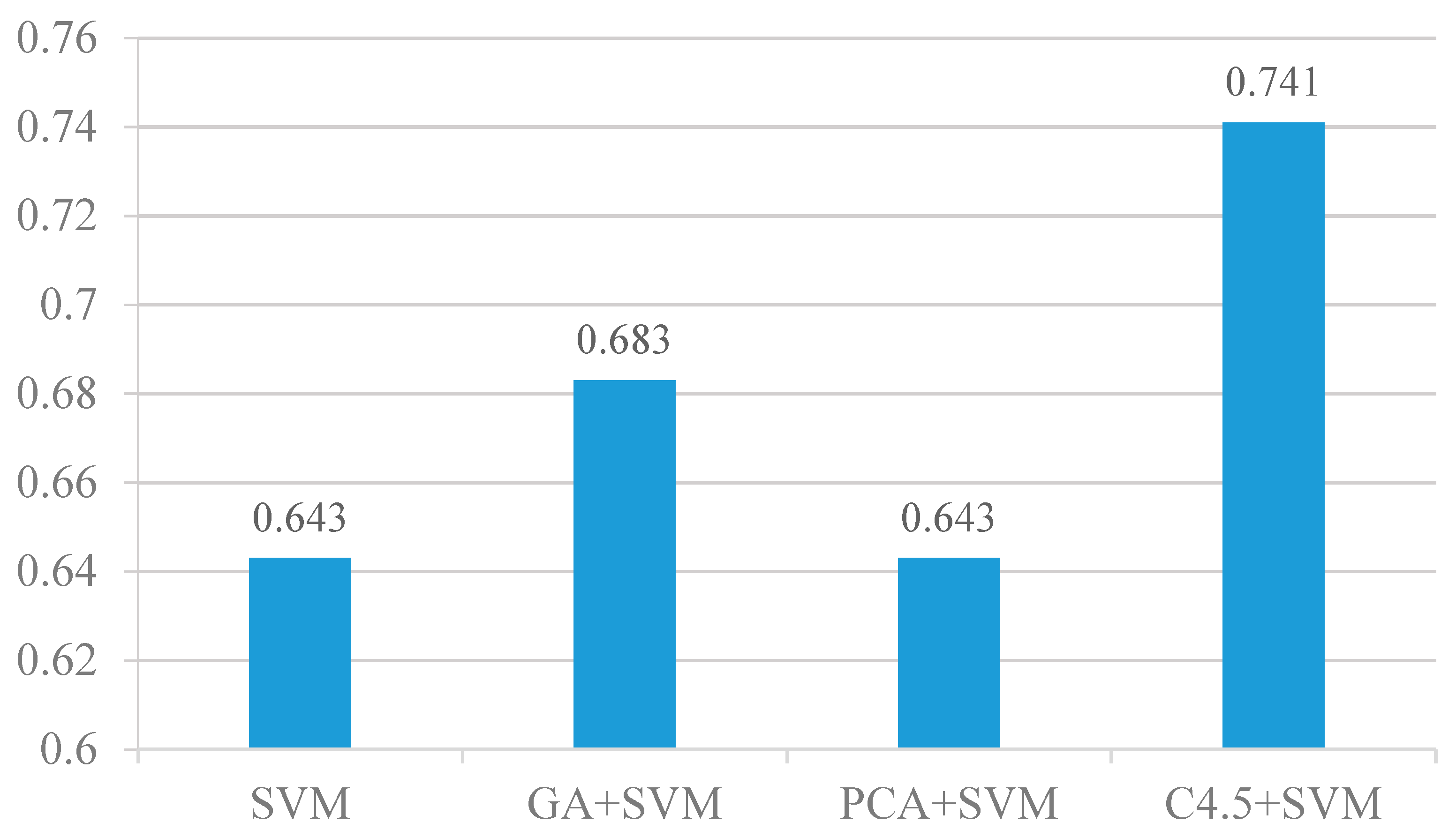
4.3. Feature Selection Analysis
4.4. Results of the Feature Selection Analysis
5. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fritschi, C.; Quinn, L. Fatigue in patients with diabetes: a review. J. Psychosom. Res. 2010, 69, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.A.; Brooks, S.V.; Zebra, E. Skeletal Muscle Weakness and Fatigue in Old Age: Underlying Mechanisms. In Annual Review of Gerontology and Geriatrics: Special Focus on the Biology of Aging; Cristofalo, V.J., Ed.; Springer Publishing: New York, NY, USA, 1990; Volume 10, pp. 147–166. [Google Scholar]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Cardiovascular Health Study Collaborative Research Group: Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, 146–156. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Economic costs of diabetes in the US in 2012. Diabetes Care 2013, 36, 1033–1046. [Google Scholar] [CrossRef]
- Ray, J.A.; Valentine, W.J.; Secnik, K.; Oglesby, A.K.; Cordony, A.; Gordois, A.; Davey, P.; Palmer, A.J. Review of the cost of diabetes complications in Australia, Canada, France, Germany, Italy and Spain. Curr. Med Res. Opin. 2005, 21, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.M.; Hahm, J.R.; Kim, T.K.; Choi, W.H. Factors affecting fatigue in patients with type II diabetes mellitus in Korea. Asian Nurs Res. (Korean Soc. Nurs Sci.) 2015, 9, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Thakkinstian, A.; Anothaisintawee, T.; Chontong, S.; Borel, A.-L.; Perfect, M.M.; Janovsky, C.C.; Kessler, R.; Schultes, B.; Harsch, I.A.; et al. Sleep characteristics in type 1 diabetes and associations with glycemic control: systematic review and meta-analysis. Sleep Med. 2016, 23, 26–45. [Google Scholar] [CrossRef]
- Goedendorp, M.M.; Tack, C.J.; Steggink, E.; Bloot, L.; Bazelmans, E.; Knoop, H. Chronic fatigue in type 1 diabetes: highly prevalent but not explained by hyperglycemia or glucose variability. Diabetes Care 2014, 37, 73–80. [Google Scholar] [CrossRef]
- Hamilton, M.T.; Hamilton, D.G.; Zderic, T.W. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 2007, 56, 2655–2667. [Google Scholar] [CrossRef]
- Avlund, K. Fatigue in older adults:an early indicator of the aging process? Aging Clin. Exp. Res. 2011, 22, 100–115. [Google Scholar] [CrossRef]
- Bot, M.; Pouwer, F.; de Jonge, P.; Tack, C.; Geelhoed-Duijvestijn, P.; Snoek, F. Differential associations between depressive symptoms and glycaemic control in outpatients with diabetes. Diabet. Med. 2013, 30, e115–e122. [Google Scholar] [CrossRef]
