Implementation of High Gas Barrier Laminated Films Based on Cellulose Nanocrystals for Food Flexible Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Carboxylated and Esterified Cellulose Nanocrystals Preparation
2.2.2. Charges Density Assessment
2.2.3. CNCs Morphology Assessment
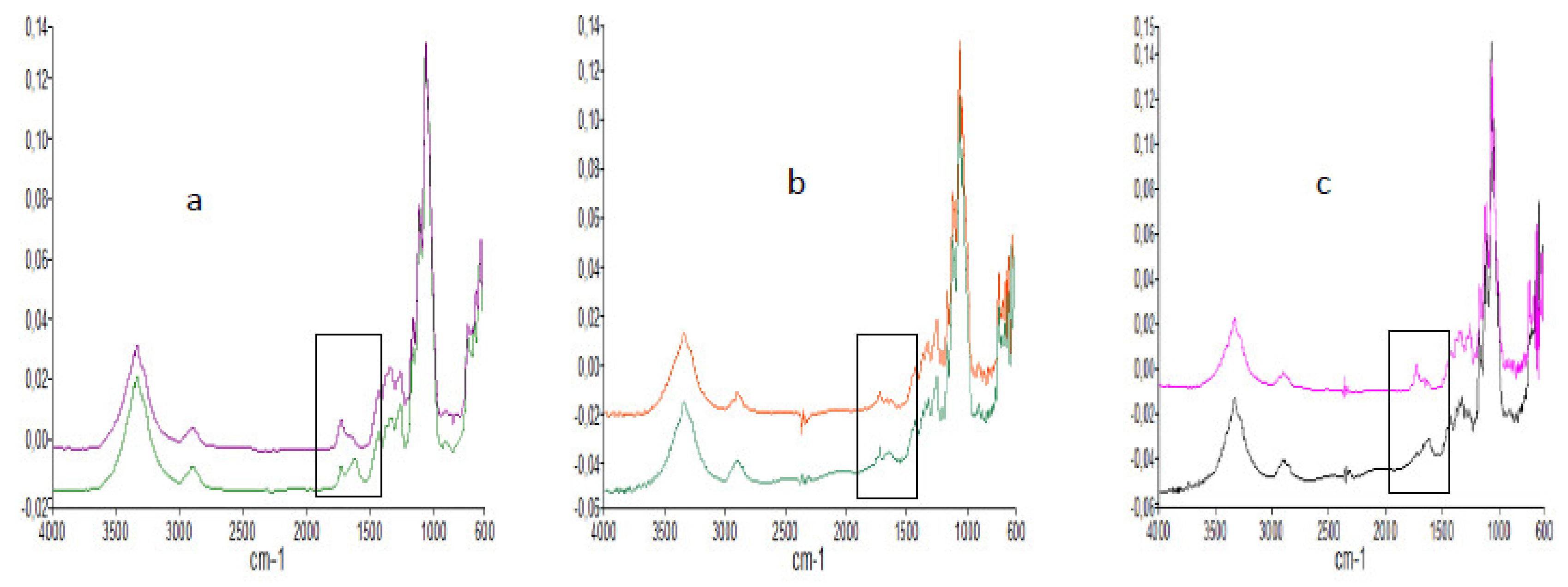
2.2.4. FTIR and Raman Analysis
2.2.5. Wide Angle X-ray Scattering (WAXS)
2.2.6. Thermogravimetric Analysis (TGA)
2.2.7. CNCs Coatings of Plastic Films (P-CNC)
2.2.8. Optical Properties of Coated Film
2.2.9. Z Potential of Uncoated and CNCs-Coated PET Films
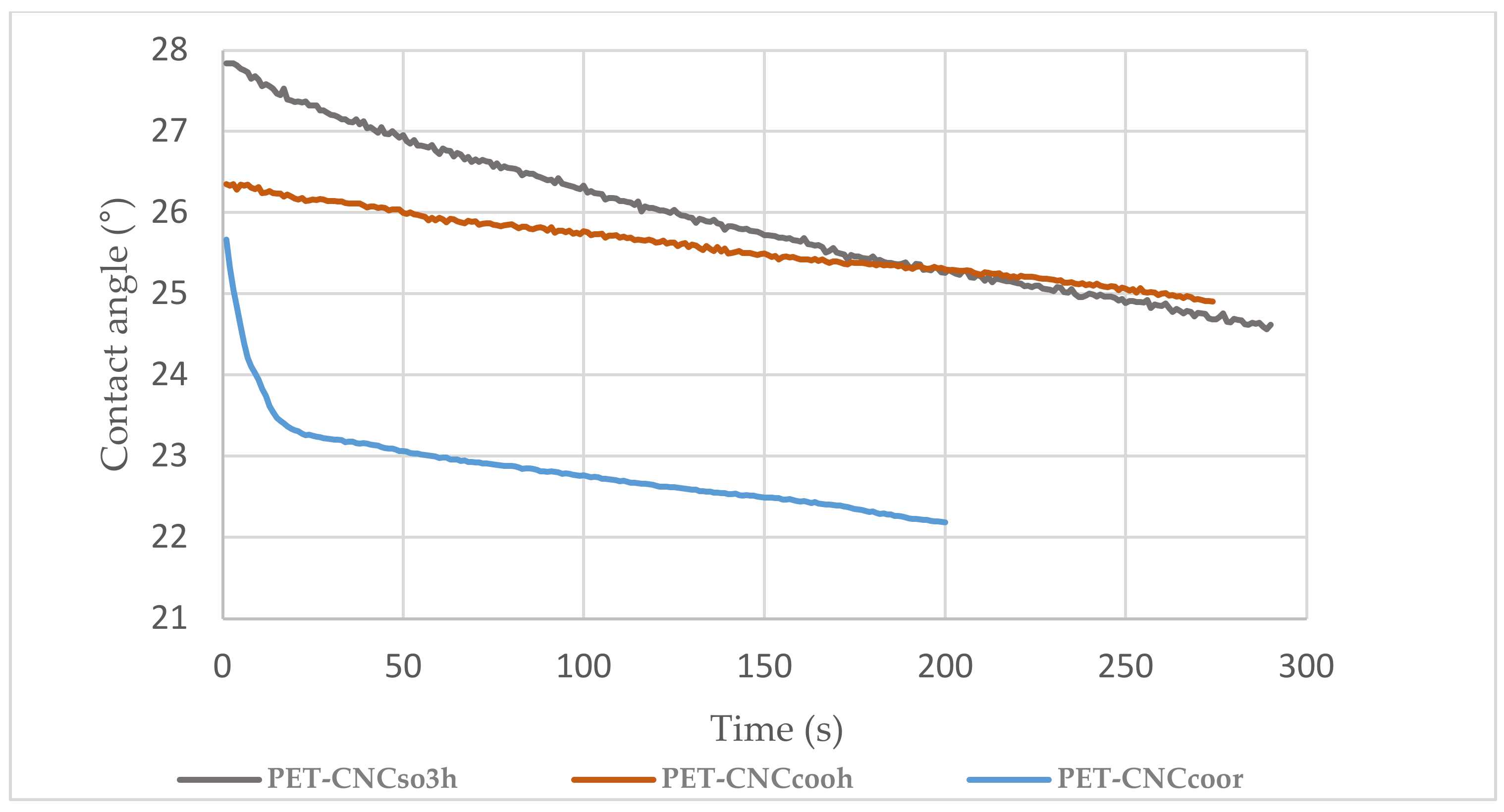
2.2.10. Water Contact Angles Assessment
2.2.11. Corona-Treatment of CNCs-Coated Films and Lamination
2.2.12. Gas Permeability Measurement and Peeling Test
3. Results and Discussion
3.1. Characterization of CNCs Properties
3.1.1. Light Scattering Characterization
3.1.2. FTIR and Raman Spectra of Dried Cellulose Nanocrystals
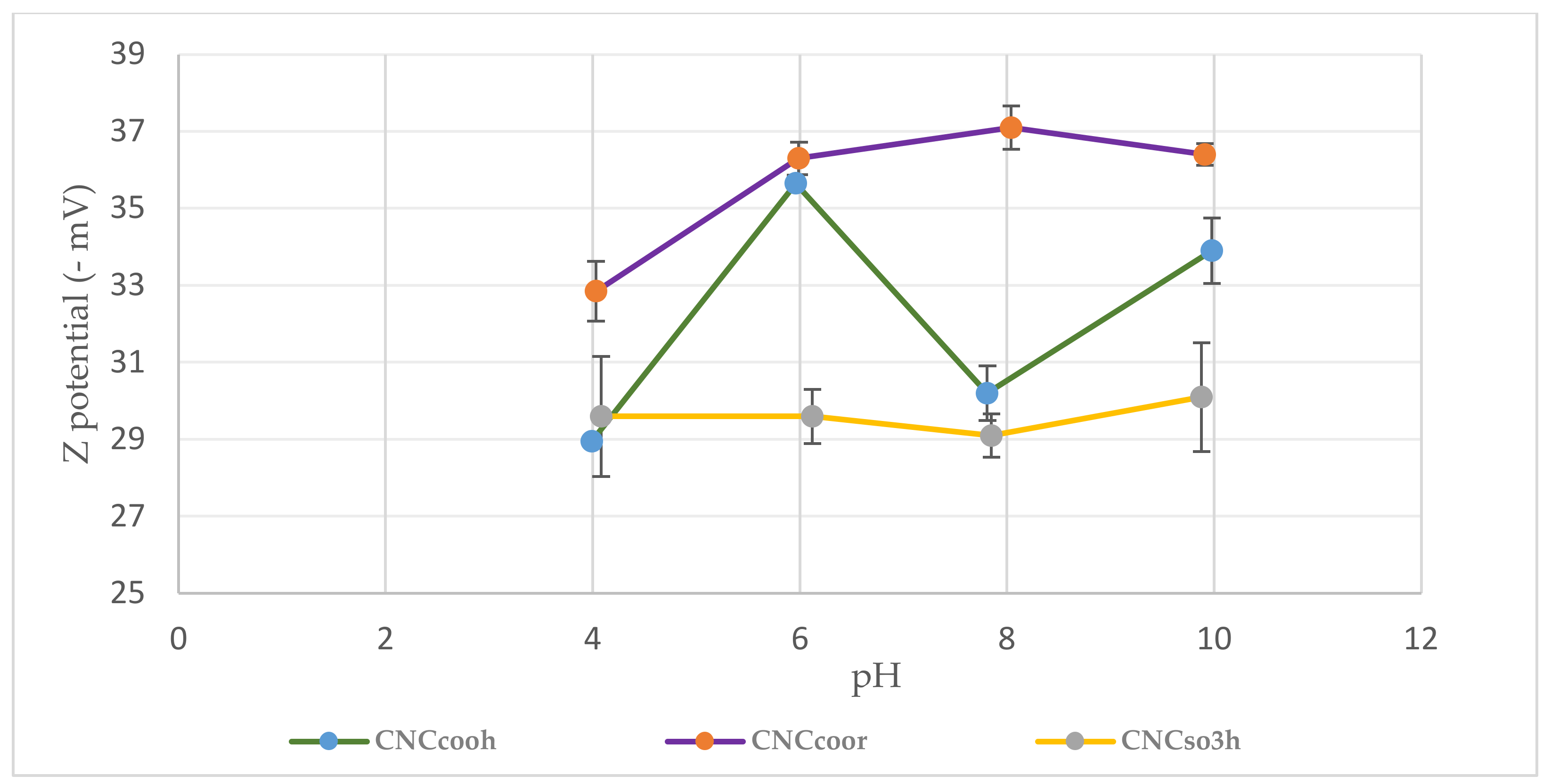
3.1.3. Z Potential Versus pH of Dispersed Cellulose Nanocrystals
3.1.4. Charges Density by Conductometric Titration
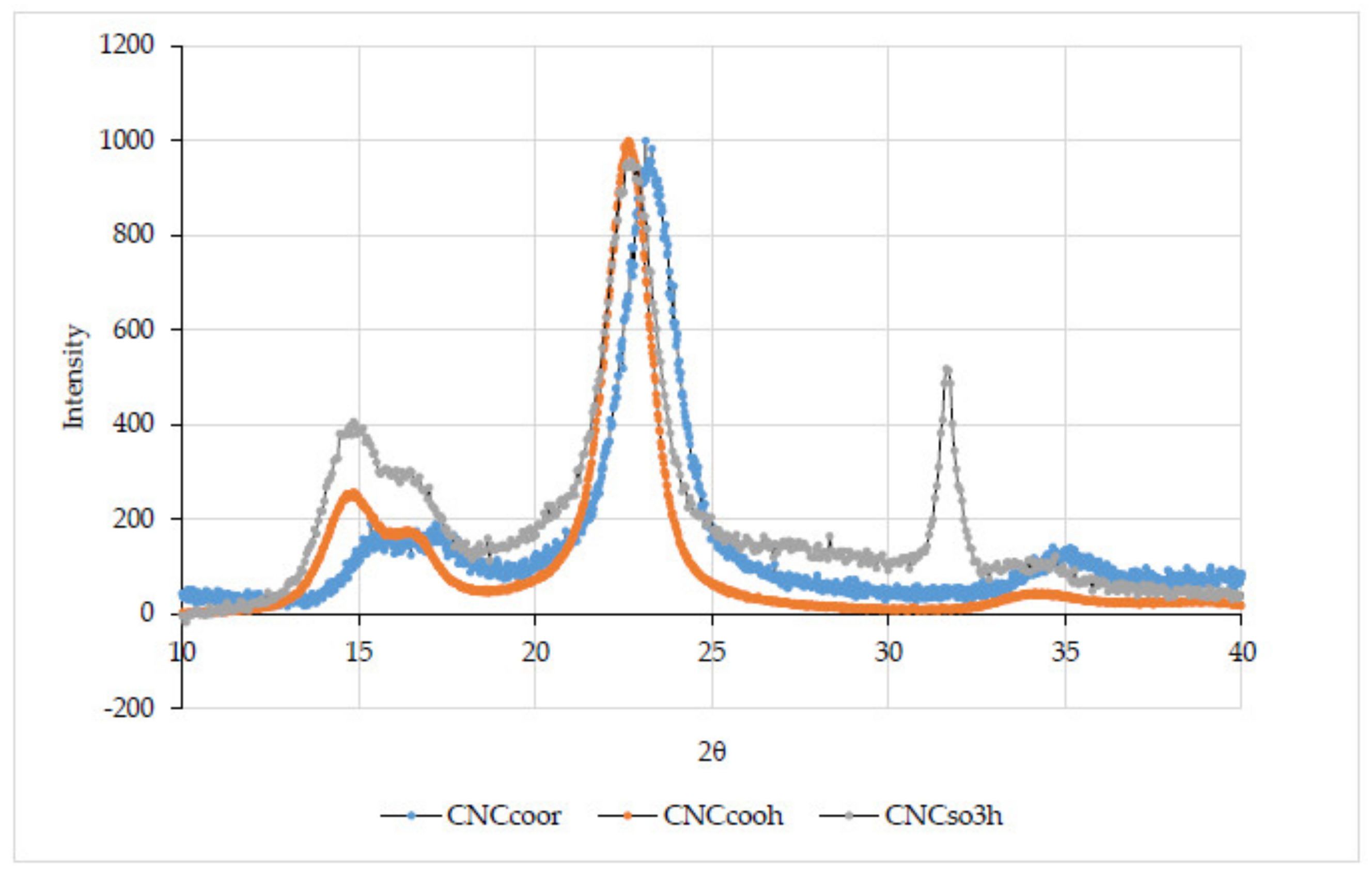
3.1.5. Wide Angle X-ray Scattering (WAXS)
3.1.6. Thermogravimetric Analysis (TGA)
3.2. Characterization of CNCs-Coated Plastics
3.2.1. Z Potential of CNCs Coated and Uncoated PET Film Versus pH
3.2.2. FTIR of Corona-Treated and Untreated CNCs-Coated PET Film
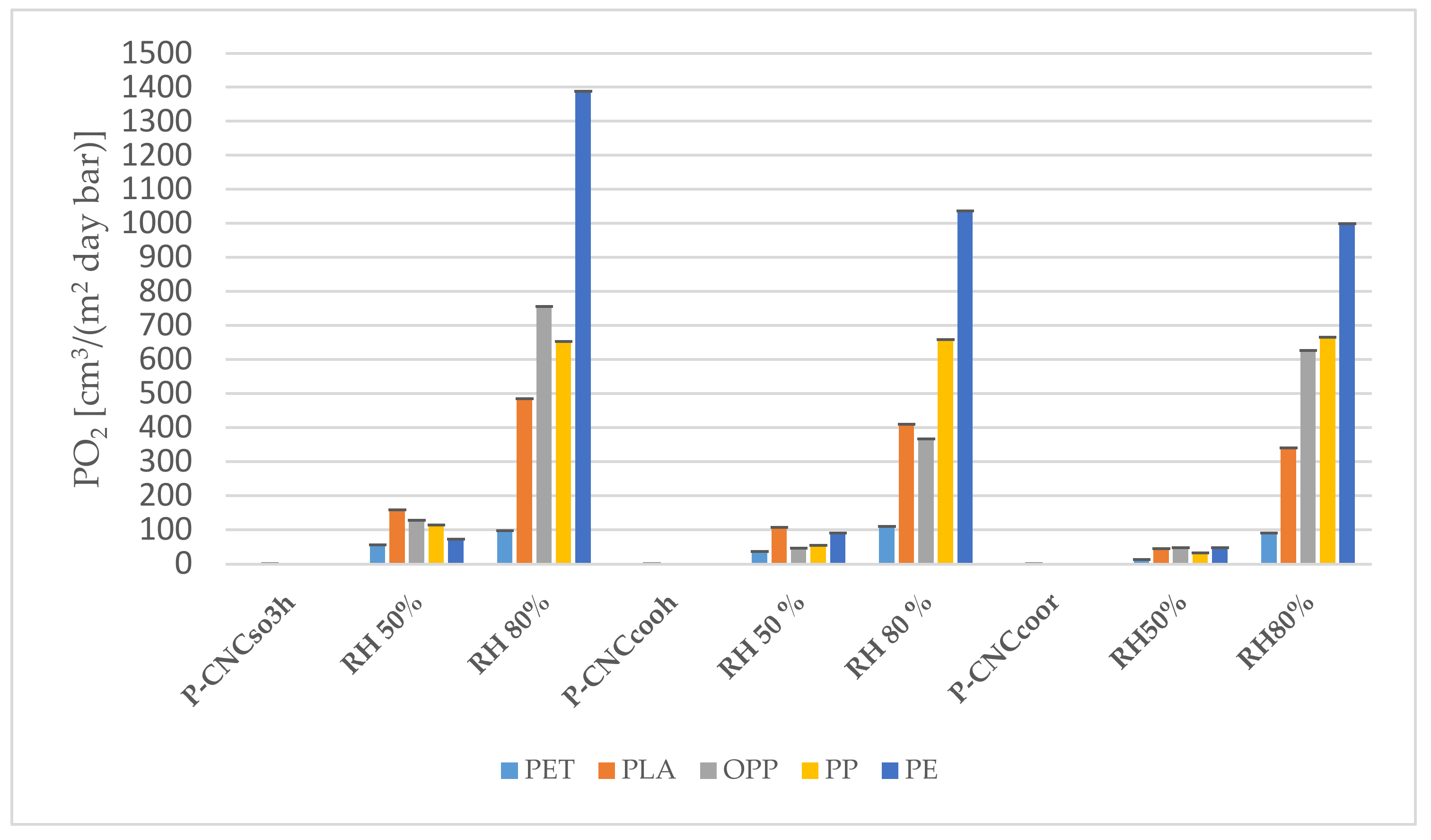
3.2.3. Oxygen Permeability of Coated Polymers (P-CNC)
