Functional Roles of Saccades for a Hand Movement
Abstract
1. Introduction
2. Materials and Methods
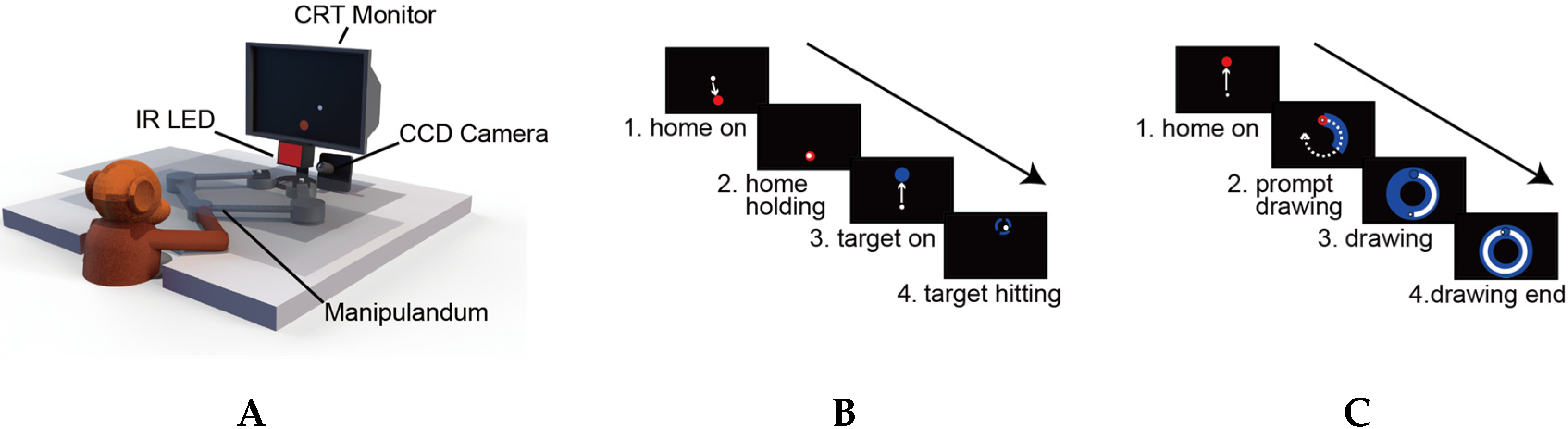
2.1. Subjects and Apparatus
2.2. Behavioral Tasks
2.3. Data Recording and Analysis
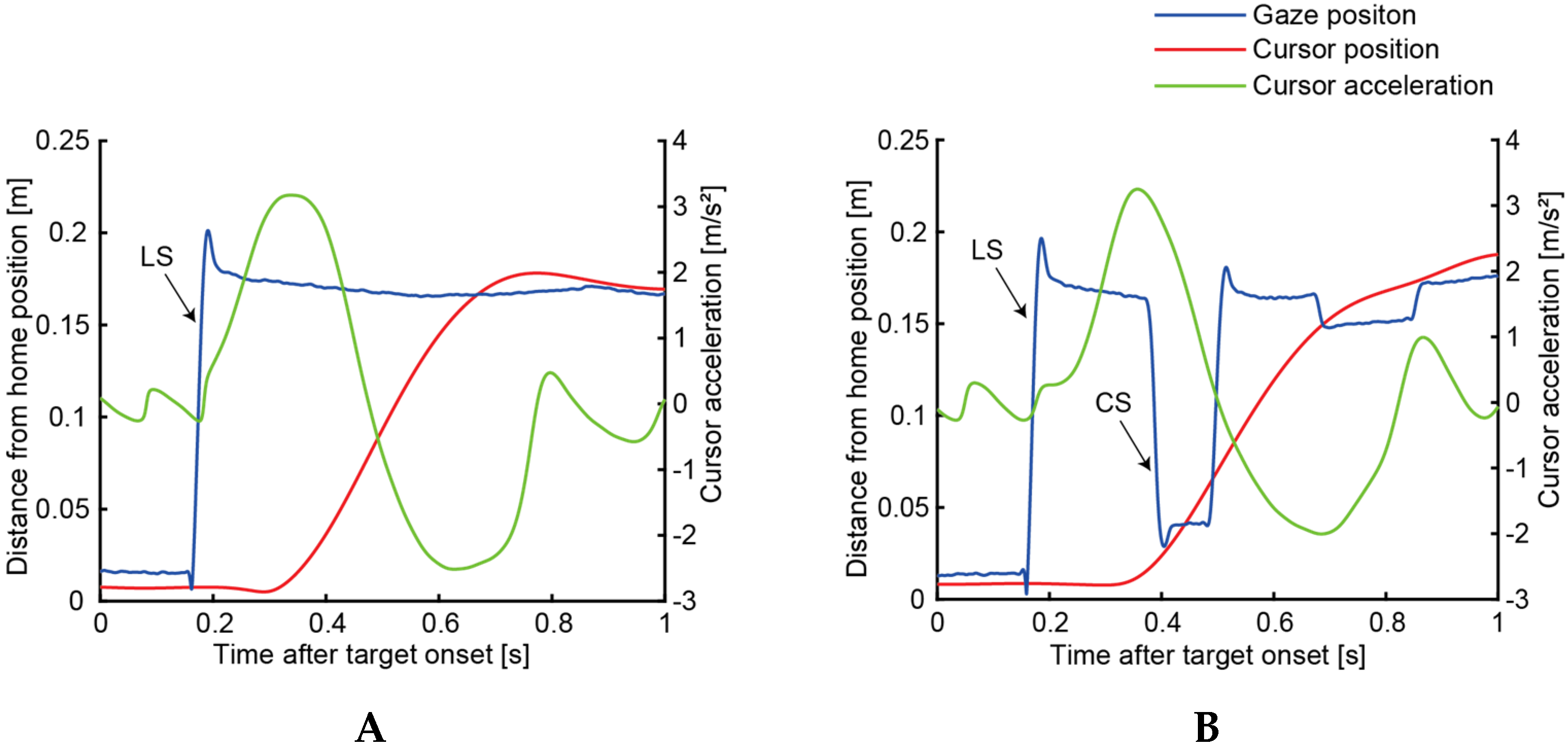
2.3.1. Saccade
2.3.2. Hand Movement
3. Results
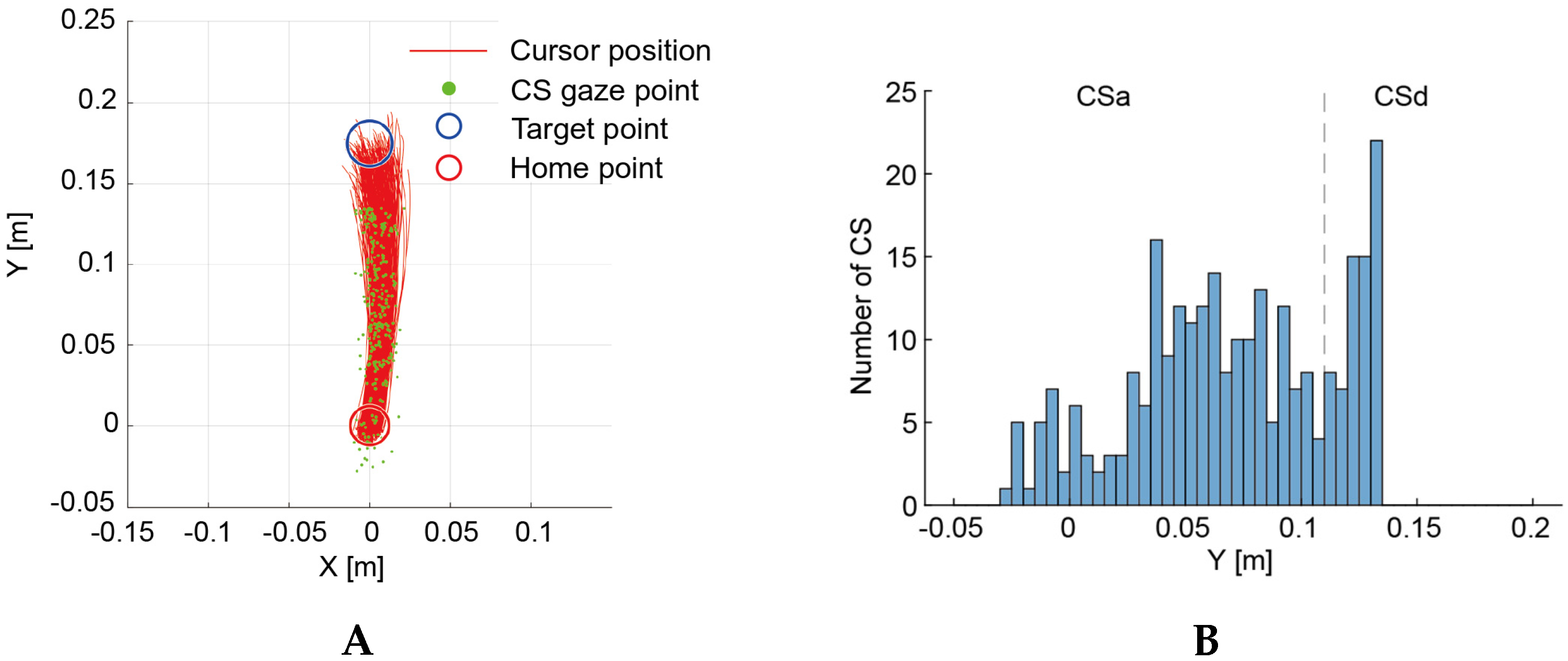
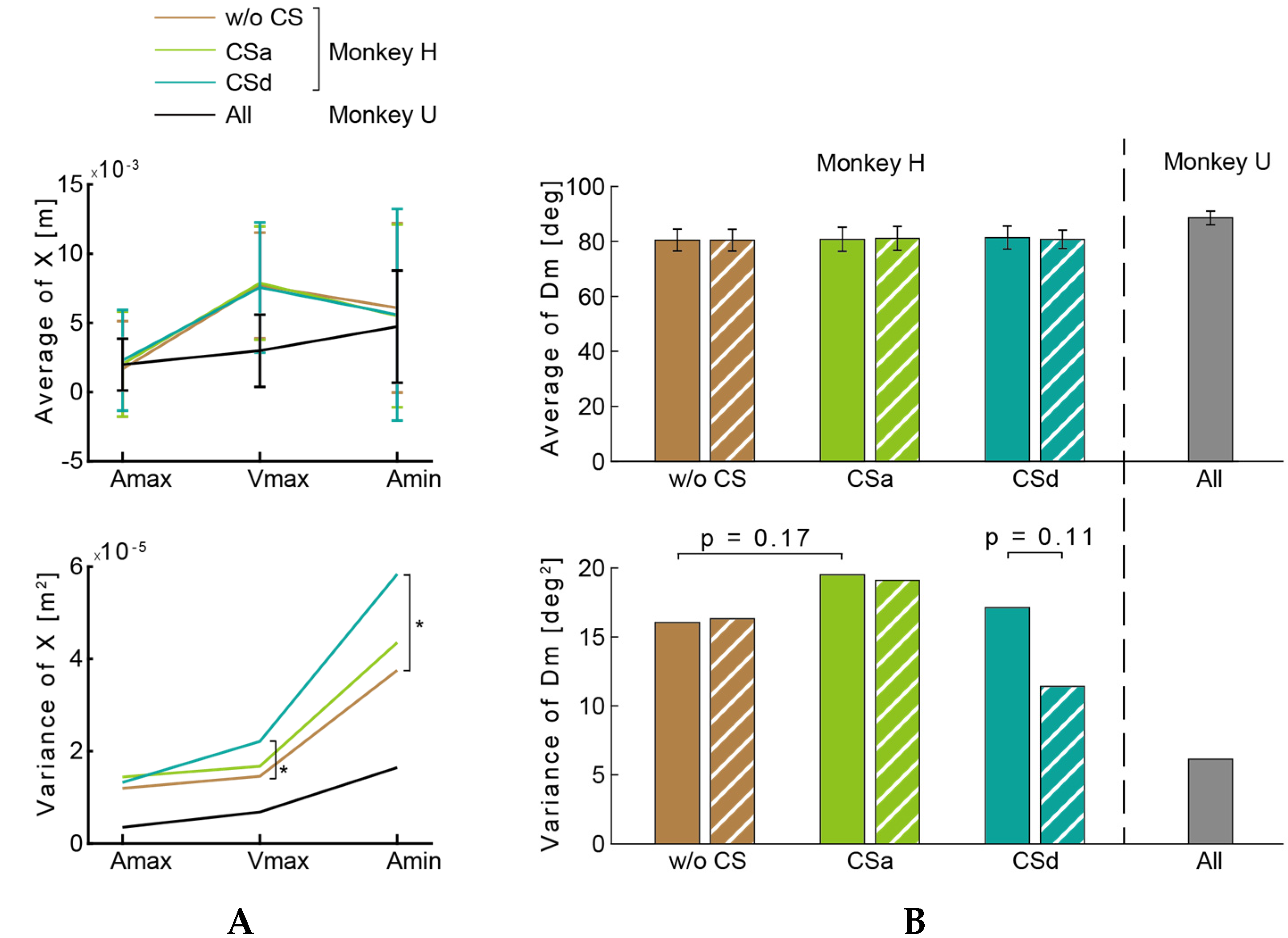
3.1. Hitting Task
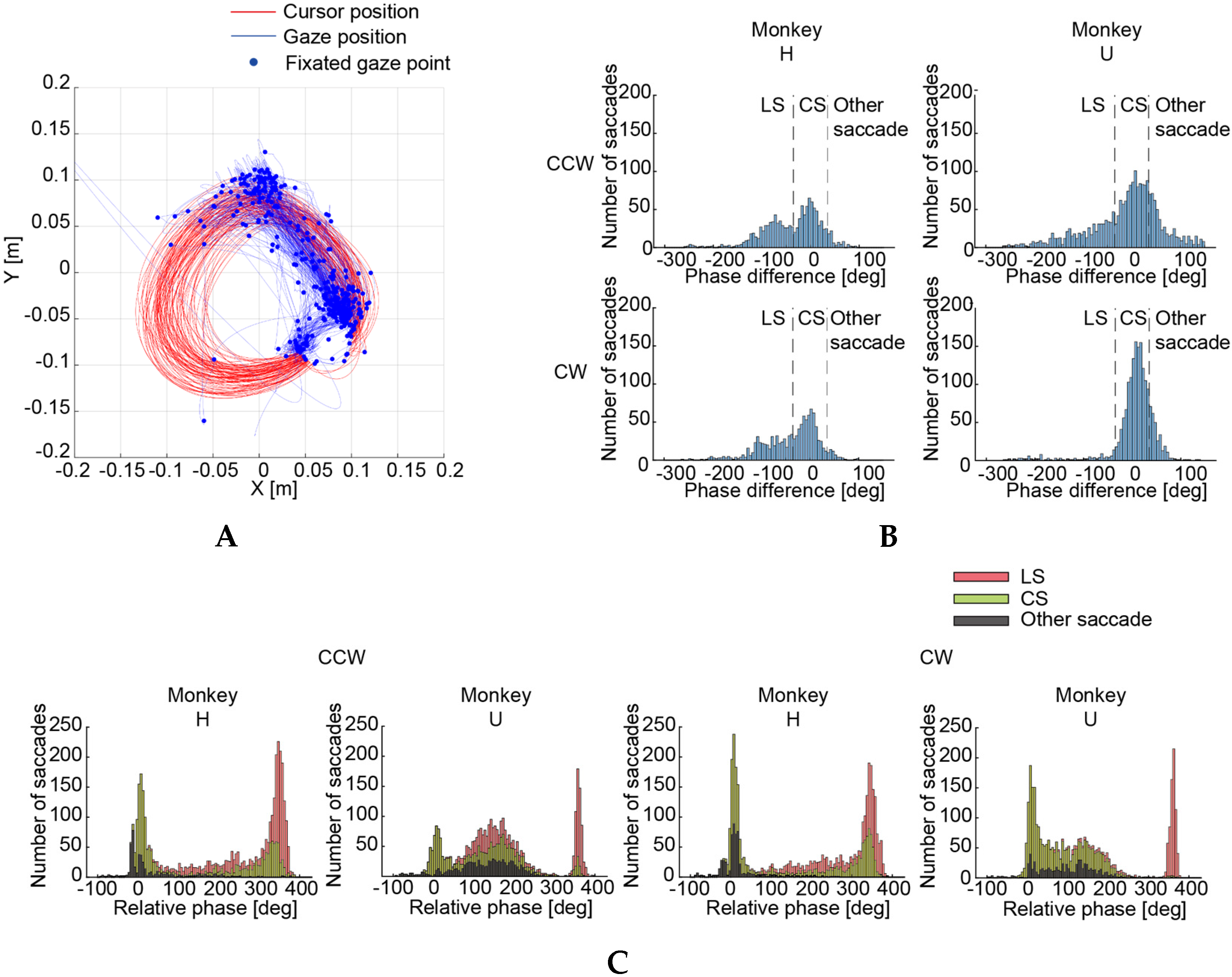
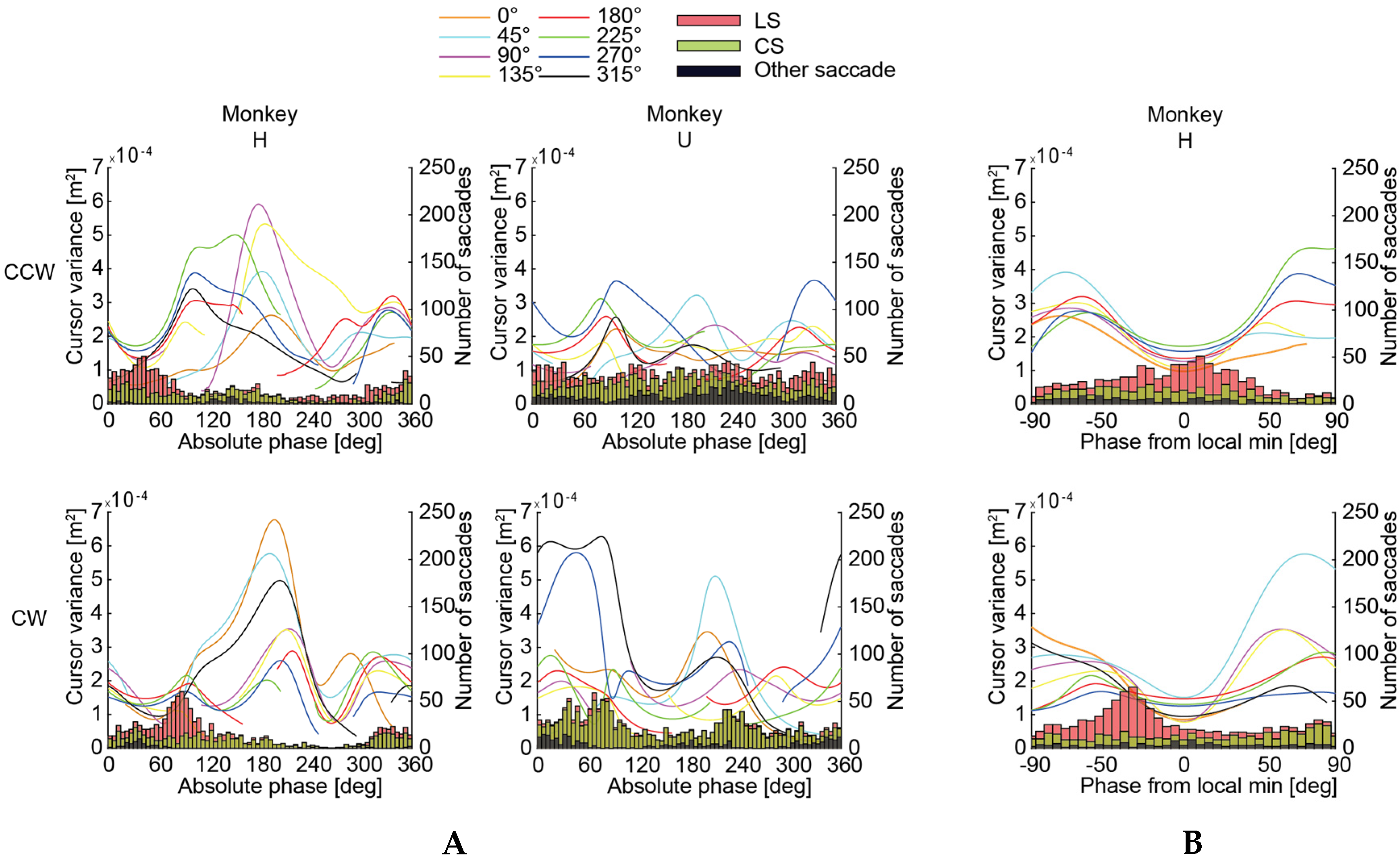
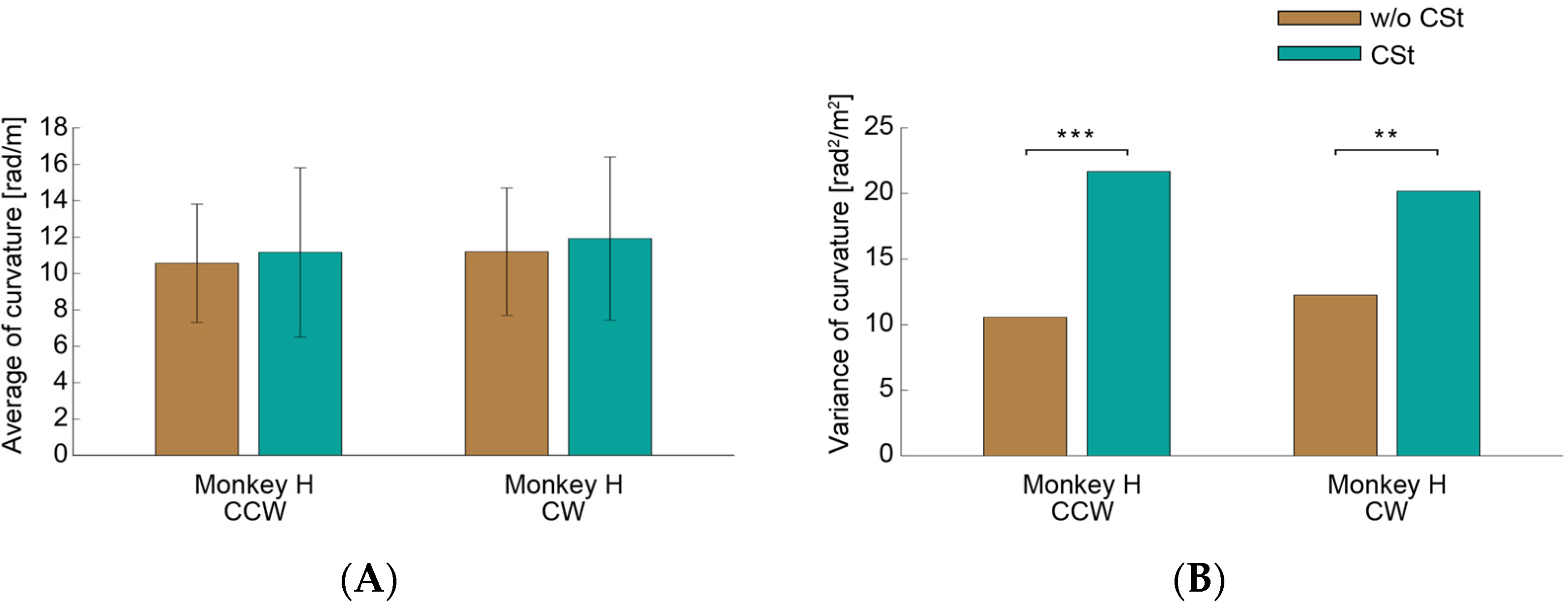
3.2. Circle-Drawing Task
4. Discussion
4.1. Functional Roles of the Saccades
4.2. The Saccades and the Control Model of an Arm Movement
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morasso, P.; Mussa Ivaldi, F.A. Trajectory formation and handwriting: A computational model. Biol. Cybern. 1982, 45, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Viviani, P.; Cenzato, M. Segmentation and coupling in complex movements. J. Exp. Psychol. Hum. Percept. Perform. 1985, 11, 828–845. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Kawato, M. A via-point time optimization algorithm for complex sequential trajectory formation. Neural Netw. 2004, 17, 353–364. [Google Scholar] [CrossRef]
