Development of Delpazolid for the Treatment of Tuberculosis
Abstract
1. Linezolid, the First Oxazolidinone Antibacterial Agent
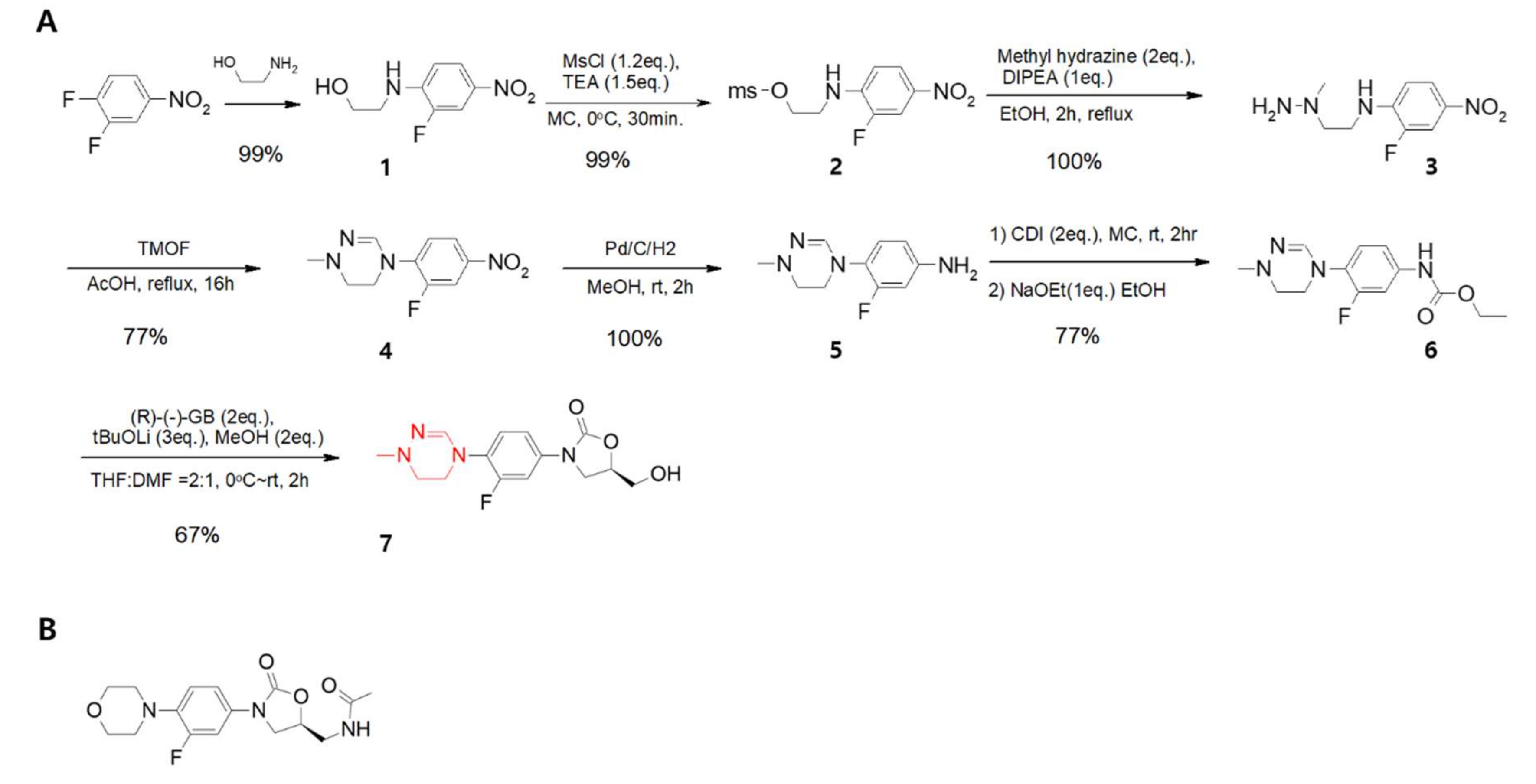
2. Development of Delpazolid (LCB01-0371)
3. Safety Evaluation in the Phase 1 Clinical Trial as PO
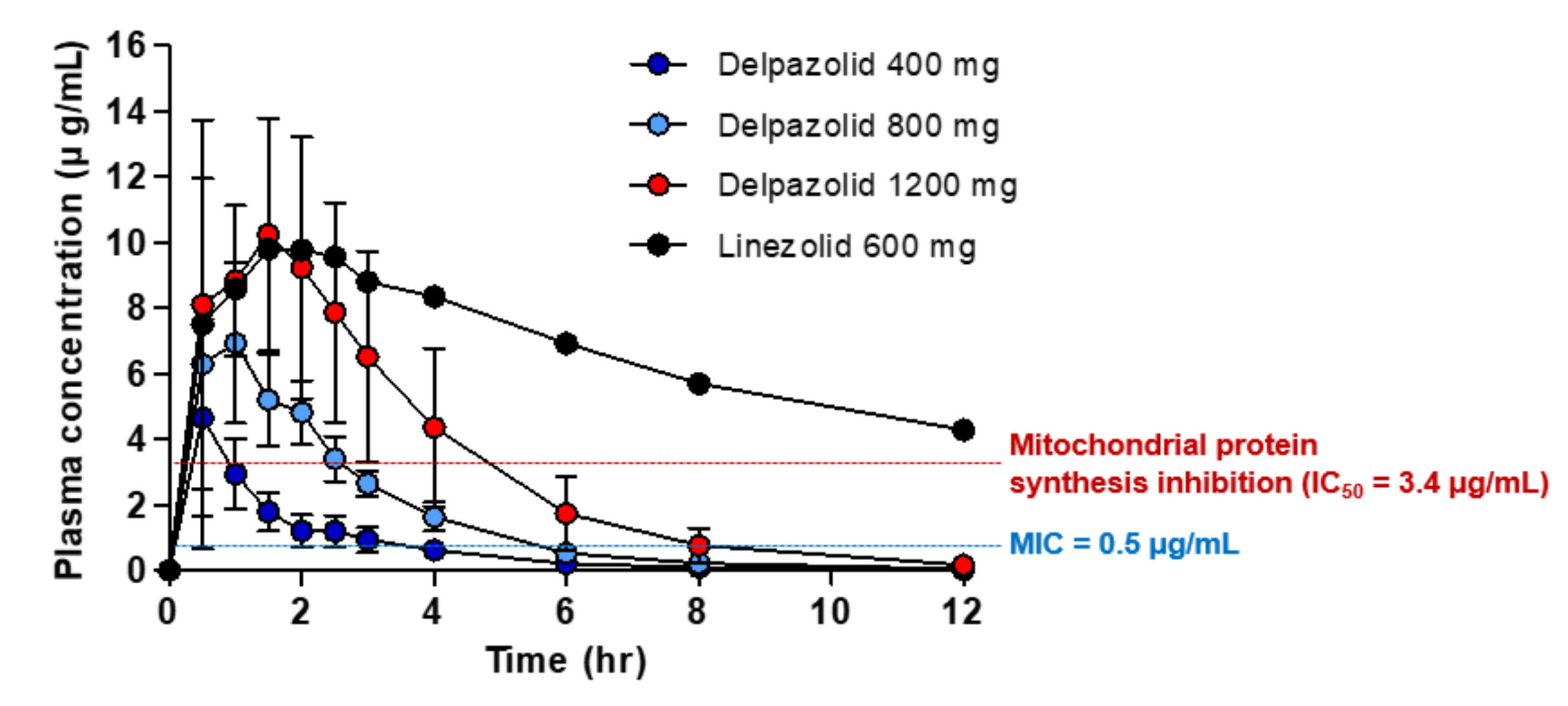
4. Poor PK Profiles but Safe for Humans
5. Toxicology
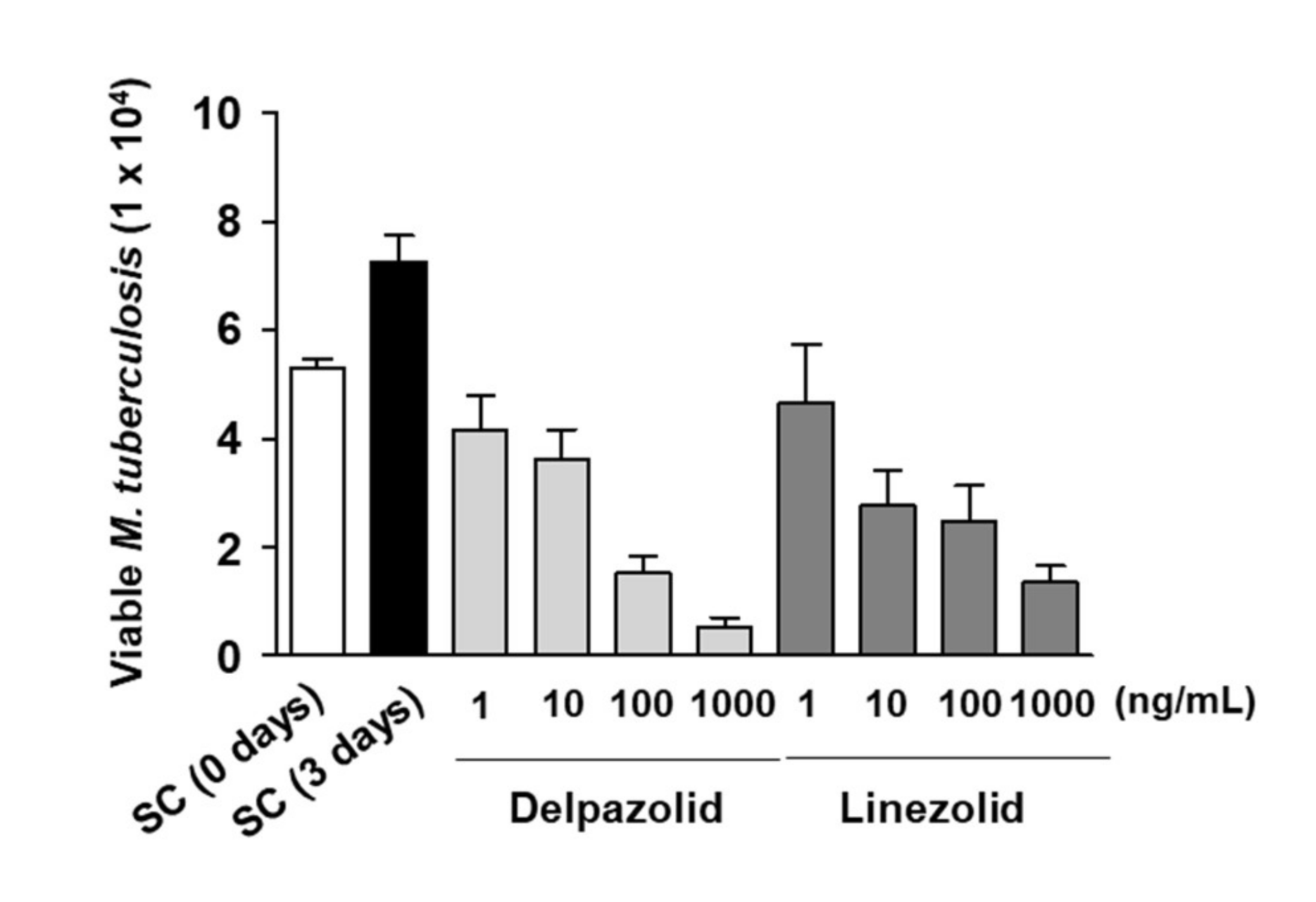
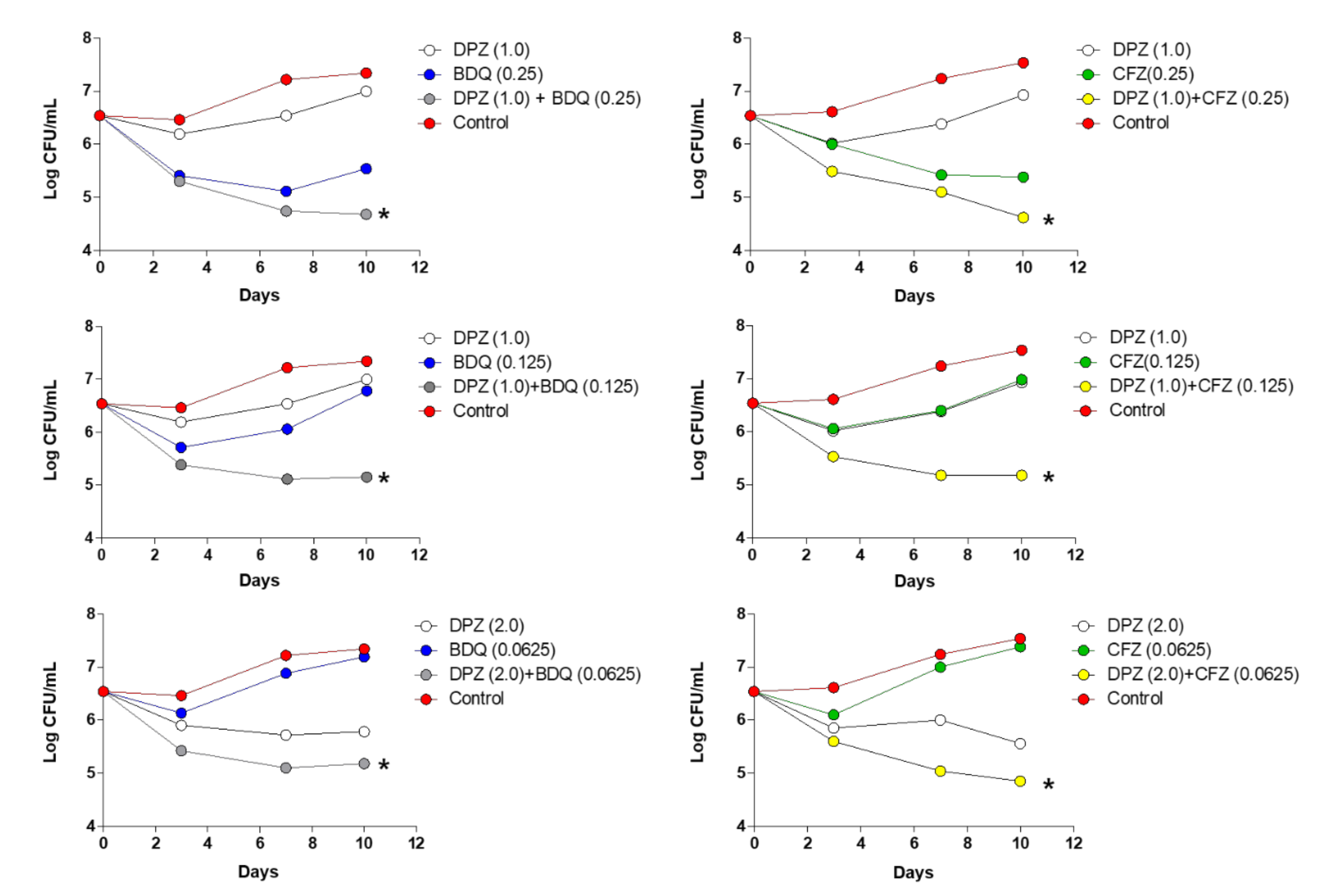
6. Activity Against TB and Combination Study of Delpazolid with Other Anti-TB Agents
7. Activity of Delpazolid on Nontuberculous Mycobacteria
8. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bozdogan, B.; Appelbaum, P.C. Oxazolidinones: Activity, mode of action, and mechanism of resistance. Int J Antimicrob Agents. 2004, 23, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Schito, G.C. The oxazolidinones as a new family of antimicrobial agent. Clin Microbiol Infect. 2001, 4, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Moellering, R.C. Linezolid: The first oxazolidinone antimicrobial. Ann Intern Med. 2003, 138, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Millard, J.; Pertinez, H.; Bonnett, L.; Hodel, E.M.; Dartois, V.; Johnson, J.L.; Caws, M.; Tiberi, S.; Bolhuis, M.; Alffenaar, J.C.; et al. Linezolid pharmacokinetics in MDR-TB: A systematic review, meta-analysis and Monte Carlo simulation. J Antimicrob Chemother. 2018, 73, 1755–1762. [Google Scholar] [CrossRef]