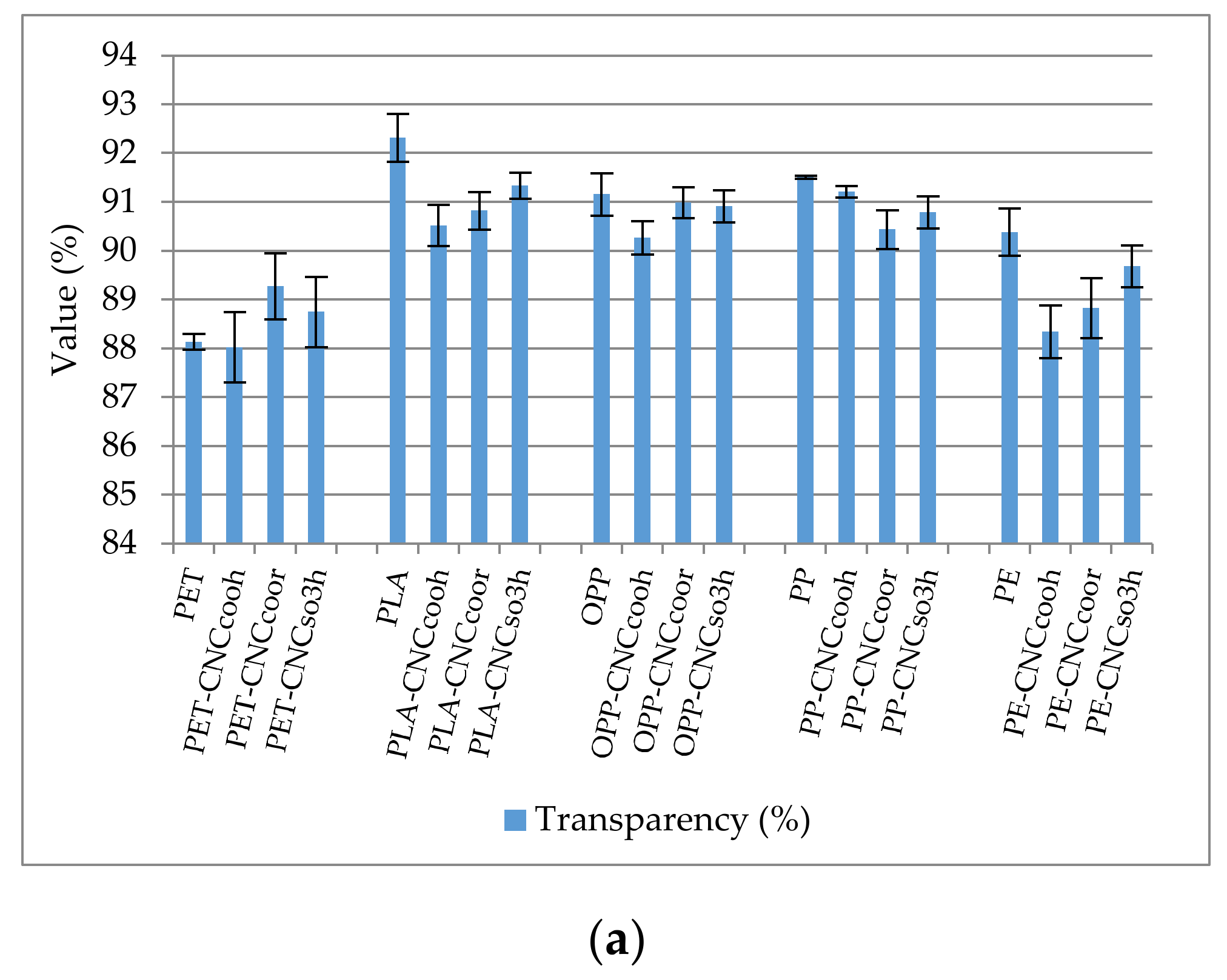
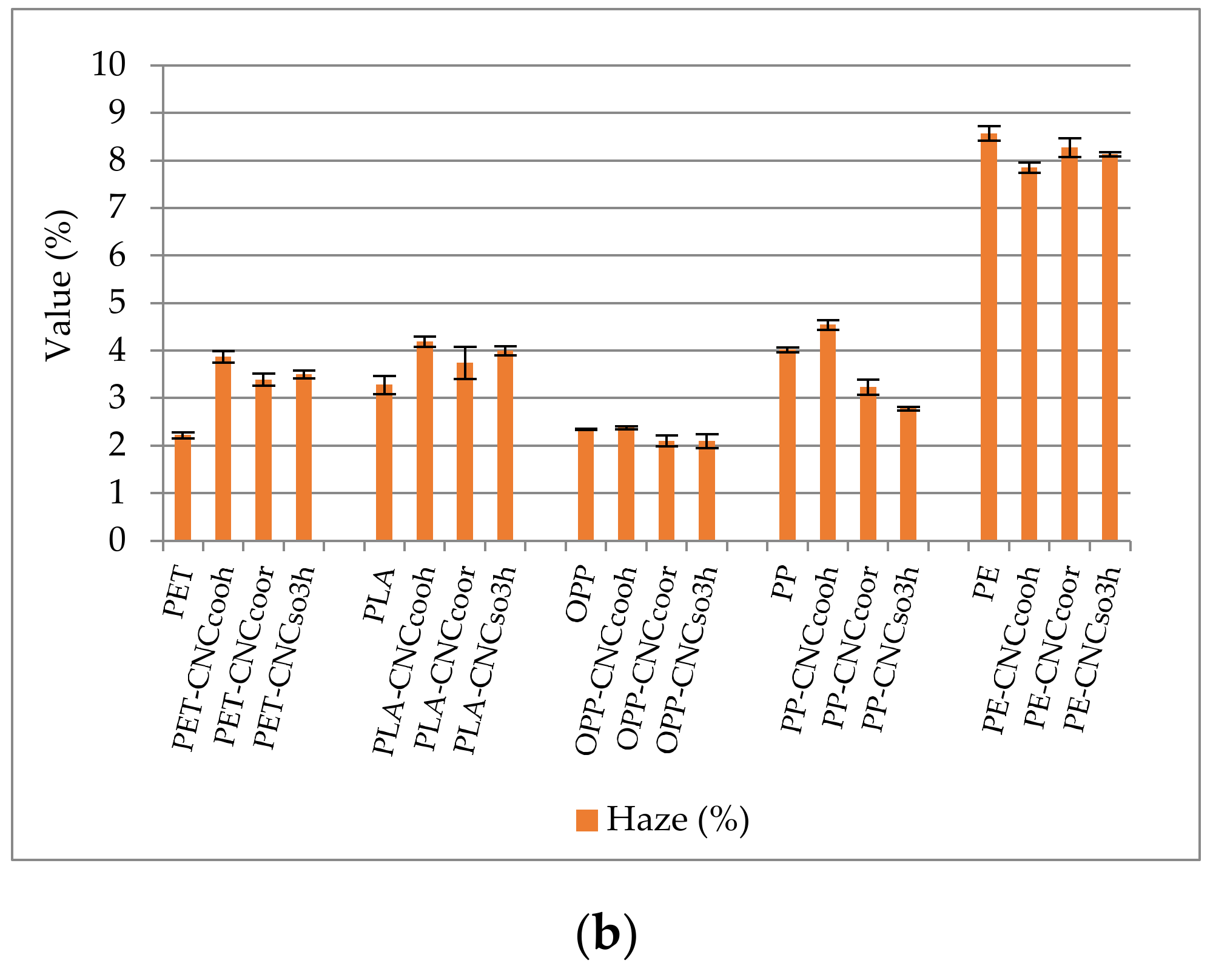
3.2.4. Haze and Transparency of Coated and Uncoated Polymers
3.3. Characterization of Laminated CNCs-Coated PET Films (P-CNC-P)
3.3.1. Delamination Test
3.3.2. Oxygen Permeability of Laminates with CNCs Coated Films (P-CNC-P)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Araki, J.; Wada, M.; Kuga, S. Steric Stabilization of a Cellulose Microcrystal Suspension by Poly(ethylene glycol) Grafting. Langmuir 2001, 17, 21–27. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of Reaction Conditions on the Properties and Behavior of Wood Cellulose Nanocrystal Suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef]
- Cherhal, F.; Cousin, F.; Capron, I. Influence of Charge Density and Ionic Strength on the Aggregation Process of Cellulose Nanocrystals in Aqueous Suspension, as Revealed by Small-Angle Neutron Scattering. Langmuir 2015, 31, 5596–5602. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.S.; Villalobos, M.; Cranston, E.D. Benchmarking Cellulose Nanocrystals: From the Laboratory to Industrial Production. Langmuir 2016, 33, 1583–1598. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.C.W.; Hrapovic, S.; Lam, E.; Male, K.B.; Mahmoud, K.A.; Liu, Y.; Luong, J.H.T. Characteristics and Properties of Carboxylated Cellulose Nanocrystals Prepared from a Novel One-Step Procedure. Small 2010, 7, 302–305. [Google Scholar] [CrossRef]
