Evaluation of new Seawater-based Mouth Rinse Versus Chlorhexidine 0.2% Reducing Plaque and Gingivitis Indexes. A Randomized Controlled Pilot Study
Abstract
Featured Application
Abstract
1. Introduction
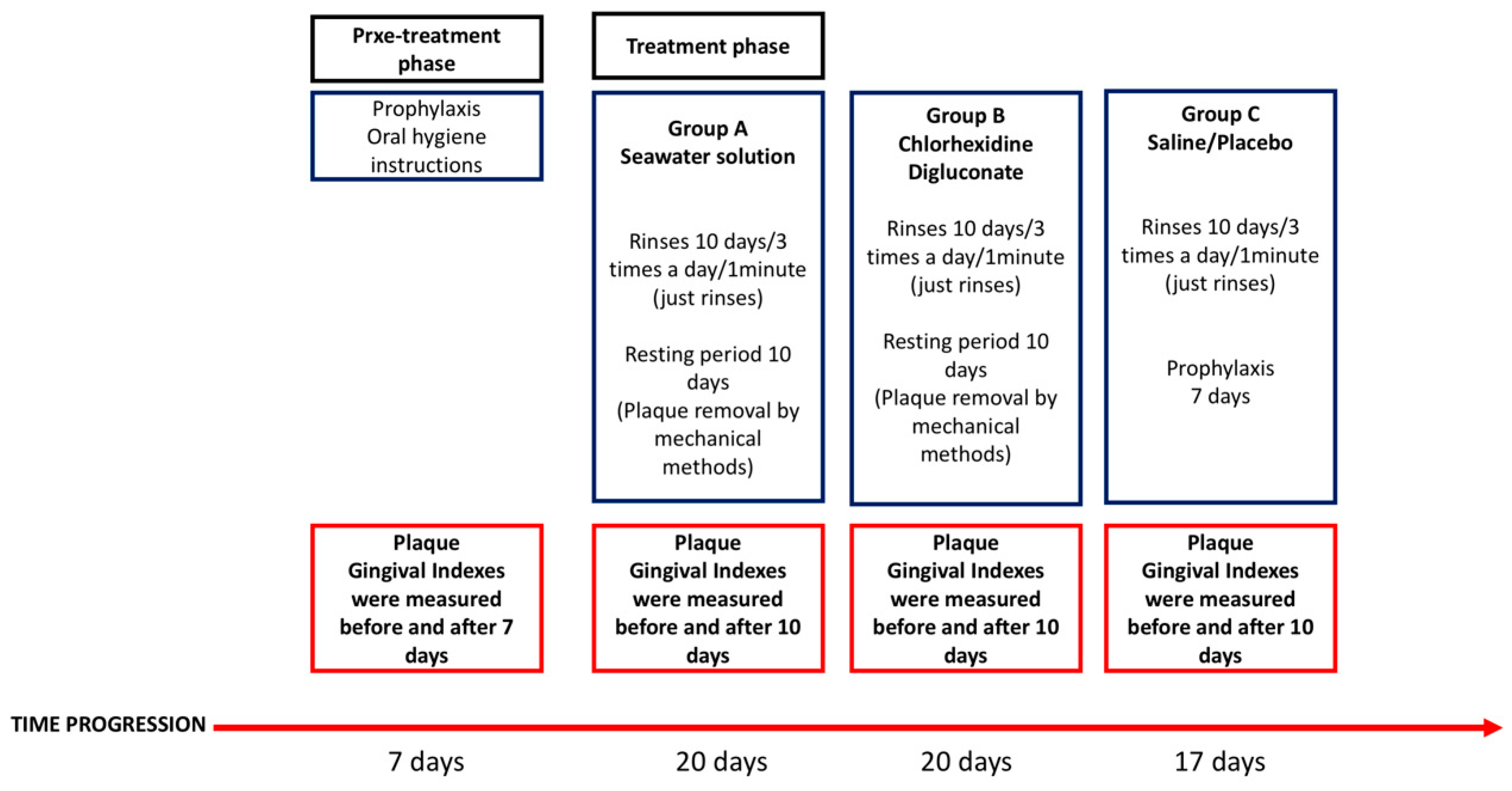
2. Material and Methods
- -
- Healthy patients with at least 26–32 permanent teeth;
- -
- Non-smokers, with good oral health, presenting probing depths ≤ 3 mm, no active dental caries, no fixed or removable prostheses, and orthodontic devices.
