Abstract
Perovskite and perovskite related oxides are important materials with applications ranging from solid oxide fuel cells, electronics, batteries and high temperature superconductors. The investigation of physical properties at the atomic scale such as self-diffusion is important to further improve and/or miniaturize electronic or energy related devices. In the present review we examine the oxygen self-diffusion and defect processes in perovskite and perovskite related oxides. This contribution is not meant to be an exhaustive review of the literature but rather aims to highlight the important mechanisms and ways to tune self-diffusion in this important class of energy materials.
1. Introduction
Diffusion is a fundamental process that impacts material and device properties in semiconductors, oxides and metals [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. Perovskite (refer to Figure 1) and perovskite related oxides have been widely studied for applications including electronics, batteries, solid oxide fuel cells (SOFC) and superconductors [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. As paradigms of the insights of the governing dynamics gained by the application of atomistic modelling, we consider here perovskite and perovskite related oxides employed in SOFC technology and batteries.
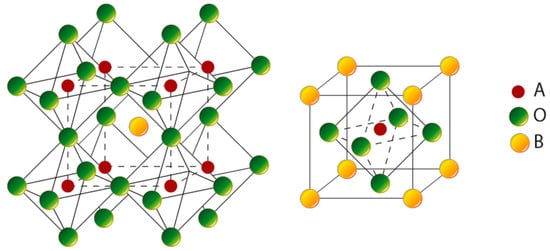
Figure 1.
A schematic of the cubic perovskite structure ABO3.
The importance of SOFCs is their potential for high efficiency energy conversion accompanied with reduced emission of greenhouse gases as compared to fossil fuel-based power generation [61,62]. For high operating temperatures (up to 1000 °C), SOFCs can operate with hydrogen and/or natural gas efficiently converting the chemical energy to electricity. SOFCs are applied in combined heat and power applications, but the high operating temperatures can result to materials issues and increased cost [63,64,65]. In particular, high temperatures lead to thermal cycling, performance degradation and the use of expensive materials in interconnects [65]. To alleviate these issues, the community aims to lower the operating temperatures of SOFCs to the intermediate temperature range (500–700 °C). Regrettably, however, this reduction in temperature leads to the increase in the losses for reaction and transport kinetics in the active layers of the SOFC, and in particular the cathode and electrolyte. This in turn motivated the community to investigate new classes of materials that have high oxygen diffusivities at 500–700 °C. It should be stressed that oxygen diffusivities are significant because at the intermediate temperature range the oxygen reduction reaction in the cathode and the oxygen diffusion in the cathode and electrolyte have to be accelerated (to alleviate for the lower temperature). This can be represented by the following reaction:
This relation describes the reduction of oxygen on the cathode surface, the diffusion of oxygen in the cathode and finally the diffusion of oxygen on the electrolyte. It is acknowledged that in the traditional SOFC materials (for example La1−xSrxMnO3−δ) the reduction of temperature will lead to electrical energy losses [66]. A way to overcome this problem is to use alternative materials such as the mixed ionic-electronic conductors (MIEC) [33,66,67], in which the oxygen reduction kinetics in MIEC electrodes is contributed by both the oxygen surface exchange and diffusion. Although oxygen diffusion needs also to be high at electrolytes there low, electronic conductivity is needed. Numerous studies in recent years have addressed oxygen diffusion in cathode and electrolyte materials [51,68,69,70,71,72,73,74].
The second example considered in the present review is battery materials. Demand for electrical energy storage is ever increasing particularly for mobile applications. As the requirement is to have high energy densities and capacities there is a lot of scientific and technological interest on solid state rechargeable Li-ion batteries [75]. In these devices, the solid electrolyte is a key component and this has been the driving force for numerous theoretical and experimental studies [75]. Increasing the ionic conductivity by replacing the solvent electrolytes with solid materials is a way to overcome the restrictions emerging in electrochemical applications. Additionally, the electrolyte has to be compatible with the electrode materials and be able to withstand the diffusion of ions during the battery life cycle [76].
Ionic diffusion can be influenced and even tuned by numerous parameters including the (i.e., crystal structure, doping and composition) and external parameters (i.e., elastic strain). Atomic scale modelling has the advantage that it can deconvolute the individual contributions of these parameters thus offering a way to gain insights on their impact in physical properties such as self-diffusion. This in turn can lead to progress in the selection of materials of technological importance and the rapid tuning of their properties, leading experimental work to the most promising systems [77,78].
In the present review article, we focus on ionic diffusion mechanisms and the related energetics of SOFC and battery materials. The main emphasis is to demonstrate how atomic scale modelling can provide insights of ionic diffusion in structurally complicated systems. The review is structured as follows. First, we briefly discuss diffusion mechanisms and the atomistic scale methodologies. Thereafter, cathode and electrolyte SOFC materials are considered with particular focus on the Ruddlesden–Popper series (for example La2NiO4) and double perovskites (for example GdBaCo2O5+δ). Then the focus is on disordered oxides such as lithium lanthanum titanates (La2/3-xLi3xTiO3) and the importance of methodological advances to gain insights on their complicated mechanisms of diffusion. The final part is concerned on the future directions to optimise the ionic diffusion including the promising ideas of grain boundary engineering.
2. Diffusion Mechanisms
Diffusion in materials is a fundamental and complicated phenomenon. The added complexity in oxide materials, as compared to metals, is the anion and cation sublattices. These sublattices can restrict the ionic diffusion to its own sublattice, however, the cation sublattice may have a significant impact upon the anion diffusion. In oxides oxygen self-diffusion is typically orders of magnitude faster than cation self-diffusion [79,80,81,82].
For net diffusion to occur in a crystalline material atoms have to migrate away from their equilibrium positions towards neighbouring equilibrium positions. For this process to occur point defects (interstitials and/or vacancies) are necessary [34]. For the materials considered is the present review there are mainly three mechanisms: the interstitial, the interstitialcy and the vacancy mechanisms. There are also mechanisms, which are based upon the point defects but also involve cooperative motion of the local polyhedral structures.
Considering first the vacancy mechanism of diffusion, in which an ion diffuses by migrating to a neighbouring vacancy [81,83]. The vacancy mechanism necessitates the presence of lattice vacancies, with their concentration in the lattice and migration energy barriers effectively controlling the transport kinetics. The vacancy mechanism is common for oxygen self-diffusion in a number of oxygen hypostoichiometric oxides that are of interest to SOFC (for example fluorite- and perovskite-related systems) [84].
In the interstitial mechanism, interstitial ions migrate from one interstitial site to a neighbouring interstitial site. Conversely, to the vacancy mechanism in the interstitial mechanism when a jump between interstitial sites is completed there is no net displacement of the other ions. Additionally, the interstitial mechanism does only require the interstitial ions and no other point defects. Finally, the interstitialcy mechanism is distinct from the interstitial mechanism, as the interstitial ion displaces another ion from its equilibrium lattice site. Following this the displaced ion moves to another interstitial site and so on.
In the vacancy mechanism, the migration enthalpies for the thermally activated vacancy hopping is the critical factor determining how low a temperature the electrolyte and cathode can effectively function at. According to transition state theory, the rate of vacancy hops, ν, is given by the Boltzmann relationship,
where EM is the migration barrier and νo is a constant.
The vacancy diffusivity, Dv, is related to the mean square displacement of the vacancies via the Einstein relation,
It should be noted that It should be noted that Dv exponentially depends on the energy barrier for oxygen vacancy migration. The oxygen diffusivity, DO, is connected to Dv via the following relation,
where cv is the vacancy concentration fraction.
3. Atomistic Simulation Methodology
As the focus of the present review is the diffusion in energy materials, we will briefly introduce here molecular dynamics (MD) and density functional theory (DFT) methods. The quantum mechanical formulation offers the most complete description, however, the analytical solution of Schrödinger’s equation for a large number of electrons is practically impossible due to the complexity of many-electron interactions [85]. DFT is in essence an approximation that allows the efficient modelling of solids [86,87]. Presently in DFT, the common way to address the exchange-correlation energy is by the local density approximation (LDA), the generalized gradient approximation (GGA) and hybrid functional, which incorporate a part of the exact exchange from Hartree–Fock theory [88]. Typically, a plane-wave basis set is used with the pseudopotential method, with the core electrons being described by effective potentials (known as pseudopotentials) and the valence electrons evolving explicitly. Diffusion energetics and mechanisms can be investigated using DFT as the activation energy of diffusion can be calculated by identifying the minimum energy path, using methods such as the nudged elastic band (NEB) [89]. The main issue of DFT simulations is the relatively small number of atoms that can be modelled (presently a few hundred atoms) and this is an issue particularly when modelling complicated diffusion mechanisms (for example, involving the cooperative motion of local polyhedral structures in the lattice).