- Habibi, Y.; Chanzy, H.; Vignon, M.R. TEMPO-mediated surface oxidation of cellulose whiskers. Cellul. 2006, 13, 679–687. [Google Scholar] [CrossRef]
- Reid, M.S.; Villalobos, M.; Cranston, E. Cellulose Nanocrystal Interactions Probed by Thin Film Swelling to Predict Dispersibility. Nanoscale 2016, 8, 12247–12257. [Google Scholar] [CrossRef]
- Shimizu, M.; Saito, T.; Isogai, A. Bulky quaternary alkylammonium counterions enhance thenanodispersibility of 2,2,6,6-tetramethylpiperidine-1-oxyl-oxidized cellulose in diverse solvents. Biomacromolecules 2014, 15, 1904–1909. [Google Scholar] [CrossRef]
- Cheng, N.; Wen, Y.; Wang, L.; An, X.; Zhu, X.; Ni, Y. Adsorption of polyethylene glycol (PEG) onto cellulose nano-crystals to improve its dispersity. Carbohydr. Polym. 2015, 123, 157–163. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Casari, A.C.A.; Mariano, M.; Yamashita, F.; Innocentini-Mei, L.H.; Soldi, V.; Martelli, S.M. Effect of a gelatin-based edible coating containing cellulose nanocrystals (CNC) on the quality and nutrient retention of fresh strawberries during storage. IOP Conf. Ser. Mater. Sci. Eng. 2014, 64, 012024. [Google Scholar] [CrossRef]
- Fotie, G.; Limbo, S.; Piergiovanni, L. Effectiveness of cellulose nanocrystals (cncs) application as bio-based oxygen barrier for shelled walnuts shelf-life extension. Ital. J. Food Sci. 2017, 6. [Google Scholar]
- Fotie, G.; Amoroso, L.; Limbo, S.; Muratore, G.; Piergiovanni, L. Food life extension by cellulose nanocrystals coatings. Ital. J. Food Sci. 2019, 8–14. [Google Scholar]