- Morasso, P. Spatial Control of Arm Movements. Exp. Brain Res. 1981, 42, 223–227. [Google Scholar] [CrossRef]
- Gomi, H.; Kawato, M. Human arm stiffness and equilibrium-point trajectory during multi-joint movement. Biol. Cybern. 1997, 76, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, A.P.; Kalaska, J.F.; Caminiti, R.; Massey, J.T. On the Relations between the Direction of Two-Dimensional Arm Movements and Cell Discharge in Primate Motor Cortex. J. Neurosci. 1982, 2, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.H.; Kalaska, J.F. Changes in motor cortex activity during reaching movements with similar hand paths but different arm postures. J. Neurophysiol. 1995, 73, 2563–2567. [Google Scholar] [CrossRef] [PubMed]
- Klein Breteler, M.D.; Hondzinski, J.M.; Flanders, M. Drawing sequences of segments in 3D: Kinetic influences on arm configuration. J. Neurophysiol. 2003, 89, 3253–3263. [Google Scholar] [CrossRef]
- Abrams, R.A.; Meyer, D.E.; Kornblum, S. Eye-hand coordination: Oculomotor control in rapid aimed limb movements. J. Exp. Psychol. Hum. Percept. Perform. 1990, 16, 248–267. [Google Scholar] [CrossRef]
- Frens, M.A.; Erkelens, C.J. Coordination of hand movements and saccades: Evidence for a common and a separate pathway. Exp. Brain Res. 1991, 85, 682–690. [Google Scholar] [CrossRef]
- Bekkering, H.; Adam, J.J.; van den Aarssen, A.; Kingma, H.; Whiting, H.T. Interference between saccadic eye and goal-directed hand movements. Exp. Brain Res. 1995, 106, 475–484. [Google Scholar] [CrossRef]
- Helsen, W.F.; Elliott, D.; Starkes, J.L.; Ricker, K.L. Temporal and spatial coupling of point of gaze and hand movements in aiming. J. Motor Behav. 1998, 30, 249–259. [Google Scholar] [CrossRef]
- Lunenburger, L.; Kutz, D.F.; Hoffmann, K.P. Influence of arm movements on saccades in humans. Eur. J. Neurosci. 2000, 12, 4107–4116. [Google Scholar] [CrossRef]
- Diaz, G.; Cooper, J.; Rothkopf, C.; Hayhoe, M. Saccades to future ball location reveal memory-based prediction in a virtual-reality interception task. J. Vis. 2013, 13, 20. [Google Scholar] [CrossRef]