MD using classical potentials is a very common method to study the energetics of diffusion in energy materials. MD the state of the system can be described by the positions and the momenta of all the constituent particles, with Newton’s equations of motion for an ensemble of particles being solved iteratively. The interactions between particles is through potential energy functions, typically within the classical Born-like description of the crystal lattice [90]. In these the ionic interactions are defined by a long-range Coulombic term and by a short-range parameterized pair potential (for example the Buckingham potential [91]). The key advantage of MD as compared to DFT is that extended systems can be modelled, however, this is not a method that can be used when the description of the electronic structure of the system becomes important [92,93,94,95,96]. In essence, not unlike experimental techniques different methodologies are more appropriate for different length scales and properties.
Finally, it should be stressed that the use of thermodynamic models (for example the cBΩ model) can be used to gain further insights from the experimental and computational modelling methods as it was demonstrated for other systems in previous work [97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113].
4. Ruddlesden–Popper Layered Oxides
Ruddlesden–Popper series oxides have been considered for intermediate temperature oxide fuel cells and in particular cathodes for a number of years [114,115,116,117]. They can be described by the formula An+1BnO3n+1. The first members (n = 1) of the series include the widely studied La2NiO4. La2NiO4 is tetragonal (space group I4/mmm) [117] at a wide temperature range (423–1073 K) including the temperature that are important of Intermediate temperature solid oxide fuel cells (IT-SOFC). For completeness, La2NiO4 is orthorhombic (Cmca) at room temperature [118]. As a side line it should be mentioned La2NiO4 is in the K2NiF4 structure that is widely known as it is the structure of La2−xBaxCuO4 the archetypal material for high temperature superconductivity [119].
The intricacy in these materials is that oxygen concentration can have a significant impact on the oxygen diffusion properties. This is because self-diffusion is dominated by oxygen and oxygen vacancies (in oxygen deficient or hypostoichiometric oxides) or oxygen interstitials (in oxygen excess or hyperstoichiometric oxides) act as the vehicles for diffusion. More formally and in Kröger–Vink notation [120] this can be described by:
where is the oxygen diffusion coefficient, is the vacancy diffusion coefficient, is the interstitial diffusion coefficient, is the oxygen vacancy concentration and is the oxygen interstitial concentration.
Considering La2NiO4 is oxygen hypostoichiometric, the excess oxygen is typically related to the experimental conditions of the samples [70,121,122]. For numerous years the experimentally determined activation energies of diffusion in lanthanum nikelate were in a wide range 0.19–0.90 eV [40,115,122,123,124,125], whereas there was no agreement on the diffusion mechanism. Classical molecular dynamics work [124] calculated an activation energy of diffusion of 0.51 eV in excellent agreement with the time of flight secondary ion mass spectrometry (ToF-SIMS) study of Sayers et al. [122] (0.54 eV). Additionally, the MD study proposed the interstitialcy in the a-b plane diffusion mechanism for oxygen self-diffusion (refer to Figure 2) [125]. An analogous interstitialcy mechanism in the a-b plane was also proposed in the experimental (neutron scattering experiments with analysis based on the maximum entropy method) work by Yashima et al. [74] in the related tetragonal (Pr0.9La0.1)2(Ni0.74Cu0.21Ga0.05)O4+δ.
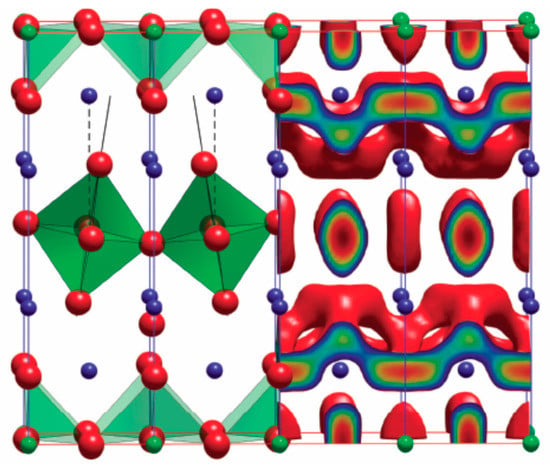
Figure 2.
In the left part of the figure the tetragonal Pr2NiO4+ δ where O is in red, NiO6 octahedra in green and Ni in blue (c-axis is the vertical axis). The right part of the figure represents the isosurface connecting the O diffusion sites in the a-b plane as derived using molecular dynamics at 1100 K and δ = 0.09875 Reproduced by permission from the Owner Societies [125].
It should be noted that research on the Ruddlesden–Popper series oxides continues to attract the interest of the research community and although self-diffusion is well characterised theoretically and experimentally, the focus is on proton diffusivity, doping and structural/electronic/magnetic properties [126,127,128,129,130,131,132,133,134,135].
5. Double Perovskites
The interest on double perovskite materials (AA’B2O5-δ) as cathodes for SOFC was motivated by the seminal studies of Taskin et al. [69,136]. The secondary ion mass spectrometry work by Tarancon et al. [73] confirmed the high oxygen self-diffusion of oxygen in GdBaCo2O5+δ and determined a tracer diffusion coefficient of 0.6 eV. This is also in agreement with the classical molecular dynamics work of Parfitt et al. [137] with the calculated activation energy of diffusion of 0.5 eV as the 0.1 eV difference can be traced in the methodological errors. Additionally, Parfitt et al. [137] showed that the oxygen self-diffusion mechanism is in the a-b- plane. In particular the MD calculations revealed that oxygen migration in this double perovskite is highly anisotropic taking place in the Gd-O and the adjacent Co-O layers. The energetics and mechanisms of oxygen self-diffusion in double perovskites are also supported by the MD studies of Hermet et al. [138] and experimental work [139]. The classical atomistic and MD studies of Seymour et al. [53] showed that oxygen diffusion in PrBaCo2O5+δ is analogous but with a lower activation energy of diffusion (0.35 eV) constituting this material very promising. An interesting feature of the double perovskites is the antisite disorder in the cation sublattice. The impact of cation ordering on oxygen diffusion was experimentally observed by Taskin et al. [69,136] in the related material Gd0.5Ba0.5MnO3±δ. In particular, Taskin et al. [69,136] determined a significant increase in the oxygen diffusion coefficient in the cation ordered perovskite as compared to the cation disordered perovskite. The question that arises is: how does the antisite disorder in the cation sublattice impact the energetics and mechanism of oxygen self-diffusion in the double perovkite system? Parfitt et al. [137] used classical MD calculations to show that the self-diffusion of oxygen in GdBaCo2O5+δ is highly dependent on the cation disorder. In particular, for ordered GdBaCo2O5.5, the oxygen diffusion mechanism is anisotropic along the a-b plane, whereas disorder in the cation sublattice (formation of Ba and Gd antisites) results to a reduction of the oxygen diffusivity and diffusion in the c-axis (refer to Figure 3) [137]. The higher the disorder in the cation sublattice, the more isotropic the mechanism of oxygen self-diffusion [137]. From a technological viewpoint this implies that the thermal history of the material and sample preparation are important parameters that could impact the energetics and mechanism of diffusion of these systems. For SOFC applications ordered double perovskites should be preferred.
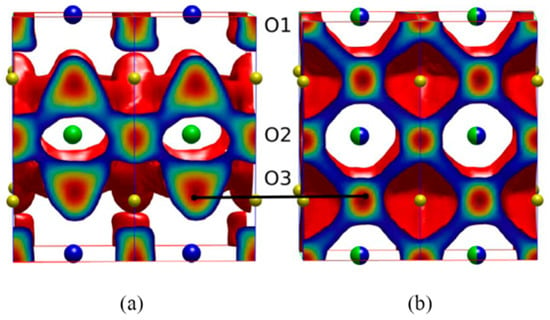
Figure 3.
A schematic representation of the MD calculated oxygen density profiles for GdBaCo2O5.5. (a) when ordered (anisotropic diffusion) and (b) fully disordered (isotropic diffusion) [137]. Blue and green spheres represent Ba and Gd ions respectively (Copyright 2011 Royal Society of Chemistry).