- Rampazzo, R.; Alkan, D.; Gazzotti, S.; Ortenzi, M.A.; Piva, G.; Piergiovanni, L. Cellulose Nanocrystals from Lignocellulosic Raw Materials, for Oxygen Barrier Coatings on Food Packaging Films. Packag. Technol. Sci. 2017, 30, 645–661. [Google Scholar] [CrossRef]
- Belbekhouche, S.; Bras, J.; Siqueira, G.; Chappey, C.; Lebrun, L.; Khelifi, B.; Marais, S.; Dufresne, A. Water sorption behavior and gas barrier properties of cellulose whiskers and microfibrils films. Carbohydr. Polym. 2011, 83, 1740–1748. [Google Scholar] [CrossRef]
- Gicquel, E.; Martin, C.; Yanez, J.G.; Bras, J. Cellulose nanocrystals as new bio-based coating layer for improving fiber-based mechanical and barrier properties. J. Mater. Sci. 2016, 52, 3048–3061. [Google Scholar] [CrossRef]
- Fotie, G.; Rampazzo, R.; Ortenzi, M.A.; Checchia, S.; Fessas, D.; Piergiovanni, L. The Effect of Moisture on Cellulose Nanocrystals Intended as a High Gas Barrier Coating on Flexible Packaging Materials. Polymers 2017, 9, 415. [Google Scholar] [CrossRef] [PubMed]
- Eyley, S.; Thielemans, W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Sabapathi, S. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef]
- Lam, E.; Male, K.B.; Chong, J.H.; Leung, A.C.; Luong, J.H.T. Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends Biotechnol. 2012, 30, 283–290. [Google Scholar] [CrossRef]