- -
- Subjects with systemic diseases, caries, or active periodontal disease;
- -
- Subjects with crowns, or an orthodontic treatment;
- -
- Subjects taking antibiotics or anti-inflammatory drugs, or any other medication;
- -
- Subjects with an allergy to any of the ingredients of the mouth rinse.
2.1. Experimental Protocol
2.2. Calibration Trial
2.3. Patient Variables
2.4. Statistical Analysis
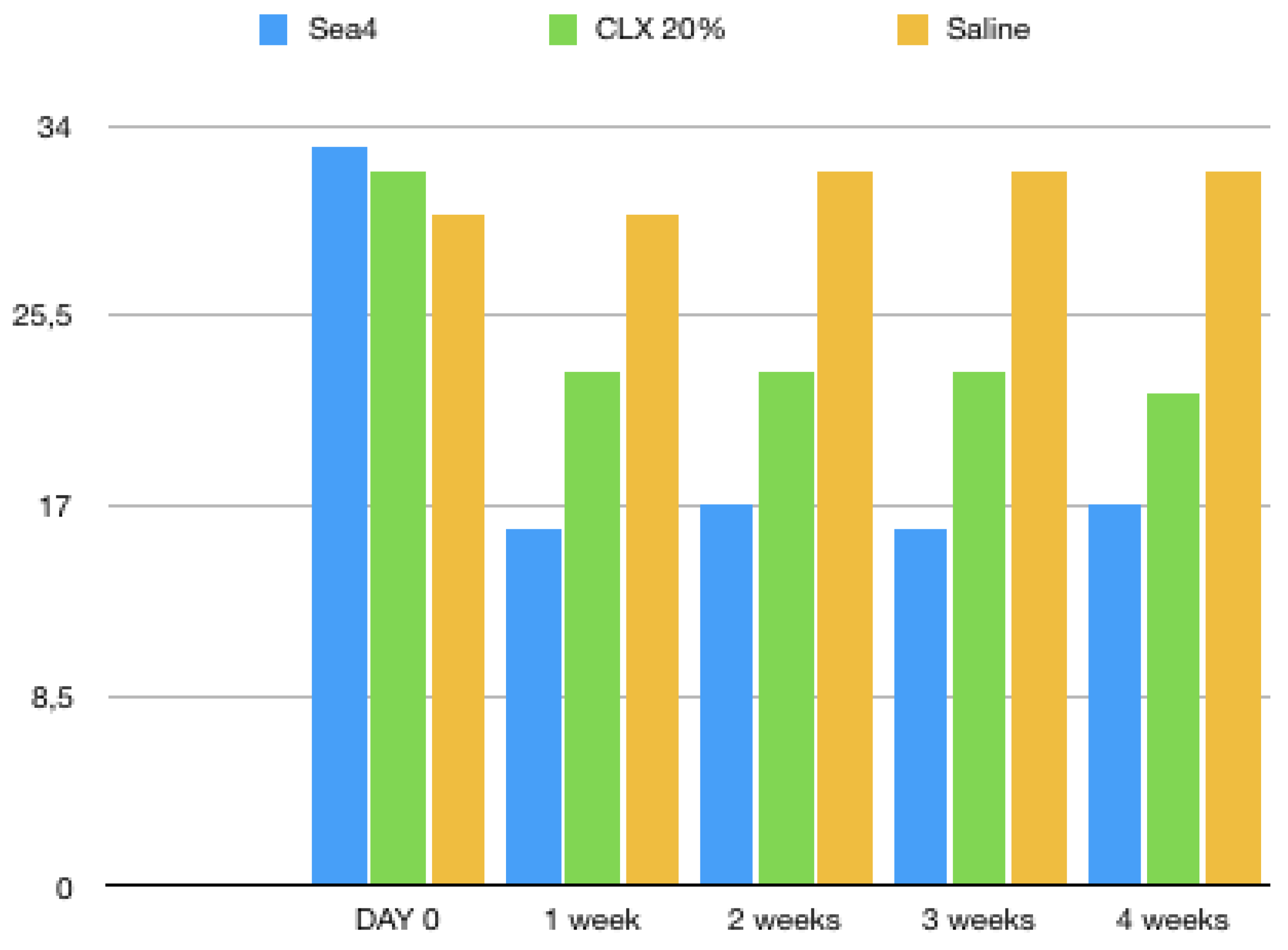
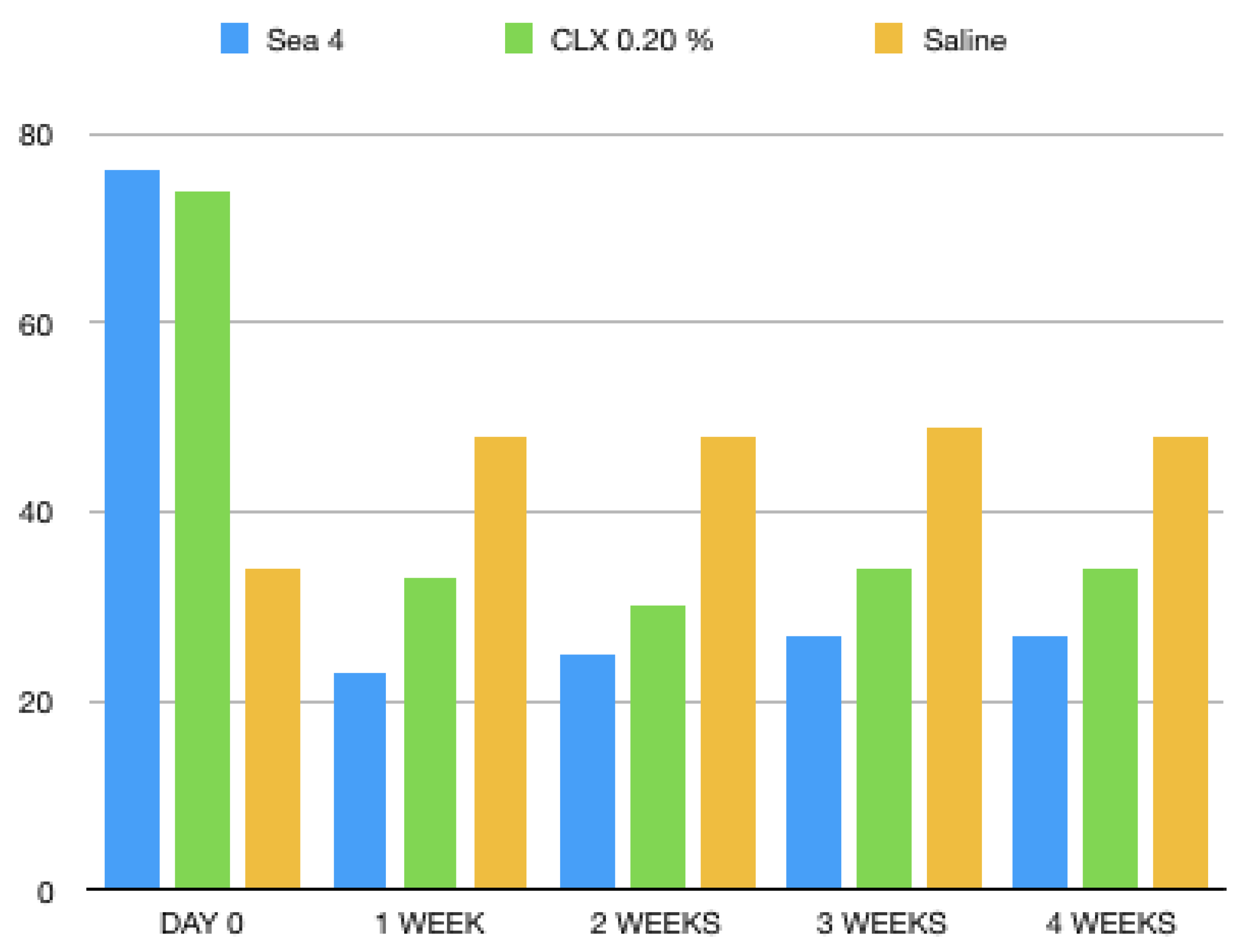
3. Results
Patients’ Perception of Mouth Rinses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lang, N.P.; Cumming, B.R.; Le, H. Toothbrushing frequency as it relates to plaque development and gingival health. J. Periodontol. 1973, 44, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Okamoto, H.; Yoneyama, T.; Haffajee, A.; Socransky, S.S. Longitudinal changes in periodontal disease in untreated subjects. J. Clin. Periodontol. 1989, 16, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, M.P.C.; Slot, D.E.; Van der Weijden, G.A. The effect of an essential-oils mouthrinse as compared to a vehicle solution on plaque and gingival Inflammation: A systematic review and meta-analysis. Int. J. Dent. Hyg. 2014, 12, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Stoeken, J.E.; Paraskevas, S.; Van DerWeijden, G.A. The long-term effect of a mouthrinse containing essential oils on dental plaque and gingivitis: A systematic review. J. Periodontol. 2007, 78, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Stoeken, J.E.; Versteeg, P.A.; Rosema, N.A.; Timmerman, M.F.; van der Velden, U.; van der Weijden, G.A. Inhibition of “de novo” plaque formation with 0.12% chlorhexidine spray compared to 0.2% spray and 0.2% chlorhexidine mouthwash. J. Periodontol. 2007, 78, 899–904. [Google Scholar] [CrossRef]
- Haas, A.N.; Wagner, T.P.; Muniz, F.W.; Fiorini, T.; Cavagni, J. Celeste RK Essential oils-containing mouthwashes for gingivitis and plaque: Meta-analyses and meta-regression. J. Dent. 2016, 55, 7–15. [Google Scholar] [CrossRef]