- Eaton, W.W.; Armenian, H.; Gallo, J.; Pratt, L.; Ford, D.E. Depression and risk for onset of type II diabetes: a prospective population-based study. Diabetes Care 1996, 19, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Fritschi, C.; Quinn, L.; Hacker, E.D.; Penckofer, S.M.; Wang, E.; Foreman, M.; Ferrans, C.E. Fatigue in women with type 2 diabetes. Diabetes Educ. 2012, 38, 662–672. [Google Scholar] [CrossRef] [PubMed]
- EI_Jerjawi, N.S.; Abu-Naser, S.S. Diabetes Prediction Using Artificial Neural Network. Int. J. Adv. Sci. Technol. 2018, 121, 55–64. [Google Scholar]
- Eswari, T.; Sampath, P.; Lavanya, S. Predictive methodology for diabetic data analysis in big data. Procedia Comput. Sci. 2015, 50, 203–208. [Google Scholar]
- Jain, D.; Singh, V. Feature selection and classification systems for chronic disease prediction: A review. Egypt. Inform. J. 2018, 19, 179–189. [Google Scholar] [CrossRef]
- Ronksley, P.E.; Hemmelgarn, B.R.; Heitman, S.J.; Hanly, P.J.; Faris, P.D.; Quan, H.; Tsai, W.H. Obstructive sleep apnoea is associated with diabetes in sleepy subjects. Thorax 2009, 64, 834–839. [Google Scholar] [CrossRef]
- Cuellar, N.G.; Ratcliffe, S.J. A comparison of glycemic control, sleep, fatigue, and depression in type 2 diabetes with and without restless legs syndrome. J. Clin. Sleep Med. 2008, 4, 50–56. [Google Scholar] [CrossRef]
- Paschalides, C.; Wearden, A.J.; Dunkerley, R.; Bundy, C.; Davies, R.; Dickens, C.M. The associations of anxiety, depression and personal illness representations with glycaemic control and health-related quality of life in patients with type 2 diabetes mellitus. J. Psychosom. Res. 2004, 57, 557–564. [Google Scholar] [CrossRef]
- Reutens, A.T.; Atkins, R.C. Epidemiology of diabetic nephropathy. Diabetes Kidney 2011, 170, 1–7. [Google Scholar]
- Singh, R.; Teel, C.; Sabus, C.; McGinnis, P.; Kluding, P. Fatigue in type 2 diabetes: impact on quality of life and predictors. PLoS ONE 2016, 11, e0165652. [Google Scholar] [CrossRef]
- Gandhi, G.Y.; Murad, M.H.; Fujiyoshi, A.; Mullan, R.J.; Flynn, D.N.; Elamin, M.B.; Swiglo, B.A.; Isley, W.L.; Guyatt, G.H.; Montori, V.M. Patient-important outcomes in registered diabetes trials. JAMA 2008, 299, 2543–2549. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Tiesinga, L.J.; Dassen, T.W.; Halfens, R.J. Fatigue: a summary of the definitions, dimensions, and indicators. Int. J. Nurs. Termin. Classif. 1996, 7, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.P. Application of Orem’s Self-Care Deficit Theory and Standardized Nursing Languages in a Case Study of a Woman with Diabetes. Int. J. Nurs. Terminol. Classif. 2007, 18, 103–110. [Google Scholar] [CrossRef]
- Chandrashekar, G.; Sahin, F. A survey on feature selection methods. Comput. Electr. Eng. 2014, 40, 16–28. [Google Scholar] [CrossRef]