- Mann, D.L.; Nakamoto, H.; Logt, N.; Sikkink, L.; Brenner, E. Predictive eye movements when hitting a bouncing ball. J. Vis. 2019, 19, 28. [Google Scholar] [CrossRef]
- Beaubaton, D.; Hay, L. Contribution of Visual Information to Feedforward and Feedback Processes in Rapid Pointing Movements. Hum. Mov. Sci. 1986, 5, 19–34. [Google Scholar] [CrossRef]
- Desmurget, M.; Grafton, S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn. Sci. 2000, 4, 423–431. [Google Scholar] [CrossRef]
- Saunders, J.A.; Knill, D.C. Humans use continuous visual feedback from the hand to control fast reaching movements. Exp. Brain Res. 2003, 152, 341–352. [Google Scholar] [CrossRef]
- Thaler, L.; Goodale, M.A. The role of online visual feedback for the control of target-directed and allocentric hand movements. J. Neurophysiol. 2011, 105, 846–859. [Google Scholar] [CrossRef][Green Version]
- Tchalenko, J. Eye movements in drawing simple lines. Perception 2007, 36, 1152–1167. [Google Scholar] [CrossRef]
- Ueyama, Y.; Miyashita, E. Devising a Robotic Arm Manipulandum for Normal and Altered Reaching Movements to Investigate Brain Mechanisms of Motor Control. Instrum. Sci. Technol. 2013, 41, 251–273. [Google Scholar] [CrossRef]
- Hinder, M.R.; Tresilian, J.R.; Riek, S.; Carson, R.G. The contribution of visual feedback to visuomotor adaptation: How much and when? Brain Res. 2008, 1197, 123–134. [Google Scholar] [CrossRef]
- Milner, T.E. A model for the generation of movements requiring endpoint precision. Neuroscience 1992, 49, 487–496. [Google Scholar] [CrossRef]
- Plamondon, R.; Alimi, A.M. Speed/accuracy trade-offs in target-directed movements. Behav. Brain Sci. 1997, 20, 279. [Google Scholar] [CrossRef]
- Matthis, J.S.; Yates, J.L.; Hayhoe, M.M. Gaze and the Control of Foot Placement When Walking in Natural Terrain. Curr. Biol. 2018, 28, 1224–1233. [Google Scholar] [CrossRef]
- Todorov, E.; Jordan, M.I. Optimal feedback control as a theory of motor coordination. Nat. Neurosci. 2002, 5, 1226–1235. [Google Scholar] [CrossRef]
- Liu, D.; Todorov, E. Evidence for the flexible sensorimotor strategies predicted by optimal feedback control. J. Neurosci. 2007, 27, 9354–9368. [Google Scholar] [CrossRef]