- Gerson, S.L.; Kaplan, S.L.; Bruss, J.B.; Le, V.; Arellano, F.M.; Hafkin, B.; Kuter, D.J. Hematologic effects of linezolid: Summary of clinical experience. Antimicrob Agents Chemother. 2002, 46, 2723–2726. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.W.; Kim, A.; Jang, K.; Nam, H.; Cho, Y.L.; Yu, K.S.; Jang, I.J. Chung, J.Y. Safety, tolerability and pharmacokinetics of 21 day multiple oral administration of a new oxazolidinone antibiotic, LCB01-0371, in healthy male subjects. J Antimicrob Chemother. 2018, 73, 183–190. [Google Scholar] [CrossRef]
- De Freitas Ferreira, R.; Schapira, M. A systematic analysis of atomic protein-ligand interactions in the PDB. Medchemcomm. 2017, 8, 1970–1981. [Google Scholar] [CrossRef]
- Singh, B.; Cocker, D.; Ryan, H.; Sloan, D.J. Linezolid for drug-resistant pulmonary tuberculosis. Cochrane Database Syst Rev. 2019, 3, CD012836. [Google Scholar] [CrossRef]
- Cho, Y.S.; Lim, H.S.; Cho, Y.L.; Nam, H.S.; Bae, K.S. Multiple-dose Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Oral LCB01-0371 in Healthy Male Volunteers. Clin Ther. 2018, 40, 2050–2064. [Google Scholar] [CrossRef]
- Cho, Y.S.; Lim, H.S.; Lee, S.H.; Cho, Y.L.; Nam, H.S.; Bae, K.S. Pharmacokinetics, Pharmacodynamics, and Tolerability of Single-Dose Oral LCB01-0371, a Novel Oxazolidinone with Broad-Spectrum Activity, in Healthy Volunteers. Antimicrob Agents Chemother. 2018, 62, e00451-18. [Google Scholar] [CrossRef]