6. Others Perovskite Systems
It has been 34 years since the discovery of high temperature superconductivity by Bednorz and Müller [119] and many thousands of studies have taken place but there is still no unambiguous explanation of superconductivity and related phenomena (i.e., pseudo gap state, linear dependence of electrical resistance) [140,141,142,143,144,145,146,147,148,149]. Although LnBa2Cu3O7-δ are very important perovskite-related materials (crystal structure is a combination of perovskite and rock salt units) with superconducting properties (high critical temperatures) [140,141,142,143,144,145,146,147,148,149] they will not be reviewed in detail here as the focus is on materials with fast oxygen self-diffusion. Oxygen vacancies (in the Cu-O planes) and oxygen vacancy diffusion in these materials is of importance as it can impact the structure and superconducting properties [140,141,142,143,144,145,146,147,148,149]. The activation energies of oxygen self-diffusion of LnBa2Cu3O7-δ are typically in the range 0.76-0.98 eV [150,151,152,153,154,155]. An important observation is that in LnBa2Cu3O7-δ there is an increase in the activation energy and pre-exponential factor of oxygen self-diffusion (refer to [51] and references therein).
There is a large amount of experimental and theoretical investigations on the materials properties of perovskite and related materials. As it was demonstrated in the Ruddlesden–Popper series example, the experimental determination of the self-diffusion properties and in particular the diffusion mechanism can be a challenge and, in that respect, computational modelling work can provide useful and complementary information. This becomes even more pronounced when considering disordered perovskite related structures such as lithium lanthanum titanates (La2/3-xLi3xTiO3, LLTO). In what follows we will describe how computational modelling techniques involving the fruitful marriage of classical molecular dynamics with genetic algorithms can describe the complicated Li self-diffusion in LLTO. La2/3-xLi3xTiO3 has high ionic conductivities and exhibits non-Arrhenius behaviour. Through MD analysis insights can be gained on the three-dimensional network of diffusion pathways via which ions migrate in different directions. Importantly in this perovskite cations form low La content (Lapoor) and high La content (Larich) atomic layers, which in turn will impact the lithium migration [156]. Jay et al. [59] introduced the ordering degree in the crystal using the following relation [59]:
where Rdis. (R(La-rich)) is the occupancies of the A-sites by the exact amount of La3+ ions for the disordered (La-rich) layered structure. Therefore, for S = 0 the system’s structure is completely disordered. Figure 4 represents LLTO with Lapoor and Larich layers coloured in blue and yellow respectively. For S = 0 the MD calculations by Jay et al. [59] revealed that lithium diffusion is completely homogeneous and isotropic. From an atomistic point of view this can be explained as lithium diffusion in LLTO is facilitated by vacancies, therefore the stoichiometry and the ordering degree that constitute the percolating network (refer to Figure 4) are key [59]. Given the number of possible combination of Lapoor and Larich layers it becomes computationally intractable to consider all the different possibilities for large number of atoms. To overcome this hurdle Jay et al. [59] investigated the impact of the structure on the conductivity of LLTO using MD combined with a genetic algorithm (GA) (refer to Figure 5). This approach was the first to reproduce the experimental results in this material [157], and more importantly it is an effective defect engineering strategy to maximise the lithium diffusivity by tailoring the lanthanum content of the layers. This approach is a paradigm and in essence transferable thus it can lead to the design of advanced materials with optimised properties particularly in disordered systems with a wide range of possible compositions and stoichiometry [158,159,160,161,162,163]. The trend is on machine learning techniques to encounter difficult problems in science and engineering [164].
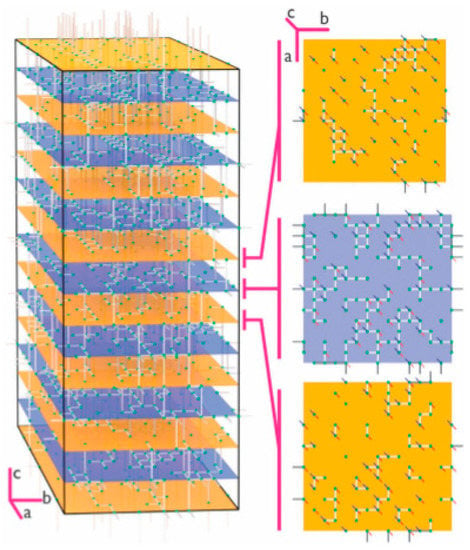
Figure 4.
A schematic representation of diffusion pathways in lithium lanthanum titanate. Here the pathways in the ab directions are shown for every Lapoor and Larich layer. This is an example of how insights can be gained by the synergetic application of molecular dynamics with genetic algorithms. Reproduced by permission from the Owner Societies [59].
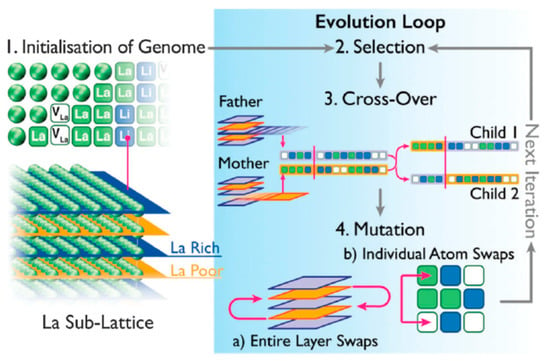
Figure 5.
A schematic representation describing how the genetic algorithm favors “inheritance” from structures having high diffusivity to yield the iterative refinement of structures. Reproduced by permission from the Owner Societies [59].
7. Summary
In the present review, we have considered the diffusion processes of perovskite and related oxides. These materials are technologically important for a range of applications including sensors, SOFC and batteries. Although they have been investigated by the community for numerous decades, recent experimental and theoretical methodological advances (such as ToF-SIMS and DFT) provided insight and have accelerated progress.
Considering the paradigm of disordered ionic conductors can be an effective way to proceed given the advantages (for example blocked pathways do not affect diffusion) and the new compositional and structural possibilities for the discovery of new materials. The latter could benefit by the fruitful combination of atomistic simulation (MD and/or DFT) with machine learning.
Author Contributions
All authors contributed to the writing and Editing of the review. All authors have read and agreed to the published version of the manuscript.