- Bendahou, A.; Hajlane, A.; Dufresne, A.; Boufi, S.; Kaddami, H. Esterification and amidation for grafting long aliphatic chains on to cellulose nanocrystals: A comparative study. Res. Chem. Intermed. 2014, 41, 4293–4310. [Google Scholar] [CrossRef]
- Berlioz, S.; Molina-Boisseau, S.; Nishiyama, Y.; Heux, L. Gas-Phase Surface Esterification of Cellulose Microfibrils and Whiskers. Biomacromolecules 2009, 10, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Gazzotti, S.; Farina, H.; Lesma, G.; Rampazzo, R.; Piergiovanni, L.; Ortenzi, M.A.; Silvani, A. Polylactide/cellulose nanocrystals: The in situ polymerization approach to improved nanocomposites. Eur. Polym. J. 2017, 94, 173–184. [Google Scholar] [CrossRef]
- Braun, B.; Dorgan, J.R. Single-Step Method for the Isolation and Surface Functionalization of Cellulosic Nanowhiskers. Biomacromolecules 2009, 10, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Çetin, N.S.; Tingaut, P.; Özmen, N.; Henry, N.; Harper, D.P.; Dadmun, M.D.; Sèbe, G. Acetylation of Cellulose Nanowhiskers with Vinyl Acetate under Moderate Conditions. Macromol. Biosci. 2009, 9, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López-Martínez, J.; Kenny, J.M. PLA-PHB/cellulose based films: Mechanical, barrier and disintegration properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Fortunati, E.; Peltzer, M.; Armentano, I.; Torre, L.; Jiménez, A.; Kenny, J.M. Effects of modified cellulose nanocrystals on the barrier and migration properties of PLA nano-biocomposites. Carbohydr. Polym. 2012, 90, 948–956. [Google Scholar] [CrossRef]