- Sunwoo, J.; Kim, Y.K.; Choi, Y.; Yu, K.S.; Nam, H.; Cho, Y.L.; Yoon, S.; Chung, J.Y. Effect of food on the pharmacokinetic characteristics of a single oral dose of LCB01-0371, a novel oxazolidinone antibiotic. Drug Des Devel Ther. 2018, 12, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Choe, J.H.; Kim, Y.J.; Yang, C.S.; Kwon, H.J.; Jeong, J.; Kim, G.; Park, D.E.; Jo, E.K.; Cho, Y.L.; et al. Activity of LCB01-0371, a Novel Oxazolidinone, against Mycobacterium abscessus. Antimicrob Agents Chemother. 2017, 61, e02752-16. [Google Scholar] [CrossRef] [PubMed]
- Hällberg, B.M.; Larsson, N.G. Making proteins in the powerhouse. Cell Metab. 2014, 20, 226–240. [Google Scholar] [CrossRef] [PubMed]
- McKee, E.E.; Ferguson, M.; Bentley, A.T.; Marks, T.A. Inhibition of mammalian mitochondrial protein synthesis by oxazolidinones. Antimicrob Agents Chemother. 2006, 50, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- De Vriese, A.S.; Coster, R.V.; Smet, J.; Seneca, S.; Lovering, A.; Van Haute, L.L.; Vanopdenbosch, L.J.; Martin, J.J.; Groote, C.C.; Vandecasteele, S.; et al. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin Infect Dis. 2006, 42, 1111–1117. [Google Scholar] [CrossRef]
- Nagiec, E.E.; Wu, L.; Swaney, S.M.; Chosay, J.G.; Ross, D.E.; Brieland, J.K.; Leach, K.L. Oxazolidinones inhibit cellular proliferation via inhibition of mitochondrial protein synthesis. Antimicrob Agents Chemother. 2005, 49, 3896–3902. [Google Scholar] [CrossRef]
- Jeong, J.W.; Jung, S.J.; Lee, H.H.; Kim, Y.Z.; Park, T.K.; Cho, Y.L.; Chae, S.E.; Baek, S.Y.; Woo, S.H.; Lee, H.S.; et al. In vitro and in vivo activities of LCB01-0371, a new oxazolidinone. Antimicrob Agents Chemother. 2010, 54, 5359–5362. [Google Scholar] [CrossRef]