Funding
Alexander Chroneos is grateful for funding from the Lloyd’s Register Foundation, a charitable foundation helping to protect life and property by supporting engineering-related education, public engagement and the application of research.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Werner, M.; Mehrer, H.; Hochheimer, H.D. Effect of hydrostatic pressure, temperature, and doping on self-diffusion in germanium. Phys. Rev. B 1985, 32, 3930–3937. [Google Scholar] [CrossRef]
- Giese, A.; Stolwijk, N.A.; Bracht, H. Double-hump diffusion profiles of copper and nickel in germanium wafers yielding vacancy-related diffusion. Appl. Phys. Lett. 2000, 77, 642–644. [Google Scholar] [CrossRef]
- Chui, C.O.; Gopalakrishnan, K.; Griffin, P.B.; Plummer, J.D.; Saraswat, K.C. Activation and diffusion of ion-implanted p and n dopants in germanium. Appl. Phys. Lett. 2003, 83, 3275–3277. [Google Scholar] [CrossRef]
- Chroneos, A.; Skarlatos, D.; Tsamis, C.; Christofi, A.; McPhail, D.S.; Hung, R. Implantation an diffusion of phosphorous in germanium. Mater. Sci. Semicond. Proc. 2006, 9, 640–643. [Google Scholar] [CrossRef]
- Janke, C.; Jones, R.; Coutinho, J.; Öberg, S.; Briddon, P.R. Ab initio investigation of phosphorus diffusion paths in germanium. Phys. Rev. B 2008, 77, 195210. [Google Scholar] [CrossRef]
- Brotzmann, S.; Bracht, H.; Lundsgaard Hansen, J.; Nylandsted Larsen, A.; Simoen, E.; Haller, E.E.; Christensen, J.S.; Werner, P. Diffusion and defect reactions between donors, C, and vacancies in Ge. I Experimental results. Phys. Rev. B 2008, 77, 235207. [Google Scholar] [CrossRef]
- Chroneos, A.; Grimes, R.W.; Uberuaga, B.P.; Bracht, H. Diffusion and defect reactions between donors, C, and vacancies in Ge. II Atomistic calculations of related complexes. Phys. Rev. B 2008, 77, 235208. [Google Scholar] [CrossRef]
- Chroneos, A.; Bracht, H.; Grimes, R.W.; Uberuaga, B.P. Vacancy-mediated dopant diffusion activation enthalpies for germanium. Appl. Phys. Lett. 2008, 92, 172103. [Google Scholar] [CrossRef]
- Bruno, E.; Mirabella, S.; Scapellato, G.; Impellizzeri, G.; Terrasi, A.; Priolo, F.; Napolitani, E.; De Salvador, D.; Mastramatteo, M.; Carnera, A. Mechanism of B diffusion in crystalline Ge under proton irradiation. Phys. Rev. B 2009, 80, 033204. [Google Scholar] [CrossRef]
- Chroneos, A. Effect of germanium substrate loss and nitrogen on dopant diffusion in germanium. J. Appl. Phys. 2009, 105, 056101. [Google Scholar] [CrossRef]
- Kube, R.; Bracht, H.; Chroneos, A.; Posselt, M.; Schmidt, B. Intrinsic and extrinsic diffusion of indium in germanium. J. Appl. Phys. 2009, 106, 063534. [Google Scholar] [CrossRef]
- Bracht, H.; Schneider, S.; Klug, J.N.; Liao, C.Y.; Hansen, J.L.; Haller, E.E.; Larsen, A.N.; Bougeard, D.; Posselt, M.; Wundisch, C. Interstitial-mediated diffusion in germanium under proton irradiation. Phys. Rev. Lett. 2009, 103, 255501. [Google Scholar] [CrossRef] [PubMed]
- Chroneos, A.; Grimes, R.W.; Bracht, H. Fluorine codoping in germanium to suppress donor diffusion and deactivation. J. Appl. Phys. 2009, 106, 063707. [Google Scholar] [CrossRef]
- Rupasov, D.; Chroneos, A.; Parfitt, D.; Kilner, J.A.; Grimes, R.W.; Istomin, S.Y.; Antipov, E.V. Oxygen diffusion in Sr0.75Y0.25CoO2.625: A molecular dynamics study. Phys. Rev. B 2009, 79, 172102. [Google Scholar] [CrossRef]
- Kushima, A.; Yildiz, B. Oxygen ion diffusivity in strained yttria stabilized zirconia: Where is the fastest strain? J. Mater. Chem. 2010, 20, 4809–4819. [Google Scholar] [CrossRef]
- Impellizzeri, G.; Boninelli, S.; Priolo, F.; Napolitani, E.; Spinella, C.; Chroneos, A.; Bracht, H. Fluorine effect on As diffusion in Ge. J. Appl. Phys. 2011, 109, 113527. [Google Scholar] [CrossRef]
- Tahini, H.; Chroneos, A.; Grimes, R.W.; Schwingenschlögl, U.; Bracht, H. Diffusion of E centers in germanium predicted using GGA+U approach. Appl. Phys. Lett. 2011, 99, 072112. [Google Scholar] [CrossRef][Green Version]
- Chroneos, A.; Schwingenschlögl, U.; Dimoulas, A. Impurity diffusion, point defect engineering and surface/interface passivation in germanium. Ann. Phys. (Berl.) 2012, 524, 123–132. [Google Scholar]
- Ruprecht, B.; Wilkening, M.; Uecker, R.; Heitjans, P. Extremely slow Li ion dynamics in monoclinic Li2TiO3—Probing macroscopic jump diffusion via 7Li NMR stimulated echoes. Phys. Chem. Chem. Phys. 2012, 14, 11974–11980. [Google Scholar] [CrossRef]
- Rushton, M.J.D.; Chroneos, A.; Skinner, S.J.; Kilner, J.A.; Grimes, R.W. Effect of strain on the oxygen diffusion in yttria and gadolinia co-doped ceria. Solid State Ion. 2013, 230, 37–42. [Google Scholar] [CrossRef]
- Schneider, S.; Bracht, H.; Klug, J.N.; Hansen, J.L.; Larsen, A.N.; Bougeard, D.; Haller, E.E. Radiation-enhanced self- and boron diffusion in germanium. Phys. Rev. B. 2013, 87, 115202. [Google Scholar] [CrossRef]
- Tahini, H.A.; Chroneos, A.; Grimes, R.W.; Schwingenschlögl, U.; Bracht, H. Point defect engineering strategies to retard phosphorous diffusion in germanium. Phys. Chem. Chem. Phys. 2013, 15, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Wohlmuth, D.; Epp, V.; Bottke, P.; Hanzu, I.; Bitschnau, B.; Letofsky-Papst, I.; Kriechbaum, M.; Amenitsch, H.; Hofer, F.; Wilkening, M. Order vs. disorder-a huge increase in ionic conductivity of nanocrystalline LiAlO2 embedded in an amorphous-like matrix of lithium aluminate. J. Mater. Chem. A 2014, 2, 20295–20306. [Google Scholar]
- Rushton, M.J.D.; Chroneos, A. Impact of uniaxial strain and doping on oxygen diffusion in CeO2. Sci. Rep. 2014, 4, 6068. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Bredow, T. Interstitial lithium diffusion pathways in γ-LiAlO2: A computational study. J. Phys. Chem. Lett. 2015, 6, 4622–4626. [Google Scholar] [CrossRef]
- Chroneos, A.; Sgourou, E.N.; Londos, C.A.; Schwingenschlögl, U. Oxygen defect processes in silicon and silicon germanium. Appl. Phys. Rev. 2015, 2, 021306. [Google Scholar] [CrossRef]
- Wang, X.W.; Chen, J.G.; Tian, Y.W.; Wang, X.E.; Zhang, B.H.; Chang, X.H. Lattice strain dependent on ionic conductivity of Ce0.8+xY0.2−2xSrxO1.9 (x=0–0.08) electrolyte. Solid State Ion. 2016, 296, 85–89. [Google Scholar]
- Sgourou, E.N.; Panayiotatos, Y.; Vovk, R.V.; Chroneos, A. Toward defect engineering strategies to optimize energy and electronic materials. Appl. Sci. 2017, 7, 674. [Google Scholar] [CrossRef]
- Kuganathan, N.; Kordatos, A.; Anurakavan, S.; Iyngaran, P.; Chroneos, A. Li3SbO4 lithium-ion battery material: Defects, lithium ion diffusion and tetravalent dopants. Mater. Chem. Phys. 2019, 225, 34–41. [Google Scholar]
- Kuganathan, N.; Kordatos, A.; Chroneos, A. Li2SnO3 as a Cathode Material for Lithium-ion Batteries: Defects, Lithium Ion Diffusion and Dopants. Sci. Rep. 2018, 8, 12621. [Google Scholar]
- Kuganathan, N.; Tsoukalas, L.H.; Chroneos, A. Defects, dopants and Li-ion diffusion in Li2SiO3. Solid State Ion. 2019, 335, 61–66. [Google Scholar] [CrossRef]