- Ferrer, A.; Pal, L.; Hubbe, M.A. Nanocellulose in packaging: Advances in barrier layer technologies. Ind. Crop. Prod. 2017, 95, 574–582. [Google Scholar] [CrossRef]
- Zhang, Z.; Britt, I.J.; Tung, M.A. Permeation of oxygen and water vapor through EVOH films as influenced by relative humidity. J. Appl. Polym. Sci. 2001, 82, 1866–1872. [Google Scholar] [CrossRef]
- Shah, G.P. Oxygen Barrier Biaxially Oriented Film. U.S. Patent 5,004,647, 2 April 1991. [Google Scholar]
- Agarwal, U.P.; Atalla, R.H.; Isogai, A. Raman Spectroscopy in the Analysis of Cellulose Nanomaterials. Chem. Stud. Success Field-Tested Evid. Based Guide 2017, 1251, 75–90. [Google Scholar] [CrossRef]
- Hasani, M.; Cranston, E.D.; Westman, G.; Gray, D.G. Cationic surface functionalization of cellulose nanocrystals. Soft Matter 2008, 4, 2238–2244. [Google Scholar] [CrossRef]
- Jiang, H.; Wu, Y.; Han, B.; Zhang, Y. Effect of oxidation time on the properties of cellulose nanocrystals from hybrid poplar residues using the ammonium persulfate. Carbohydr. Polym. 2017, 174, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, C.-W.; Han, S.-Y.; Kwon, G.-J.; Kim, N.H.; Lee, S.-H. Effects of pH on Nanofibrillation of TEMPO-Oxidized Paper Mulberry Bast Fibers. Polym. 2019, 11, 414. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, L.; Rohrer, J.S. (Eds.) Applications of Ion Chromatography for Pharmaceutical and Biological Products; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Lamaming, J.; Hashim, R.; Leh, C.P.; Sulaiman, O. Properties of cellulose nanocrystals from oil palm trunk isolated by total chlorine free method. Carbohydr. Polym. 2017, 156, 409–416. [Google Scholar] [CrossRef]