- Haas, A.N.; Pannuti, C.M.; Andrade, A.K.; Escobar, E.C.; Almeida, E.R.; Costa, F.O.; Cortelli, J.R.; Cortelli, S.C.; Rode, S.D.; Pedrazzi, V.; et al. Mouthwashes for the control of supragingival biofilm and gingivitis in orthodontic patients: Evidence-based recommendations for clinicians. Braz. Oral Res. 2014, 28, 1–8. [Google Scholar] [CrossRef]
- Haas, A.N.; Silva-Boghossian, C.M.; Colombo, A.P.; Albandar, J.; Oppermann, R.V.; Rösing, C.K.; Susin, C. Predictors of clinical outcomes after periodontal treatment of aggressive periodontitis: A 12-month randomized trial. Braz. Oral Res. 2016, 20, 30–31. [Google Scholar] [CrossRef]
- Haffajee, A.D.; Roberts, C.; Murray, L.; Veiga, N.; Martin, L.; Teles, R.P.; Letteri, M.; Socransky, S.S. Effect of herbal, essential oil, and chlorhexidine mouthrinses on the composition of the subgingival microbiota and clinical periodontal parameters. J. Clin. Dent. 2009, 20, 211–217. [Google Scholar]
- Surathu, N.; Kurumathur, A.V. Traditional therapies in the management of periodontal disease in India and China. Periodontol. 2000 2011, 56, 14–24. [Google Scholar] [CrossRef]
- Chen, Y.; Wong, R.W.; Seneviratne, C.J.; Hagg, U.; McGrath, C.; Samaranayake, L.P. The effects of a natural compound containing mouth rinses on patients with fixed orthodontic appliance treatment: Clinical and microbiological outcomes. Int. J. Pediatr. Dent. 2013, 23, 452–459. [Google Scholar]
- Laing, E.; Ashley, P.; Gill, D.; Naini, F. An update on oral hygiene products and techniques. Dent. Update 2008, 35, 270–279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zimmer, S.; Korte, P.; Verde, P.; Ohmann, C.; Naumova, E.; Jordan, R.A. Randomized controlled trial on the efficacy of new alcohol-free chlorhexidine mouthrinses after 8 weeks. Int. J. Dent. Hyg. 2015, 13, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wong, R.W.; McGrath, C.; Hagg, U.; Seneviratne, C.J. Natural compounds containing mouthrinses in the management of dental plaque and gingivitis: A systematic review. Clin. Oral Investig. 2014, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Garcıa-Gargallo, M.; Zurlohe, M.; Montero, E.; Alonso, B.; Serrano, J.; Sanz, M.; Herrera, D. Evaluation of new Chlorhexidine- and cetylpyridinium chloride-based mouth rinse formulations adjunctive to scaling and root planing: A pilot study. Int. J. Dent. Hyg. 2017, 15, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Poggi, P.; Rodriguez y Baena, R.; Rizzo, S.; Rota, M.T. Mouthrinses with alcohol: Cytotoxic effects on human gingival fibroblasts in vitro. J. Periodontol. 2003, 74, 623–629. [Google Scholar] [CrossRef]