- Zong, Z.; Jing, W.; Shi, J.; Wen, S.; Zhang, T.; Huo, F.; Shang, Y.; Liang, Q.; Huang, H.; Pang, Y. Comparison of In Vitro Activity and MIC Distributions between the Novel Oxazolidinone Delpazolid and Linezolid against Multidrug-Resistant and Extensively Drug-Resistant Mycobacterium tuberculosis in China. Antimicrob Agents Chemother. 2018, 62, e00165-18. [Google Scholar] [CrossRef]
- Kerantzas, C.A.; Jacobs, W.R., Jr. Origins of Combination Therapy for Tuberculosis: Lessons for Future Antimicrobial Development and Application. mBio. 2017, 8, e01586-16. [Google Scholar] [CrossRef]
- Abate, G.; Hamzabegovic, F.; Eickhoff, C.S.; Hoft, D.F. BCG Vaccination Induces M. avium and M. abscessus Cross-Protective Immunity. Front Immunol. 2019, 10, 234. [Google Scholar] [CrossRef]
- Johnson, M.M.; Odell, J.A. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis. 2014, 6, 210–220. [Google Scholar] [PubMed]
- DeStefano, M.S.; Shoen, C.M.; Sklaney, M.R.; Cynamon, M.H. The In Vitro Activity of Delpazolid (LCB01-0371) against Several Non-Tuberculous Mycobacteria (NTM); (Poster Number AAR-731, Friday, June 21, 2019); ASM Microbe: San Francisco, CA, USA, 2019. [Google Scholar]
| Clinical Trial Phase | Experimental Design and Adverse Effects Reported |
|---|---|
| Phase 1a [9] (SAD) | Study design: Double blind, randomized, placebo control, first-in-human design |
| N=64, 8 subject per group (6 active + 2 placebo) | |
| Doses: 50, 100, 200, 400, 800, 1,600, 2,400, and 3,200 mg | |