- Wada, M.; Okano, T.; Sugiyama, J. AIIomorphs of native crystalline cellulose I evaluated by two equatorial d-spacings. J. Wood Sci. 2001, 47, 124–128. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.; Martin, A.; Conrad, C.; Jr, A.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]



| CNCs Properties | CNCSO3H | CNCCOOH | CNCCOOR |
|---|---|---|---|
| Hydrodynamic diameter (nm) | 130.55 ± 2.35 | 176.78 ± 3.52 | 175.02 ± 0.53 |
| Z potential at pH (mV) | −29.60 ± 0.71 | −35.65 ± 0.21 | −36.30 ± 0.42 |
| Conductivity (mS cm−1) | 1.31 ± 0.05 | 0.82 ± 0.04 | 1.37 ± 0.07 |
| Polydispersityindex (%) | 27.53 ± 0.51 | 25.24 ± 0.48 | 25.3 ± 0.53 |
| Types of Cellulose Nanocrystals | Total Charges Density (mmol/kg) |
|---|---|
| CNCSO3H | 0.30 ± 0.04 |
| CNCCOOH | 0.52 ± 0.07 |
| CNCCOOR | 0.88 ± 0.06 |
| Laminated Polymers | P-CNCSO3H-P | P-CNCCOOH-P | P-CNCCOOR-P |
|---|---|---|---|
| Elastic Modulus (N/m) | 13,000–36,000 | 14,000–43,000 | 18,000–28,000 |
| Breaking Strength (N/m) | 14–20 | 16–60 | 22–74 |
| Stiffness (N/m) | 650–7000 | 3600–5000 | 3500–8300 |
| P-CNCSO3H-P | 50% RH | 80% RH |
|---|---|---|
| OPP- CNCSO3H/PE | <0.06 | 1.2 ± 0.25 |
| OPP- CNCSO3H/PLA | 40 ± 0.15 | 320 ± 3.5 |
| PET- CNCSO3H/PE | 0.25 ± 0.15 | 1.107 ± 0.25 |