- Mizusaki, J.; Mima, Y.; Yamauchi, S.; Fueki, K.; Tagawa, H. Nonstoichiometry of the perovskite-type oxides La1−xSrxCoO3−δ. J. Solid State Chem. 1989, 80, 102–111. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.M.; Kruidhof, H.; Burggraaf, A.J. Importance of the surface exchange kinetics as rate-limiting step in oxygen permeation through mixed-conducting oxides. Solid State Ion. 1994, 72, 185–194. [Google Scholar] [CrossRef]
- Adler, S.B. Mechanism and kinetics of oxygen reduction on porous La1−xSrxCoO3−δ electrodes. Solid State Ion. 1998, 111, 125–134. [Google Scholar] [CrossRef]
- De Souza, R.A.; Kilner, J.A. Oxygen transport in La1−xSrxMn1−yCoyO3±δ perovskites: Part I. Oxygen tracer diffusion. Solid State Ion. 1998, 106, 175–187. [Google Scholar] [CrossRef]
- Kharton, V.V.; Viskup, A.P.; Kovalevsky, A.V.; Naumovich, E.N.; Marques, F.M.B. Ionic transport in oxygen-hyperstoichiometric phases with K2NiF4-type structure. Solid State Ion. 2001, 143, 337–353. [Google Scholar] [CrossRef]
- Horita, T.; Yamaji, K.; Sakai, N.; Yokokawa, H.; Weber, A.; Ivers-Tiffee, E. Oxygen reduction mechanism at porous La1−xSrxCoO3−δ cathodes/La0.8Sr0.2Ga0.8Mg0.2O2.8 electrolyte interface for solid oxide fuel cells. Electrochim. Acta 2001, 46, 1837–1845. [Google Scholar] [CrossRef]
- Kawada, T.; Suzuki, J.; Sase, M.; Kaimai, A.; Yashiro, K.; Nigara, Y.; Mizusaki, J.; Kawamura, K.; Yugami, H. Determination of oxygen vacancy concentration in a thin film of La0.6Sr0.4CoO3−δ by an electrochemical method. J. Electrochem. Soc. 2002, 149, E252–E259. [Google Scholar] [CrossRef]
- Van der Haar, L.M.; den Otter, M.W.; Morskate, M.; Bouwmeester, H.J.M.; Verweij, H. Chemical diffusion and oxygen surface transfer of La1−xSrxCoO3−δ studied with electrical conductivity relaxation. J. Electrochem. Soc. 2002, 149, J41–J46. [Google Scholar] [CrossRef]
- Bassat, J.M.; Odier, P.; Villesuzanne, A.; Marin, C.; Pouchard, M. Anisotropic ionic transport properties in La2NiO4+δ single crystals. Solid State Ion. 2004, 167, 341–347. [Google Scholar] [CrossRef]
- Esquirol, A.; Brandon, N.P.; Kilner, J.A.; Mogensen, M. Electrochemical characterization of La0.6Sr0.4Co0.2. Fe0.8O3 cathodes for intermediate-temperature SOFCs. J. Electrochem. Soc. 2004, 151, A1847–A1855. [Google Scholar] [CrossRef]
- Munnings, C.N.; Skinner, S.J.; Amow, G.; Whitfield, P.S.; Davidson, I.J. Oxygen transport in the La2Ni1−xCoxO4+δ system. Solid State Ion. 2005, 176, 1895–1901. [Google Scholar] [CrossRef]
- Baumann, F.S.; Fleig, J.; Habermeier, H.U.; Maier, J. Impedance spectroscopic study on well-defined (La,Sr)(Co,Fe)O3−δ model electrodes. Solid State Ion. 2006, 177, 1071–1081. [Google Scholar] [CrossRef]
- Yashiro, K.; Nakamura, T.; Sase, M.; Hermes, F.; Sato, K.; Kawada, T.; Mizusaki, J. Electrode performance at hetero-interface of perovskite-related oxides, (La,Sr)CoO3−δ/(La,Sr)2CoO4−δ. Ecs Trans. 2007, 7, 1287–1292. [Google Scholar]
- Smadici, S.; Abbamonte, P.; Bhattacharya, A.; Zhai, X.; Jiang, B.; Rusydi, A.; Eckstein, J.N.; Bader, S.D.; Zuo, J.M. Electronic reconstruction at SrMnO3-LaMnO3 superlattice interfaces. Phys. Rev. Lett. 2007, 99, 196404. [Google Scholar] [CrossRef]
- Baumann, F.S.; Maier, J.; Fleig, J. The polarization resistance of mixed conducting SOFC cathodes: A comparative study using thin film model electrodes. Solid State Ion. 2008, 179, 1198–1204. [Google Scholar] [CrossRef]
- Garcia-Barriocanal, J.; Rivera-Calzada, A.; Varela, M.; Sefrioui, Z.; Iborra, E.; Leon, C.; Pennycook, S.J.; Santamaria, J. Colossal ionic conductivity at interfaces of epitaxial ZrO2:Y2O3/SrTiO3 heterostructures. Science 2008, 321, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Sase, M.; Hermes, F.; Yashiro, K.; Sato, K.; Mizusaki, J.; Kawada, T.; Sakai, N.; Yokokawa, H. Enhancement of oxygen surface exchange at the hetero-interface of (La,Sr)CoO3/(La,Sr)2CoO4 with PLD-layered films. J. Electrochem. Soc. 2008, 155, B793–B797. [Google Scholar] [CrossRef]
- LaO’, G.J.; Ahn, S.J.; Crumlin, E.; Orikasa, Y.; Biegalski, M.D.; Christen, H.M.; Shao-Horn, Y. Catalytic activity enhancement for oxygen reduction on epitaxial perovskite thin films for solid-oxide fuel cells. Angew. Chem. 2010, 49, 5344–5347. [Google Scholar]
- Kushima, A.; Yip, S.; Yildiz, B. Competing strain effects in reactivity of LaCoO3 with oxygen. Phys. Rev. B. 2010, 82, 115435. [Google Scholar] [CrossRef]
- Chroneos, A.; Vovk, R.V.; Goulatis, I.L.; Goulatis, L.I. Oxygen transport in perovskite and related oxides: A brief review. J. Alloy. Compd. 2010, 494, 190–195. [Google Scholar] [CrossRef]
- Berenov, A.V.; Atkinson, A.; Kilner, J.A.; Bucher, E.; Sitte, W. Oxygen tracer diffusion and surface exchange kinetics in La0.6Sr0.4CoO3−δ. Solid State Ion. 2010, 181, 819–826. [Google Scholar] [CrossRef]
- Seymour, I.D.; Chroneos, A.; Kilner, J.A.; Grimes, R.W. Defect processes in orthorhombic LnBaCo2O5.5 double perovskites. Phys. Chem. Chem. Phys. 2011, 13, 15305–15310. [Google Scholar]
- Santiso, J.; Burriel, M. Deposition and characterisation of epitaxial oxide thin films for SOFCs. J. Solid State Electrochem. 2011, 15, 985–1006. [Google Scholar] [CrossRef]
- Crumlin, E.J.; Ahn, S.J.; Lee, D.; Mutoro, E.; Biegalski, M.D.; Christen, H.M.; Shao-Horn, Y. Oxygen electrocatalysis on epitaxial La0.6Sr0.4CoO3−δ perovskite thin films for solid oxide fuel cells. J. Electrochem. Soc. 2012, 159, F219–F225. [Google Scholar] [CrossRef]
- Kubicek, M.; Cai, Z.; Ma, W.; Yildiz, B.; Hutter, H.; Fleig, J. Tensile lattice strain accelerates oxygen surface exchange and diffusion in La1–xSrxCoO3−δ thin films. ACS Nano 2013, 7, 3276–3286. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, Y.L.; Grimaud, A.; Hong, W.T.; Biegalski, M.D.; Morgan, D.; Shao-Horn, Y. Strontium influence on the oxygen electrocatalysis of La2−xSrxNiO4±δ (0.0 ≤ xSr ≤ 1.0) thin films. J. Mater. Chem. A 2014, 2, 6480–6487. [Google Scholar] [CrossRef]
- Yildiz, B. “Stretching” the energy landscape of oxides-Effects on electrocatalysis and diffusion. MRS Bull. 2014, 39, 147–156. [Google Scholar] [CrossRef]
- Jay, E.E.; Rushton, M.J.D.; Chroneos, A.; Grimes, R.W.; Kilner, J.A. Genetics of superionic conductivity in lithium lanthanum titanates. Phys. Chem. Chem. Phys. 2015, 17, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, Y.L.; Wang, X.R.; Morgan, D.; Shao-Horn, Y. Enhancement of oxygen surface exchange on epitaxial La0.6Sr0.4Co0.2Fe0.8O3−δ thin films using advanced heterostructured oxide interface engineering. MRS Commun. 2016, 6, 204–209. [Google Scholar] [CrossRef]