- Fine, D.H.; Markowitz, K.; Furgang, D.; Goldsmith, D.; Charles, C.H.; Lisante, T.A.; Lynch, M.C. Effect of an essential oil-containing antimicrobial mouthrinse on specific plaque bacteria in vivo. J. Clin. Periodontol. 2007, 34, 652–657. [Google Scholar] [CrossRef]
- Papaioannou, W.; Vassilopoulos, S.; Vrotsos, I.; Margaritis, V.; Panis, V. A comparison of a new alcohol-free 0.2% chlorhexidine oral rinse to an established 0.2% chlorhexidine rinse with alcohol for the control of dental plaque accumulation. Int. J. Dent. Hyg. 2016, 14, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Mor-Reinoso, C.; Pascual, A.; Nart, J.; Quirynen, M. Inhibition of de novo plaque growth by a new 0.03% chlorhexidine mouth rinse formulation applying a non-brushing model: A randomized, double-blind clinical trial. Clin. Oral Investig. 2016, 20, 1459–1467. [Google Scholar] [CrossRef]
- Van Maanen-Schakel, N.W.; Slot, D.E.; Bakker, E.W.; Van der Weijden, G.A. The effect of an oxygenating agent on chlorhexidine-induced extrinsic tooth staining: A systematic review. Int. J. Dent. Hyg. 2012, 10, 198–208. [Google Scholar] [CrossRef]
- Yoshizawa, Y.; Tanojo, H.; Kim, S.J.; Maibach, H.I. Seawater or its components alter experimental irritant dermatitis in man. Skin Res. Technol. 2001, 7, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.; Michel, M.G.; Nadan, J.; Nowzari, H. The street children of Manila are affected by early-in-life periodontal infection: Description of a treatment modality: Sea salt. Refuat Hapeh Vehashinayim 2013, 30, 6–13, 67. [Google Scholar]
- Van der Weijden, F.A.; Van der Sluijs, E.; Ciancio, S.G.; Slot, D.E. Can Chemical Mouthwash Agents Achieve Plaque/Gingivitis Control? Dent. Clin. N. Am. 2015, 59, 799–829. [Google Scholar] [CrossRef] [PubMed]