- Izawa, J.; Rane, T.; Donchin, O.; Shadmehr, R. Motor adaptation as a process of reoptimization. J. Neurosci. 2008, 28, 2883–2891. [Google Scholar] [CrossRef]
- Nagengast, A.J.; Braun, D.A.; Wolpert, D.M. Optimal control predicts human performance on objects with internal degrees of freedom. PLoS Comput. Biol. 2009, 5, e1000419. [Google Scholar] [CrossRef]
- Ueyama, Y.; Miyashita, E. Optimal Feedback Control for Predicting Dynamic Stiffness During Arm Movement. IEEE Trans. Ind. Electron. 2014, 61, 1044–1052. [Google Scholar] [CrossRef]
- Shadmehr, R.; Mussa-Ivaldi, F.A. Adaptive representation of dynamics during learning of a motor task. J. Neurosci. 1994, 14, 3208–3224. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, J.W.; Pine, Z.M.; Ghilardi, M.F.; Ghez, C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J. Neurosci. 2000, 20, 8916–8924. [Google Scholar] [CrossRef] [PubMed]
- Osu, R.; Burdet, E.; Franklin, D.W.; Milner, T.E.; Kawato, M. Different mechanisms involved in adaptation to stable and unstable dynamics. J. Neurophysiol. 2003, 90, 3255–3269. [Google Scholar] [CrossRef] [PubMed]