- Singhal, S.C. Advances in solid oxide fuel cell technology. Solid State Ion. 2000, 135, 305–313. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Minh, N.Q.; Takahashi, T. Science and Technology of Ceramic Fuel Cells; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Fleig, J. Solid oxide fuel cells: Polarization mechanisms and modelling of the electrochemical performance. Annu. Rev. Mater. Res. 2003, 33, 361–382. [Google Scholar] [CrossRef]
- Jacobson, A.J. Materials for solid oxide fuel cells. Chem. Mater. 2010, 22, 660–674. [Google Scholar] [CrossRef]
- Tarancón, A.; Burriel, M.; Santiso, J.; Skinner, S.J.; Kilner, J.A. Advances in layered oxide cathodes for intermediate temperature solid oxide fuel cells. J. Mater. Chem. 2010, 20, 3799–3813. [Google Scholar] [CrossRef]
- Adler, S.B.; Lane, J.A.; Steele, B.C.H. Electrode kinetics of porous mixed-conducting oxygen electrodes. J. Electrochem. Soc. 1996, 143, 3554–3564. [Google Scholar] [CrossRef]
- Boehm, E.; Bassat, J.M.; Dordor, P.; Mauvy, F.; Grenier, J.C. Oxygen transport properties of La2Ni1−xCuxO4+δ mixed donducting oxides. Solid State Sci. 2003, 5, 973–981. [Google Scholar] [CrossRef]
- Taskin, A.A.; Lavrov, A.N.; Ando, Y. Achieving fast oxygen diffusion in perovskites by cation ordering. Appl. Phys. Lett. 2005, 86, 091910. [Google Scholar] [CrossRef]
- Boehm, E.; Bassat, J.M.; Dordor, P.; Mauvy, F.; Grenier, J.C.; Stevens, P. Oxygen diffusion and transport properties in non-stoichiometric Ln2−xNiO4+δ oxides. Solid State Ion. 2005, 176, 2717–2725. [Google Scholar] [CrossRef]
- Frayret, C.; Villesuzanne, A.; Pouchard, M. Application of density functional theory to the modelling of the mixed ionic and electronic conductor La2NiO4+δ: Lattice relaxation, oxygen mobility, and energetics of Frenkel defects. Chem. Mater. 2005, 17, 6538–6544. [Google Scholar] [CrossRef]
- Kim, G.; Wang, S.; Jacobson, A.J.; Reimus, L.; Brodersen, P.; Mims, C.A. Rapid oxygen ion diffusion and surface exchange kinetics in PrBaCo2O5+x with a perovskite related structure and ordered A cations. J. Mater. Chem. 2007, 17, 2500–2505. [Google Scholar] [CrossRef]
- Tarancón, A.; Skinner, S.J.; Chater, R.J.; Hernádez-Ramírez, F.; Kilner, J.A. Layered perovskites as promising cathodes for intermediatetemperature solid oxide fuel cells. J. Mater. Chem. 2007, 17, 3175–3181. [Google Scholar] [CrossRef]
- Yashima, M.; Enoki, M.; Wakita, T.; Ali, R.; Matsushita, Y.; Izumi, F.; Ishihara, T. Structural disorder and diffusional pathway of oxide ions in a doped Pr2NiO4+δ-based mixed conductor. J. Am. Chem. Soc. 2008, 130, 2762–2763. [Google Scholar] [CrossRef]
- Bachman, J.C.; Muy, S.; Grimaud, A.; Chang, H.H.; Pour, N.; Lux, S.F.; Paschos, O.; Maglia, F.; Lupart, S.; Lamp, P.; et al. Inorganic solid-state electrolytes for lithium batteries: Mechanisms and properties governing ion conduction. Chem. Rev. 2016, 116, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Urban, A.; Li, X.; Su, D.; Hautier, G.; Ceder, G. Unlocking the potential of cation-disordered oxides for rechargeable lithium batteries. Science 2014, 519–522. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.A.; Maier, J. A computational study of cation defects in LaGaO3. Phys. Chem. Chem. Phys. 2003, 5, 740–748. [Google Scholar] [CrossRef]
- Predith, A.; Ceder, G.; Wolverton, C.; Persson, K.; Mueller, T. Ab initio prediction of ordered ground-state structures in ZrO2-Y2O3. Phys. Rev. B 2008, 77, 144104. [Google Scholar] [CrossRef]
- Kingery, W.D.; Bowen, H.K.; Uhlmann, D.R. Introduction to Ceramics; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Chiang, Y.M.; Birnie, D.; Kingery, W.D. Physical Ceramics: Principles for Ceramic Science and Engineering; MIT Press: Cambridge, CA, USA, 1997. [Google Scholar]
- Mehrer, H. Diffusion in Solids; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Miyoshi, S.; Martin, M. B-site cation diffusivity of Mn and Cr in perovskite-type LaMnO3 with cation-deficit nonstoichiometry. Phys. Chem. Chem. Phys. 2009, 11, 3063–3070. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B. Oxide-ion electrolytes. Annu. Rev. Mater. Res. 2003, 33, 91–128. [Google Scholar] [CrossRef]
- Kilner, J.A.; Irvine, J.T.S. Handbook of Fuel Cells—Advances in Electrocatalysis, Materials, Diagnostics and Durability; Vielstich, W., Gasteiger, H.A., Yokokawa, H., Eds.; John Wiley & Sons: Chichester, England, 2009; Volume 5. [Google Scholar]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W. Nobel lecture: Electronic structure of matter—Wave functions and density functionals. Rev. Mod. Phys. 1999, 71, 1253–1266. [Google Scholar] [CrossRef]
- Koch, W.; Holthausen, M.C. A Chemist’s Guide to Density Functional Theory; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Born, M.; Mayer, J.E. Zur Gittertheorie der IonenKristalle. Z. Phys. 1932, 75, 1. [Google Scholar] [CrossRef]
- Buckingham, R.A. The classical equation of state of gaseous helium, neon and argon. Proc. R. Soc. Lond. Ser. Amath. Phys. Sci. 1938, 168, 264–283. [Google Scholar]
- Chroneos, A.; Bracht, H.; Grimes, R.W.; Uberuaga, B.P. Phosphorous clustering in germanium-rich silicon germanium. Mater. Sci. Eng. B 2008, 154–155, 72–75. [Google Scholar] [CrossRef]
- Chroneos, A. Dopant-vacancy cluster formation in germanium. J. Appl. Phys. 2010, 107, 076102. [Google Scholar] [CrossRef]
- Chroneos, A.; Jiang, C.; Grimes, R.W.; Schwingenschlögl, U.; Bracht, H. E centers in ternary Si1−x-yGexSny random alloys. Appl. Phys. Lett. 2009, 95, 112101. [Google Scholar] [CrossRef]
- Murphy, S.T.; Chroneos, A.; Grimes, R.W.; Jiang, C.; Schwingenschlögl, U. Phase stability and the arsenic vacancy defect in InxGa1−xAs. Phys. Rev. B 2011, 84, 184108. [Google Scholar] [CrossRef]
- Zhu, J.; Vasilopoulou, M.; Davazoglou, D.; Kennou, S.; Chroneos, A.; Schwingenschlögl, U. Intrinsic defects and H doping in WO3. Sci. Rep. 2017, 7, 40882. [Google Scholar] [CrossRef] [PubMed]
- Varotsos, P. Calculation of the migration volume of vacancies in ionic solids from macroscopic parameters. Phys. Stat. Sol. (a) 1978, 47, K133–K136. [Google Scholar] [CrossRef]
- Varotsos, P.; Alexopoulos, K. Thermodynamics of Point Defects and their Relation with the Bulk Properties; Amelinckx, S., Gevers, R., Nihoul, J., Eds.; North-Holland: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Varotsos, P. Comparison of models that interconnect point defect parameters in solids with bulk properties. J. Appl. Phys. 2007, 101, 123503. [Google Scholar] [CrossRef]
- Varotsos, P. Point defect parameters in β-PbF2 revisited. Solid State Ion. 2008, 179, 438–441. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, X.; Xu, J.; Zhou, R. Application of the cBΩ model for the calculation of oxygen self-diffusion coefficients in minerals. J. Appl. Phys. 2010, 108, 053505. [Google Scholar] [CrossRef]
- Vallianatos, F.; Saltas, V. Application of the cBΩ model to the calculation of diffusion parameters of He in olivine. Phys. Chem. Miner. 2014, 41, 181–188. [Google Scholar] [CrossRef]