- Guyon, I.; Elisseeff, A. An introduction to variable and feature selection. J. Mach. Learn. Res. 2003, 3, 1157–1182. [Google Scholar]
- Liu, H.; Yu, L. Toward integrating feature selection algorithms for classification and clustering. IEEE Trans. Knowl. Data Eng. 2005, 17, 491–502. [Google Scholar]
- You, D.; Wu, X.; Shen, L.; He, Y.; Yuan, X.; Chen, Z.; Deng, S.; Ma, C. Online streaming feature selection via conditional independence. Appl. Sci. 2018, 8, 2548. [Google Scholar] [CrossRef]
- Bolon-Canedo, V.; Sanchez-Marono, N.; Alonso-Betanzos, A. A review of feature selection methods on synthetic data. Knowl. Inf. Syst. 2013, 34, 483–519. [Google Scholar] [CrossRef]
- Kumar, V.; Minz, S. Feature selection: a literature review. Smart Comput. Rev. 2014, 4, 211–229. [Google Scholar] [CrossRef]
- Saeys, Y.; Inza, I.; Larranaga, P. A review of feature selection techniques in bioinformatics. Bioinformatics 2007, 23, 2507–2517. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Alelyani, S.; Liu, H. Feature selection for classification-a review. In Data Classification Algorithms and Applications; Aggarwal, C.C., Ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2014. [Google Scholar]
- Jolliffe, I.T. Principal Component Analysis; Springer Verlag: New York, NY, USA, 1986. [Google Scholar]
- de la lglesia, B. Evolutionary computation for feature selection in classification problems. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2013, 3, 381–407. [Google Scholar] [CrossRef]
- Xue, B.; Zhang, M.; Browne, W.N.; Yao, X. A survey on evolutionary computation approaches to feature selection. IEEE Trans. Evol. Comput. 2016, 20, 606–626. [Google Scholar] [CrossRef]
- Xie, S.; Li, K.; Xiao, M.; Zhang, L.; Li, W. Key quality indicators prediction for web browsing with embedded filter feature selection. Appl. Sci. 2020, 10, 2141. [Google Scholar] [CrossRef]
- Goldberg, D.E. Genetic Algorithms in Search Optimization and Machine Learning; Addition Wesley: Boston, MA, USA, 1989. [Google Scholar]
- Maldonado, S.; López, J. Dealing with high-dimensional class-imbalanced datasets: Embedded feature selection for SVM classification. Appl. Soft Comput. 2018, 67, 94–105. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, H. Spectral feature selection for supervised and unsupervised learning. In Proceedings of the 24th International Conference on Machine Learning, Corvalis Oregon, OR, USA, 20–24 June 2007; Ghahramani, Z., Ed.; ACM: New York, NY, USA, 2007; pp. 1151–1157. [Google Scholar]