- Saijo, N.; Gomi, H. Multiple motor learning strategies in visuomotor rotation. PLoS ONE 2010, 5, e9399. [Google Scholar] [CrossRef]
- Wang, J.X.; Kurth-Nelson, Z.; Kumaran, D.; Tirumala, D.; Soyer, H.; Leibo, J.Z.; Hassabis, D.; Botvinick, M. Prefrontal cortex as a meta-reinforcement learning system. Nat. Neurosci. 2018, 21, 860–868. [Google Scholar] [CrossRef]
- Sugiyama, T.; Schweighofer, N.; Izawa, J. Reinforcement meta-learning optimizes visuomotor learning. bioRxiv 2020. [Google Scholar] [CrossRef]
| Subject | Number of Trials | Saccade [Times/Trial] (Number of Trials) | ||||
|---|---|---|---|---|---|---|
| Total | LS | Total CS | CSa 1 | CSd 2 | ||
| Monkey H | 762 | 2.51 (762) | 1.77 (762) | 0.35 (242) | 0.27 (199) 3 | 0.09 (67) 3 |
| Monkey U | 556 | 1.49 (556) | 1.16 (540) | 0.01 (8) | 0.01(8) | 0.00 (0) |
| Subject | Direction 1 | Trial | Saccade [Times/Trial] (Number of Trials) All/Midway 2 | ||
|---|---|---|---|---|---|
| Total 3 | LS | CS | |||