| MTD: 2,400 mg (Up to 2,400 mg, only mild adverse events were reported) | |
| Phase 1b [8] (MAD-7 days) | Study design: Double blind, randomized, placebo control |
| N=32, 8 subject per group (6 active + 2 placebo) | |
| Doses: 400, 800, 1,200, 1,600 mg BID for 7 days | |
| MTD: 1,200 mg BID (Up to 2,400 mg/day, only mild adverse events were reported) | |
| Phase 1b [6] (MAD-21 days) | Study design: Double blind, randomized, placebo control |
| N=36, 12 subject per group (10 active + 2 placebo) | |
| Doses: 800 mg QD and BID, 1,200 mg BID for 21 days | |
| MTD: 1,200 mg BID (Up to 2,400mg/day, No SAE reported) | |
| Phase 2a a (EBA Trial) | Study design: Open label, randomized |
| N=80, 16 subject per delpazolid group; 8 patients in active control groups, HRZE and linezolid | |
| Doses: Delpazolid 400 mg BID, 800 mg QD, 800 mg BID, 1,200 mg QD, HRZE and linezolid 600 mg BID for 14 days |
| Compound | Bacteria | Human Mitochondria (IC50) | Animal Mitochondria [14] | |
|---|---|---|---|---|
| Escherichia coli | K562 cell | AC16 cell | Rat, Rabbit (liver & heart) | |
| Delpazolid | 2.6 μM (0.8 μg/mL) | 4.8 μM (1.5 μg/mL) | 10.9 μM (3.4 μg/mL) | NA |
| Linezolid | 11.6 μM (3.9 μg/mL) | 3.1 μM (1.0 μg/mL) | 10.0 μM (3.4 μg/mL) | 12.8 μM |
| General Toxicity | Status | PO | IV |
| Single dose acute toxicity study in rats | Completed | MTD = 2000 mpk | MTD = 1000 mpk |
| Single dose acute toxicity study in dogs | Completed | MTD = 1000 mpk | MTD = 500 mpk |
| 4- week toxicity study in rats with 4 -week recovery | Completed | NOAEL = 60 mpk | NOAEL = 120 mpk |