- Cortelli, S.C.; Cortelli, J.R.; Wu, M.M.; Simmons, K.; Charles, C.A. Comparative antiplaque and antigingivitis efficacy of a multipurpose essential oil-containing mouth rinse and a cetylpyridinium chloride-containing mouth rinse: A 6-month randomized clinical trial. Quintessence Int. 2012, 43, e82–e94. [Google Scholar] [PubMed]
- Shiloah, J.; Patters, M.R. DNA probe analyses of the survival of selected periodontal pathogens following scaling, root planning, and intra-pocket irrigation. J. Periodontol. 1994, 65, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, L.T.; Merugu, S.; Sudhakar, U. Comparison of intraoral distribution of two commercially available chlorhexidine mouthrinses with and without alcohol at three different rinsing periods. J. Int. Soc. Prev. Commun. Dent. 2012, 2, 20–24. [Google Scholar] [CrossRef]
- Todkar, R.; Sheikh, S.; Byakod, G.; Muglikar, S. Efficacy of chlorhexidine mouthrinses with and without alcohol—A clinical study. Oral Health Prev. Dent. 2012, 10, 291–296. [Google Scholar]
- Jose, A.; Butler, A.; Payne, D.; Maclure, R.; Rimmer, P.; Bosma, M.L. A randomized clinical study to evaluate the efficacy of alcohol-free or alcohol-containing mouth rinses with Chlorhexidine on gingival bleeding. Br. Dent. J. 2015, 219, 125–130. [Google Scholar] [CrossRef]
- Rohrer, N.; Widmer, A.F.; Waltimo, T.; Kulik, E.M.; Weiger, R.; Filipuzzi-Jenny, E.; Walter, C. Antimicrobial efficacy of 3 oral antiseptics containing octenidine, polyhexamethylene biguanide, or Citroxx: Can Chlorhexidine be replaced? Infect. Control Hosp. Epidemiol. 2010, 31, 733–739. [Google Scholar] [CrossRef]
| Questionaire | Positive Control | CHX | Sea4 ENCIAS | Intergropup Statistics | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Anova p Value | |
| Do you have stains in your teeth and tongue after rinsing? Not, yes, very much | 0.66 | 0.11 | 0.89 | 0.28 | 0.29 | 0.22 | 0.0131 * |
| Mouthwash flavour: Quality How was the taste of the Product? (very good—very bad) | 6.12 | 0.72 | 6.29 | 0.41 | 7.45 | 0.51 | 0.0351 * |
| Mouthwash flavour: Duration How long did the taste remain in the mouth? (short period of time—long period of time) | 5.45 | 0.23 | 6.81 | 0.15 | 7.78 | 0.43 | 0.451 |
| How was your taste of food and drink after mouth rinse use? | 5.42 | 0.37 | 8.56 | 1.94 | 5.54 | 0.76 | 0.0248 * |