| Monkey H | CCW | 743 | 5.22 (741)/ 1.81 (723) | 2.33 (735)/ 0.83 (457) | 2.27 (694)/ 0.86 (447) |
| CW | 751 | 4.77 (751)/ 1.72 (648) | 1.78 (714)/ 0.75 (439) | 2.22 (668)/ 0.88 (441) | |
| Monkey U | CCW | 749 | 4.70 (749)/ 3.04 (749) | 1.70 (691)/ 0.89 (531) | 1.80 (638)/ 1.35 (624) |
| CW | 769 | 4.60 (769)/ 2.29 (769) | 1.10 (679)/ 0.16 (110) | 2.60 (744)/ 1.73 (729) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakazume, Y.; Furubayashi, S.; Miyashita, E. Functional Roles of Saccades for a Hand Movement. Appl. Sci. 2020, 10, 3066. https://doi.org/10.3390/app10093066
Sakazume Y, Furubayashi S, Miyashita E. Functional Roles of Saccades for a Hand Movement. Applied Sciences. 2020; 10(9):3066. https://doi.org/10.3390/app10093066
Chicago/Turabian StyleSakazume, Yuki, Sho Furubayashi, and Eizo Miyashita. 2020. "Functional Roles of Saccades for a Hand Movement" Applied Sciences 10, no. 9: 3066. https://doi.org/10.3390/app10093066
APA StyleSakazume, Y., Furubayashi, S., & Miyashita, E. (2020). Functional Roles of Saccades for a Hand Movement. Applied Sciences, 10(9), 3066. https://doi.org/10.3390/app10093066