| 4- week toxicity study in dogs with 4 -week recovery | Completed | NOAEL (male = 20 mpk, female=10 mpk) | NOAEL = 15 mpk |
| 26- week(6 months) toxicity study in rats with 4 -week recovery | Completed | NOAEL (male = 10 mpk, female=100→75 mpk) | - |
| 39- week (9 months) toxicity study in dogs with 4 -week recovery | Completed | NOAEL = 10 mpk | - |
| Genetic Toxicity | |||
| Ames test | Completed | Negative | |
| In vitro chromosomal aberration test | Completed | Negative | |
| Rat micronucleus test | Completed | Negative | |
| Safety Pharmacology | |||
| Assessment of blockage of hERG potassium channels | Completed | Negative(IC50> 100μM) | |
| Cardiovascular telemetry study in beagle dogs | Completed | Negative | |
| Respiratory (Pulmonary) study in rats | Completed | Negative | |
| Neurobehavioral safety evaluation in rats | Completed | Negative | |
| Reproductive Toxicity | PO | ||
| Fertility and Embryonic Development to Implantation toxicity in rat | Completed | NOAEL (male = 15 mpk, female=60 mpk) | |
| Embryo-Fetal Development toxicity in rat | Completed | NOAEL = 15 mpk |
| Pharmacokinetic Parameter a | IV Infusion; 200 mg (n=6) | IV Infusion; 400 mg (n=8) | PO; 800 mg (n=8) |
|---|---|---|---|
| Cmax (μg/mL) | 2.92 ± 0.46 | 5.25 ± 0.96 | 8.20 ± 3.47 |
| Tmax (hr) | 0.83 ± 0.13 | 0.84 ± 0.13 | 1.22 ± 0.98 |
| T1/2 (hr) | 1.70 ± 0.26 | 1.48 ± 0.16 | 1.64 ± 0.48 |
| AUC0-24h (μg·hr/mL) | 5.59 ± 0.98 | 9.39 ± 1.46 | 18.65 ± 4.88 |
| AUCinf (μg·hr/mL) | 5.63 ± 1.00 | 9.42 ± 1.47 | 18.86 ± 4.99 |