- Zhang, B.; Shan, S. Application of the cBΩ model to the calculation of diffusion parameters of Si in silicates. Geochem. Geophys. Geosyst. 2015, 16, 705–718. [Google Scholar] [CrossRef]
- Chroneos, A.; Vovk, R.V. Modeling self-diffusion in UO2 and ThO2 by connecting point defect parameters with bulk properties. Solid State Ion. 2015, 274, 1–3. [Google Scholar] [CrossRef]
- Saltas, V.; Chroneos, A.; Vallianatos, F.A. Composition and temperature dependence of self-diffusion in Si1−xGex alloys. Sci. Rep. 2017, 7, 1374. [Google Scholar] [CrossRef]
- Cooper, M.W.D.; Grimes, R.W.; Fitzpatrick, M.E.; Chroneos, A. Modeling oxygen self-diffusion in UO2 under pressure. Solid State Ion. 2015, 282, 26–30. [Google Scholar] [CrossRef]
- Sarlis, N.V.; Skordas, E.S. Bulk moduli of PbSxSe1−x, PbSxTe1−x and PbSexTe1−x from the combination of the cBΩ model with the modified Born theory compared to generalized gradient approximation. Mod. Phys. Lett. B 2016, 30, 1650409. [Google Scholar] [CrossRef]
- Chroneos, A. Connecting point defect parameters with bulk properties to describe diffusion in solids. Appl. Phys. Rev. 2016, 3, 041304. [Google Scholar] [CrossRef]
- Parfitt, D.C.; Cooper, M.W.D.; Rushton, M.J.D.; Christopoulos, S.-R.G.; Fitzpatrick, M.E.; Chroneos, A. Thermodynamic calculations of oxygen self-diffusion in mixed-oxide nuclear fuels. RSC Adv. 2016, 6, 74018–74028. [Google Scholar] [CrossRef]
- Saltas, V.; Chroneos, A.; Vallianatos, F.A. A thermodynamic approach to self-diffusion in silicon: Evidence of a single diffusion mechanism? Mater. Chem. Phys. 2016, 181, 204–208. [Google Scholar] [CrossRef]
- Sarlis, N.V.; Skordas, E.S. Estimating the compressibility of osmium from recent measurements of Ir-Os alloys under high pressure. J. Phys. Chem. A 2016, 120, 1601–1604. [Google Scholar] [CrossRef]
- Saltas, V.; Chroneos, A.; Vallianatos, F.A. A thermodynamic approach of self- and hetero-diffusion in GaAs: Connecting point defect parameters with bulk properties. RSC Adv. 2016, 6, 53324–53330. [Google Scholar] [CrossRef]
- Sarlis, N.V.; Skordas, E.S. Interconnection of a thermodynamical method for point defect parameters in solids with the dynamical theory of diffusion. Solid State Ion. 2019, 335, 82–85. [Google Scholar] [CrossRef]
- Vashook, V.V.; Trofimenko, N.E.; Ullmann, H.; Makhnach, L.V. Oxygennonstoichiometry and some transport properties of LaSrNiO4−δ nickelate. Solid State Ion. 2000, 131, 329–336. [Google Scholar] [CrossRef]
- Skinner, S.J.; Kilner, J.A. Oxygen diffusion and surface exchange in La2–xSrxNiO4+δ. Solid State Ion. 2000, 135, 709–712. [Google Scholar] [CrossRef]
- Mauvy, F.; Bassat, J.M.; Boehm, E.; Manaud, J.P.; Dordor, P.; Grenier, J.C. Oxygen electrode reaction on Nd2NiO4+δ cathode materials: Impedance spectroscopy study. Solid State Ion. 2003, 158, 17–28. [Google Scholar] [CrossRef]
- Skinner, S.J. Characterisation of La2NiO4+δ using in-situ high temperature neutron powder diffraction. Solid State Sci. 2003, 5, 419–426. [Google Scholar] [CrossRef]
- Kajitani, T.; Kitagaki, Y.; Hiraga, K.; Hosoya, S.; Fukuda, T.; Yamaguchi, Y.; Wada, S.; Sugai, S.; Morii, Y.; Fuchizaki, K.; et al. Tetragonal and orthorhombic phases of La2NiO4+y. Physics C 1991, 185, 579–580. [Google Scholar] [CrossRef]
- Bednorz, J.G.; Müller, K.A. Possible high Tc superconductivity in the Ba-La-Cu-O system. Z. Phys. 1986, 64, 189–193. [Google Scholar] [CrossRef]
- Kröger, F.A.; Vink, H.J. Relations between the Concentrations of Imperfections in Crystalline Solids. In Solid State Physics; Seitz, F., Turnbull, D., Eds.; Academic Press: New York, NY, USA, 1956; Volume 3, pp. 307–435. [Google Scholar]
- Kilner, J.A.; Shaw, C.K.M. Mass transport in La2Ni1−xCoxO4+δ oxides with the K2NiF4 structure. Solid State Ion. 2002, 154–155, 523–527. [Google Scholar] [CrossRef]
- Sayers, R.; De Souza, R.A.; Kilner, J.A.; Skinner, S.J. Low temperature diffusion and oxygen stoichiometry in lanthanum nickelate. Solid State Ion. 2010, 181, 386–391. [Google Scholar] [CrossRef]
- Burriel, M.; Garcia, G.; Santiso, J.; Kilner, J.A.; Chater, R.J.; Skinner, S.J. Anisotropic oxygen diffusion properties in epitaxial thin films of La2NiO4+δ. J. Mater. Chem. 2008, 18, 416–422. [Google Scholar] [CrossRef]
- Chroneos, A.; Parfitt, D.; Kilner, J.A.; Grimes, R.W. Anisotropic oxygen diffusion in tetragonal La2NiO4+δ: Molecular dynamics calculations. J. Mater. Chem. 2010, 20, 266–270. [Google Scholar] [CrossRef]
- Parfitt, D.; Chroneos, A.; Kilner, J.A.; Grimes, R.W. Molecular dynamics study of oxygen diffusion in Pr2NiO4+δ. Phys. Chem. Chem. Phys. 2010, 12, 6834–6836. [Google Scholar] [CrossRef] [PubMed]
- Perrichon, A.; Piovano, A.; Boehm, M.; Zbiri, M.; Johnson, M.; Schoder, H.; Ceretti, M.; Paulus, W. Lattice dynamics modified by excess oxygen in Nd2NiO4+δ: Triggering low temperature oxygen diffusion. J. Phys. Chem. C. 2015, 119, 1557–1564. [Google Scholar] [CrossRef]
- Burriel, M.; Tellez, H.; Charter, R.J.; Castaing, R.; Veber, P.; Zaghrioui, M.; Ishihara, T.; Kilner, J.A.; Bassat, J.M. Influence of crystal orientation and annealing on the oxygen diffusion and surface exchange of La2NiO4+δ. J. Phys. Chem. C 2016, 120, 17927–17938. [Google Scholar] [CrossRef]
- Lee, D.; Lee, H.N. Controlling oxygen mobility in Ruddlesden-Popper oxides. Materials 2017, 10, 368. [Google Scholar] [CrossRef]
- Parfitt, D.; Kordatos, A.; Filippatos, P.P.; Chroneos, A. Diffusion in energy materials: Governing dynamics from atomistic modelling. Appl. Phys. Rev. 2017, 4, 031305. [Google Scholar] [CrossRef]
- Saher, S.; Song, J.; Vibhu, V.; Nicollet, C.; Flura, A.; Bassat, J.M.; Bouwmeester, H.J.M. Influence of annealing at intermediate temperature on oxygen transport kinetics of Pr2NiO4+δ. J. Mater. Chem. A 2018, 6, 8331–8339. [Google Scholar] [CrossRef]
- Tropin, E.S.; Ananyev, M.V.; Farlenkov, A.S.; Khodimchuk, A.V.; Berenov, A.V.; Fetisov, A.V.; Eremin, V.A.; Kolchugin, A.A. Surface defect chemistry and oxygen exchange kinetics in La2−xCaxNiO4+δ. J. Solid State Chem. 2018, 262, 199–213. [Google Scholar] [CrossRef]
- Xu, S.Z.; Jacobs, R.; Morgan, D. Factors controlling oxygen interstitial diffusion in the Ruddlesden-Popper oxide La2−xSrxNiO4+δ. Chem. Mater. 2018, 30, 7166–7177. [Google Scholar] [CrossRef]
- Maity, S.R.; Ceretti, M.; Keller, L.; Schefer, J.; Shang, T.; Pomjakushina, E.; Meven, M.; Sheptyakov, D.; Cervellino, A.; Paulus, W. Structural disorder and magnetic correlations driven by oxygen doping in Nd2NiO4+δ (δ ~ 0.11). Phys. Rev. Mater. 2019, 3, 083604. [Google Scholar] [CrossRef]
- Zhang, L.F.; Yao, F.; Meng, J.L.; Zhang, W.W.; Wang, H.C.; Liu, X.J.; Meng, J.; Zhang, H.J. Oxygen migration and proton diffusivity in transition-metal (Mn, Fe, Co, and Cu) doped Ruddlesden-Popper oxides. J. Mater. Chem. A 2019, 7, 18558–18567. [Google Scholar] [CrossRef]