- Zhu, X.; Zhang, S.; Hu, R.; Zhu, Y.; Song, J. Local and global structure preservation for robust unsupervised spectral feature selection. IEEE Trans. Knowl. Data Eng. 2018, 30, 517–529. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression shrinkage and selection via the Lasso. J. R. Stat. Soc. Ser. B (Methodological) 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Bradley, P.S.; Mangasarian, O.L. Feature selection via concave minimization and support vector machines. In Proceedings of the 15th International Conference on Machine Learning, Madison, Wisconsin, WI, USA, 24–27 July 1998; Shavlik, J.W., Ed.; Morgan Kaufmann Publishers: San Francisco, CA, USA, 1998; pp. 82–90. [Google Scholar]
- Zhu, Z.; Ong, Y.-S.; Dash, M. Wrapper-filter feature selection algorithm using a memetic framework. IEEE Trans. Syst. Man, Cybern. Part B (Cybern.) 2007, 37, 70–76. [Google Scholar] [CrossRef]
- Quinlan, J.R. Induction of decision trees. Mach. Learn. 1986, 1, 81–106. [Google Scholar] [CrossRef]
- Morchid, M.; Dufour, R.; Bousquet, P.-M.; Linares, G.; Torres-Moreno, J.-M. Feature selection using principal component analysis for massive retweet detection. Pattern Recognit. Lett. 2014, 49, 33–39. [Google Scholar] [CrossRef]
- Mori, Y.; Lizuka, M.; Tarumi, T.; Tanaka, Y. Variable selection in principal component analysis. In Statistical Methods for Biostatistics and Related Fields; Hardle, W., Mori, Y., Vieu, P., Eds.; Springer: Berlin, Germany, 2007; pp. 265–283. [Google Scholar]
- Kazemitabar, J.; Amini, A.; Bloniarz, A.; Talwalkar, A.S. Variable importance using decision trees. In Proceedings of the 31st Conference on Neural Information Processing Systems (NIPS 2017), Long Beach, CA, USA, 4–9 December 2017; pp. 425–434. [Google Scholar]
- Liu, F.; Lee, D.-H.; Lagoa, R.; Kumar, S. EEG feature selection method based on decision tree. Bio-Med Mater. Eng. 2015, 26, S1019–S1025. [Google Scholar]
- Tsai, C.-F.; Chen, Y.-C. The optimal combination of feature selection and data discretization: an empirical study. Inf. Sci. 2019, 505, 282–293. [Google Scholar] [CrossRef]
- Liang, D.; Tsai, C.-F.; Wu, H.-T. The effect of feature selection on financial distress prediction. Knowl. Based Syst. 2015, 73, 289–297. [Google Scholar] [CrossRef]
- Huang, M.-W.; Lin, W.-C.; Chen, C.-W.; Ke, S.-W.; Tsai, C.-F.; Eberle, W. Data preprocessing issues for incomplete medical datasets. Expert Syst. 2016, 33, 432–438. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V.N. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Park, H.; Park, C.; Quinn, L.; Fritschi, C. Glucose control and fatigue in type 2 diabetes: the mediating roles of diabetes symptoms and distress. J. Adv. Nurs. 2015, 71, 1650–1660. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, R.; Choudhary, P.K.; Yadav, N.; Jain, G.; Maanju, M. Study of fatigue, depression, and associated factors in type 2 diabetes mellitus in industrial workers. Ind. Psychiatry J. 2015, 24, 179–184. [Google Scholar] [CrossRef]