| Vss, Vz/F (L/kg) | 0.90 ± 0.06 | 1.05 ± 0.20 | 1.67 ± 0.79 |
| CL, Cl/F (L/hr/kg) | 0.56 ± 0.10 | 0.67 ± 0.10 | 0.69 ± 0.18 |
| MRTlast (hr) | 1.55 ± 0.21 | 1.53 ± 0.14 | 2.87 ± 0.92 |
| Cmax, norm (μg/mL) | 0.95 ± 0.15 | 0.85 ± 0.16 | 0.67 ± 0.28 |
| AUCinf, norm (μg·hr/mL) | 1.83 ± 0.33 | 1.53 ± 0.24 | 1.53 ± 0.40 |
| F (%) | - | - | 99.8 ± 20.6 |
| Drug Activities / Resistant Rate a | Linezolid | Delpazolid |
|---|---|---|
| MIC value for M. tuberculosis H37Rv (μg/mL) | 0.5 | 0.5 |
| MBC99 value for M. tuberculosis H37Rv (μg/mL) | >16 | 4 |
| MDR-TB MIC90 (μg/mL) | 1 | 0.5 |
| XDR-TB MIC90 (μg/mL) | 0.25 | 1 |
| ECOFFs (epidemiological cutoff values) (μg/mL) | 1.0 | 2.0 |
| Resistant rate of MDR-TB (%) | 6. 7 | 0.8 |
| Resistant rate of XDR-TB (%) | 4.2 | 4.2 |
| Ref. Drug | MIC (μg/mL) | Tested TB Drugs | MIC (μg/mL) | FIC index | Activity a |
|---|---|---|---|---|---|
| Delpazolid | 1 | Isoniazid | 0.13 | 1.13 | I |
| Rifampicin | 0.06 | 0.75 | Ad | ||
| Rifapentine | 0.01 | 0.75 | Ad | ||
| Ethambutol | 0.50 | 1.02 | I | ||
| Cycloserine | 4.0 | 1.02 | I | ||
| Amikacin | 0.04 | 1.02 | I | ||
| Streptomycin | 0.25 | 1.02 | I | ||
| Capreomycin | 0.31 | 1.02 | I | ||
| Moxifloxacin | 0.06 | 0.75 | Ad | ||
| Levofloxacin | 0.25 | 0.75 | Ad | ||
| Clofazimine | 0.25 | 0.52 | pS | ||
| Bedaquiline | 0.25 | 0.53 | pS | ||
| Delamanid | 0.02 | 0.75 | Ad | ||
| Ethionamide | 0.5 | 1.03 | I | ||
| p-aminosalicylic acid | 0.02 | 1 | I | ||
| Pyrazinamide b | 200 | 0.63 | pS |