- Vibhu, V.; Suchomel, M.R.; Penin, N.; Weill, F.; Grenier, J.C.; Bassat, J.M.; Rougier, A. Structural transformation of the La2−xPrxNiO4+δ system probed by high-resolution synchrotron and neutron powder diffraction. Dalton Trans. 2019, 48, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Taskin, A.A.; Lavrov, A.N.; Ando, Y. Fast oxygen diffusion in A-site ordered perovskites. Prog. Solid State Chem. 2007, 35, 481–490. [Google Scholar] [CrossRef]
- Parfitt, D.; Chroneos, A.; Tarancon, A.; Kilner, J.A. Oxygen ion diffusion in cation ordered/disordered GdBaCo2O5+δ. J. Mater. Chem. 2011, 21, 2183–2186. [Google Scholar] [CrossRef]
- Hermet, J.; Geneste, G.; Dezanneau, G. Molecular dynamics simulations of oxygen diffusion in GdBaCo2O5.5. App. Phys. Lett. 2010, 97, 174102. [Google Scholar] [CrossRef]
- Burriel, M.; Pena-Martinez, J.; Chater, R.J.; Fearn, S.; Berenov, A.V.; Skinner, S.J.; Kilner, J.A. Anisotropic oxygen ion diffusion in layered PrBaCo2O5+δ. Chem. Mater. 2012, 24, 613–621; Zapata, J.; Burriel, M.; Garcia, P.; Kilner, J.A.; Santiso, J. Anisotropic 18O tracer diffusion in epitaxial films of GdBaCo2O5+δ cathode material with different orientations. J. Mater. Chem. A. 2013, 1, 7408–7414. [Google Scholar]
- Vovk, R.V.; Obolenskii, M.A.; Zavgorodniy, A.A.; Bondarenko, A.V.; Goulatis, I.L.; Samoilov, A.V. Chroneos, Effect of high pressure on the fluctuation conductivity and the charge transfer of YBa2Cu3O7-δ single crystals. J. Alloy. Compds. 2008, 453, 69–74. [Google Scholar] [CrossRef]
- Vovk, R.V.; Obolenskii, M.A.; Zavgorodniy, A.A.; Goulatis, I.L.; Beleskii, V.I.; Chroneos, A. Structural relaxation, metal to insulator transition and pseudo-gap in oxygen deficient HoBa2Cu3O7-δ single crystals. Physics C 2009, 469, 203–206. [Google Scholar] [CrossRef]
- Vovk, R.V.; Zavgorodniy, A.A.; Obolenskii, M.A.; Goulatis, I.L.; Chroneos, A.; Simoes, V.M.P. Effect of high pressure on the metal-dielectric transition and the pseudo-gap temperature range in oxygen deficient YBa2Cu3O7-δ single crystals. J. Mater. Sci. Mater. Electron. 2011, 22, 20–24. [Google Scholar] [CrossRef]
- Vovk, R.V.; Nazyrov, Z.F.; Obolenskii, M.A.; Goulatis, I.L.; Chroneos, A.; Simoes, V.M.P. Phase separation in oxygen deficient HoBa2Cu3O7-δ single crystals: Effect of high pressure and twin boundaries. Philos. Mag. 2011, 91, 2291–2302. [Google Scholar] [CrossRef]
- Vovk, R.V.; Obolenskii, M.A.; Nazyrov, Z.F.; Goulatis, I.L.; Chroneos, A.; Simoes, V.M.P. Electro-transport and structure of 1-2-3 HTSC single crystals with different plane defects topologies. J. Mater. Sci. Mater. Electron. 2012, 23, 1255–1259. [Google Scholar] [CrossRef]
- Vovk, R.V.; Vovk, N.R.; Shekhovtsov, O.V.; Goulatis, I.L.; Chroneos, A. c-axis hopping conductivity in heavily Pr-doped YBCO single crystals. Semicond. Sci. Technol. 2013, 26, 085017. [Google Scholar] [CrossRef]
- Costa, R.M.; Dias, F.T.; Pureur, P.; Obradors, X. Multiple superconducting transition and phase separation in melt-textured YBa2Cu3O7-δ. Physics C 2013, 495, 202–207. [Google Scholar] [CrossRef]
- Slimani, Y.; Hannachi, E.; Ben Salem, M.K.; Hamrita, A.; Varilci, A.; Dachraoui, W.; Ben Salem, M.K.; Hamrita, A.; Varilci, A.; Dachraoui, W.; et al. Comparative study of nano-sized particles CoFe2O4 effects on superconducting properties of Y-123 and Y-358. Phys. B 2014, 450, 7–15. [Google Scholar] [CrossRef]
- Solovjov, A.L.; Tkachenko, M.A.; Vovk, R.V.; Chroneos, A. Fluctuation conductivity and pseudogap in HoBa2Cu3O7-δ single crystals under pressure with transport current flowing under an angle 45° to the twin boundaries. Physics C 2014, 501, 24–31. [Google Scholar] [CrossRef]
- Dzhumanov, S.; Ganiev, O.K.; Djumanov, S.S. Normal-state conductivity of underdoped to overdoped cuprate superconductors: Pseudogap effects on the in-plane and c-axis charge transports. Phys. B 2014, 440, 17–32. [Google Scholar] [CrossRef]
- Zhang, X.; Catlow, C.R.A. Molecular dynamics study of oxygen diffusion in YBa2Cu3O6.91. Phys. Rev. B 1992, 46, 457–462. [Google Scholar] [CrossRef]
- Islam, M.S.; Baetzold, R.C. Atomistic mechanisms of oxygen diffusion in YBa2Cu3O7-x and YBa2Cu4O8. J. Mater. Chem. 1994, 4, 299–303. [Google Scholar] [CrossRef]
- Kläser, M.; Kaiser, J.; Stock, F.; Müller-Vogt, G.; Erb, A. Comparitive study of oxygen diffusion in rare earth ReBa2Cu3O7-δ single crystals (RE = Y, Er, Dy) with different impurity levels. Physics C 1998, 306, 188–198. [Google Scholar]
- Conder, K. Oxygen diffusion in the superconductors of the YBaCuO family: Isotope exchange measurements and models. Mater. Sci. Eng. R. 2001, 32, 41–102. [Google Scholar] [CrossRef]
- Li, L.; Huang, D.M.; Wang, N.; Sun, Y.H.; Zhou, C. Diffusion model of oxygen in c-axis oriented YBa2Cu3O7 films. Physics C 2018, 544, 1–5. [Google Scholar] [CrossRef]
- Wang, T.G.; Cao, J.J.; Gou, X.F. Activation energy of oxygen diffusion: A possible indicator of supercurrents through YBa2Cu3O7 grain boundaries. Appl. Surf. Sci. 2019, 480, 765–769. [Google Scholar] [CrossRef]
- Ohnishi, T.; Mitsuishi, K.; Nishio, K.; Takada, K. Epitaxy of Li3xLa2/3–xTiO3 films and the influence of La ordering on Li-ion conduction. Chem. Mater. 2015, 27, 1233–1241. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Murphy, S.T.; Chroneos, A.; Jiang, C.; Schwingenschlögl, U.; Grimes, R.W. Deviations from Vegard’s law in ternary III-V alloys. Phys. Rev. B 2010, 82, 073201. [Google Scholar] [CrossRef]
- Sgourou, E.N.; Timerkaeva, D.; Londos, C.A.; Aliprantis, D.; Chroneos, A.; Caliste, D.; Pochet, P. Impact of isovalent doping on the trapping of vacancy and interstitial related defects in Si. J. Appl. Phys. 2013, 113, 113506. [Google Scholar] [CrossRef]
- Horlait, D.; Middleburgh, S.C.; Chroneos, A.; Lee, W.E. Synthesis and DFT investigation of new bismuth-containing MAX phases. Sci. Rep. 2016, 6, 18829. [Google Scholar] [CrossRef]
- Horlait, D.; Grasso, S.; Chroneos, A.; Lee, W.E. Attempts to synthesise quaternary MAX phases (Zr,M)2AlC and Zr2(Al,A)C as a way to approach Zr2AlC. Mater. Res. Lett. 2016, 4, 137–144. [Google Scholar] [CrossRef]
- Zapata-Solvas, E.; Christopoulos, S.R.G.; Ni, N.; Parfitt, D.C.; Horlait, D.; Fitzpatrick, M.E.; Chroneos, A.; Lee, W.E. Experimental synthesis and density functional theory investigation of radiation tolerance of Zr3(Al1−xSix)C2 MAX phases. J. Am. Ceram. Soc. 2017, 100, 1377–1387. [Google Scholar] [CrossRef]
- Hadi, M.A.; Roknuzzaman, M.; Chroneos, A.; Naqib, S.H.; Islam, A.K.M.A.; Vovk, R.V. Ostrikov, K. Elastic and thermodynamic properties of new Zr3−xTixAlC2 MAX phase solid solutions. Comp. Mater. Sci. 2017, 137, 318–326. [Google Scholar] [CrossRef]
- Kanarachos, S.; Christopoulos, S.R.G.; Chroneos, A.; Fitzpatrick, M.E. Detecting anomalies in time series via a deep learning algorithm combining wavelets, neural networks and Hilbert transform. Exp. Syst. Appl. 2017, 85, 292–304. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).