| Variables | Total | Nephropathy | ||
|---|---|---|---|---|
| Yes | No | |||
| (n = 307) | (n = 89) | (n = 218) | p-Value | |
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age (y) | 63.49 ± 10.42 | 64.63 ± 11.19 | 63.02 ± 10.07 | 0.219 |
| Physical status | ||||
| Waist circumference | 89.10 ± 11.12 | 94.58 ± 11.49 | 86.87 ± 10.18 | 0.000 *** |
| Hip circumference | 97.63 ± 8.56 | 100.68 ± 8.88 | 96.41 ± 8.13 | 0.000 *** |
| Waist/hip ratio | 91.16 ± 0.07 | 0.94 ± 0.76 | 0.90 ± 0.61 | 0.000 *** |
| BMI | 25.15 ± 4.20 | 26.44 ± 4.68 | 24.62 ± 3.88 | 0.001 ** |
| Systolic blood pressure | 128.49 ± 13.43 | 131.69 ± 13.49 | 127.04 ± 13.17 | 0.004 ** |
| Diastolic blood pressure | 75.53 ± 9.33 | 77.97 ± 9.03 | 74.51 ± 9.29 | 0.003 * |
| Metabolic control index | ||||
| AC sugar (preprandial sugar) | 135.13 ± 37.16 | 137.08 ± 46.20 | 134.33 ± 32.83 | 0.611 |
| PC sugar (postprandial sugar) | 195.02 ± 67.64 | 202.48 ± 61.05 | 191.98 ± 70.10 | 0.296 |
| Glycosylated hemoglobin (HbA1c) | 7.60 ± 3.86 | 8.30 ± 7.01 | 7.32 ± 0.97 | 0.197 |
| Complications | ||||
| Age-adjusted Charlson comorbidity index (CCI) | 4.54 ± 1.87 | 6.75 ± 1.26 | 3.63 ± 1.21 | 0.000 *** |
| Lifestyle | ||||
| Weekday sleep hours | 7.41 ± 2.29 | 2.13 ± 0.14 | 2.63 ± 0.28 | 0.113 |
| Weekly mean exercise count | 3.68 ± 2.67 | 2.55 ± 0.17 | 2.90 ± 0.31 | 0.047 * |
| Exercise duration (minutes) | 45.91 ± 40.26 | 39.41 ± 2.67 | 40.98 ± 4.34 | 0.008 * |
| Total exercise minutes/week | 207.25 ± 218.79 | 215.91 ± 14.62 | 223.74 ± 23.72 | 0.104 |
| Fatigue sum | 52.36 ± 9.00 | 54.2 ± 10.04 | 51.6 ± 8.45 | 0.021 * |
| Sleepiness (ESS) score | 8.77 ± 4.92 | 8.84 ± 5.53 | 8.74 ± 4.66 | 0.876 |
| sleep status (PSQI) score | 7.41 ± 3.91 | 7.48 ± 3.68 | 7.39 ± 4.00 | 0.837 |
| Quality of Life (SF-12) score | 70.56 ± 13.92 | 68.17 ± 16.82 | 71.54 ± 12.46 | 0.090 |
| PCS score | 68.56 ± 18.53 | 65.10 ± 21.77 | 69.97 ± 16.83 | 0.062 |
| General health (GH) | 35.34 ± 21.12 | 35.67 ± 23.50 | 35.21 ± 20.13 | 0.896 |
| Role physical health (RP) | 80.46 ± 27.87 | 74.72 ± 31.49 | 82.80 ± 26.01 | 0.034 * |
| Physical functioning (RF) | 73.98 ± 25.40 | 31.42 ± 30.80 | 77.01 ± 22.37 | 0.004 * |
| Body pain (BP) | 84.45 ± 21.49 | 83.43 ± 22.60 | 84.86 ± 21.07 | 0.596 |
| MCS score | 72.57 ± 12.95 | 71.54 ± 14.89 | 73.11 ± 12.09 | 0.253 |
| Mental health (MH) | 77.48 ± 22.88 | 74.30 ± 26.81 | 78.78 ± 20.99 | 0.160 |
| Vitality (VT) | 77.20 ± 18.67 | 75.56 ± 18.84 | 77.87 ± 12.60 | 0.327 |