| Was the use of the mouth rinse convenient? | 5.56 | 0.76 | 7.91 | 0.92 | 8.34 | 0.74 | 0.3729 |
| Do you feel oral numbness or a burning sensation on your mucosa due to the mouth rinse used? absolutely not—yes, very much | 1.92 | 0.23 | 5.51 | 0.66 | 3.81 | 0.56 | 0.0231 * |
| What was your perception of the plaque reduction? | 1.87 | 0.61 | 7.89 | 0.66 | 8.51 | 0.12 | 0.1689 |
| What is your overall opinion of the Mouth rinse used in this study? very bad—very good | 2.1 | 0.7 | 7.65 | 0.32 | 9.76 | 0.23 | 0.0141 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo-Guirado, J.L.; Fernández Domínguez, M.; Aragoneses, J.M.; Martínez González, J.M.; Fernández-Boderau, E.; Garcés-Villalá, M.A.; Romanos, G.E.; Delgado-Ruiz, R.A. Evaluation of new Seawater-based Mouth Rinse Versus Chlorhexidine 0.2% Reducing Plaque and Gingivitis Indexes. A Randomized Controlled Pilot Study. Appl. Sci. 2020, 10, 982. https://doi.org/10.3390/app10030982
Calvo-Guirado JL, Fernández Domínguez M, Aragoneses JM, Martínez González JM, Fernández-Boderau E, Garcés-Villalá MA, Romanos GE, Delgado-Ruiz RA. Evaluation of new Seawater-based Mouth Rinse Versus Chlorhexidine 0.2% Reducing Plaque and Gingivitis Indexes. A Randomized Controlled Pilot Study. Applied Sciences. 2020; 10(3):982. https://doi.org/10.3390/app10030982
Chicago/Turabian StyleCalvo-Guirado, José Luis, Manuel Fernández Domínguez, Juan Manuel Aragoneses, José María Martínez González, Enrique Fernández-Boderau, Miguel Angel Garcés-Villalá, Georgios E. Romanos, and Rafael Arcesio Delgado-Ruiz. 2020. "Evaluation of new Seawater-based Mouth Rinse Versus Chlorhexidine 0.2% Reducing Plaque and Gingivitis Indexes. A Randomized Controlled Pilot Study" Applied Sciences 10, no. 3: 982. https://doi.org/10.3390/app10030982
APA StyleCalvo-Guirado, J. L., Fernández Domínguez, M., Aragoneses, J. M., Martínez González, J. M., Fernández-Boderau, E., Garcés-Villalá, M. A., Romanos, G. E., & Delgado-Ruiz, R. A. (2020). Evaluation of new Seawater-based Mouth Rinse Versus Chlorhexidine 0.2% Reducing Plaque and Gingivitis Indexes. A Randomized Controlled Pilot Study. Applied Sciences, 10(3), 982. https://doi.org/10.3390/app10030982