| NTM Species (no. of strain tested) | Antibiotics | Range (μg/mL) | MIC50 (μg/mL) | MIC90 (μg/mL) |
|---|---|---|---|---|
| Mycobacterium avium (22) | Delpazolid | 8-0.125 | 2 | 8 |
| Linezolid | 8-0.125 | 2 | 8 | |
| Clarithromycin | >128-≤0.125 | >128 | >128 | |
| Mycobacterium abscessus (20) | Delpazolid | 8-0.25 | 2 | 8 |
| Linezolid | 16-0.5 | 4 | 8 | |
| Clarithromycin | 128- ≤0.125 | ≤0.125 | 1 | |
| Mycobacterium fortuitum (21) | Delpazolid | 2-0.25 | 1 | 2 |
| Linezolid | 8-0.5 | 2 | 8 | |
| Clarithromycin | 8-≤0.125 | 0.25 | 4 | |
| Mycobacterium kansasii (22) | Delpazolid | 2-0.25 | 1 | 2 |
| Linezolid | 2-0.25 | 0.5 | 2 | |
| Clarithromycin | 0.125- ≤0.125 | ≤0.125 | ≤0.125 | |
| Mycobacterium chelonae (20) | Delpazolid | 4-0.25 | 1 | 2 |
| Linezolid | 8-0.5 | 2 | 4 | |
| Clarithromycin | 0.2- ≤0.025 | 0.1 | 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.L.; Jang, J. Development of Delpazolid for the Treatment of Tuberculosis. Appl. Sci. 2020, 10, 2211. https://doi.org/10.3390/app10072211
Cho YL, Jang J. Development of Delpazolid for the Treatment of Tuberculosis. Applied Sciences. 2020; 10(7):2211. https://doi.org/10.3390/app10072211
Chicago/Turabian StyleCho, Young Lag, and Jichan Jang. 2020. "Development of Delpazolid for the Treatment of Tuberculosis" Applied Sciences 10, no. 7: 2211. https://doi.org/10.3390/app10072211
APA StyleCho, Y. L., & Jang, J. (2020). Development of Delpazolid for the Treatment of Tuberculosis. Applied Sciences, 10(7), 2211. https://doi.org/10.3390/app10072211