| Role emotional (RE) | 55.46 ± 12.41 | 57.58 ± 12.67 | 54.59 ± 11.78 | 0.055 |
| Social functioning (SF) | 80.13 ± 23.49 | 77.53 ± 26.66 | 81.19 ± 22.04 | 0.254 |
| Fatigue Score | ESS Score | PSQI Score | SF-12 Score | HbA1C | CCI | |
|---|---|---|---|---|---|---|
| r (p) | r (p) | r (p) | r (p) | r (p) | r (p) | |
| Fatigue score | 1.000 | |||||
| ESS score | 0.192 (0.001 **) | 1.000 | ||||
| PSQI score | 0.395 (0.000 ***) | 0.060 (0.292) | 1.000 | |||
| SF-12 score | −0.605 (0.000 ***) | −0.299 (0.000 ***) | −0.342 (0.000 ***) | 1.000 | ||
| HbA1c | 0.029 0.612 | −0.012 (0.840) | 0.012 (0.839) | 0.026 (0.647) | 1.000 | |
| CCI | 0.221 (0.000 ***) | 0.021 (0.714) | 0.067 (0.245) | −0.200 (0.000 ***) | 0.068 (0.238) | 1.000 |
| Univariate | Multivariate # | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | aOR | 95% CI | p | |
| Fatigue score | 1.033 | (1.004–1.062) | 0.023 * | 0.979 | (0.917–1.045) | 0.524 |
| ESS score | 1.004 | (0.955–1.056) | 0.866 | 1.010 | (0.916–1.112) | 0.847 |
| PSQI score | 1.006 | (0.945–1.072) | 0.842 | 0.898 | (0.782–1.031) | 0.126 |
| SF-12 score | 0.983 | (0.966–1.000) | 0.056 | 0.995 | (0.952–1.041) | 0.837 |
| HbA1c | 1.200 | (0.970–1.484) | 0.094 | 1.776 | (1.186–2.660) | 0.005 ** |
| CCI | 7.779 | (4.749–12.742) | 0.000 *** | 9.777 | (5.435–17.591) | 0.000 *** |
| Variable Names |
|---|
| PC sugar (postprandial 2 hours’ plasma glucose level), urine microalbumin, microalbumin-to-creatinine ratio, body height, body weight, waist circumference, hip circumference, diastolic blood pressure, alcohol drinking behavior, exercise. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lien, A.S.-Y.; Jiang, Y.-D.; Tsai, J.-L.; Hwang, J.-S.; Lin, W.-C. Prediction of Diabetic Nephropathy from the Relationship between Fatigue, Sleep and Quality of Life. Appl. Sci. 2020, 10, 3282. https://doi.org/10.3390/app10093282
Lien AS-Y, Jiang Y-D, Tsai J-L, Hwang J-S, Lin W-C. Prediction of Diabetic Nephropathy from the Relationship between Fatigue, Sleep and Quality of Life. Applied Sciences. 2020; 10(9):3282. https://doi.org/10.3390/app10093282
Chicago/Turabian StyleLien, Angela Shin-Yu, Yi-Der Jiang, Jia-Ling Tsai, Jawl-Shan Hwang, and Wei-Chao Lin. 2020. "Prediction of Diabetic Nephropathy from the Relationship between Fatigue, Sleep and Quality of Life" Applied Sciences 10, no. 9: 3282. https://doi.org/10.3390/app10093282
APA StyleLien, A. S.-Y., Jiang, Y.-D., Tsai, J.-L., Hwang, J.-S., & Lin, W.-C. (2020). Prediction of Diabetic Nephropathy from the Relationship between Fatigue, Sleep and Quality of Life. Applied Sciences, 10(9), 3282. https://doi.org/10.3390/app10093282