| PET- CNCSO3H/PP | <0.06 | <0.06 |
| P-CNCCOOH-P | 50% RH | 80% RH |
| OPP- CNCCOOH/PE | <0.06 | <0.06 |
| OPP- CNCCOOH/PLA | 12.41 ± 3.5 | 201.11 ± 3.5 |
| PET- CNCCOOH/PE | <0.06 | <0.06 |
| PET- CNCCOOH/PP | <0.06 | <0.06 |
| P-CNCCOOR-P | 50% RH | 80% RH |
| OPP- CNCCOOR/PE | <0.06 | <0.06 |
| OPP- CNCCOOR/PLA | 3.78 ± 3.5 | 103.55 ± 3.5 |
| PET- CNCCOOR/PE | <0.06 | <0.06 |
| PET-CNCCOOR/PP | <0.06 | <0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotie, G.; Gazzotti, S.; Ortenzi, M.A.; Piergiovanni, L. Implementation of High Gas Barrier Laminated Films Based on Cellulose Nanocrystals for Food Flexible Packaging. Appl. Sci. 2020, 10, 3201. https://doi.org/10.3390/app10093201
Fotie G, Gazzotti S, Ortenzi MA, Piergiovanni L. Implementation of High Gas Barrier Laminated Films Based on Cellulose Nanocrystals for Food Flexible Packaging. Applied Sciences. 2020; 10(9):3201. https://doi.org/10.3390/app10093201
Chicago/Turabian StyleFotie, Ghislain, Stefano Gazzotti, Marco Aldo Ortenzi, and Luciano Piergiovanni. 2020. "Implementation of High Gas Barrier Laminated Films Based on Cellulose Nanocrystals for Food Flexible Packaging" Applied Sciences 10, no. 9: 3201. https://doi.org/10.3390/app10093201
APA StyleFotie, G., Gazzotti, S., Ortenzi, M. A., & Piergiovanni, L. (2020). Implementation of High Gas Barrier Laminated Films Based on Cellulose Nanocrystals for Food Flexible Packaging. Applied Sciences, 10(9), 3201. https://doi.org/10.3390/app10093201







