Biodegradation and Absorption Technology for Hydrocarbon-Polluted Water Treatment
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
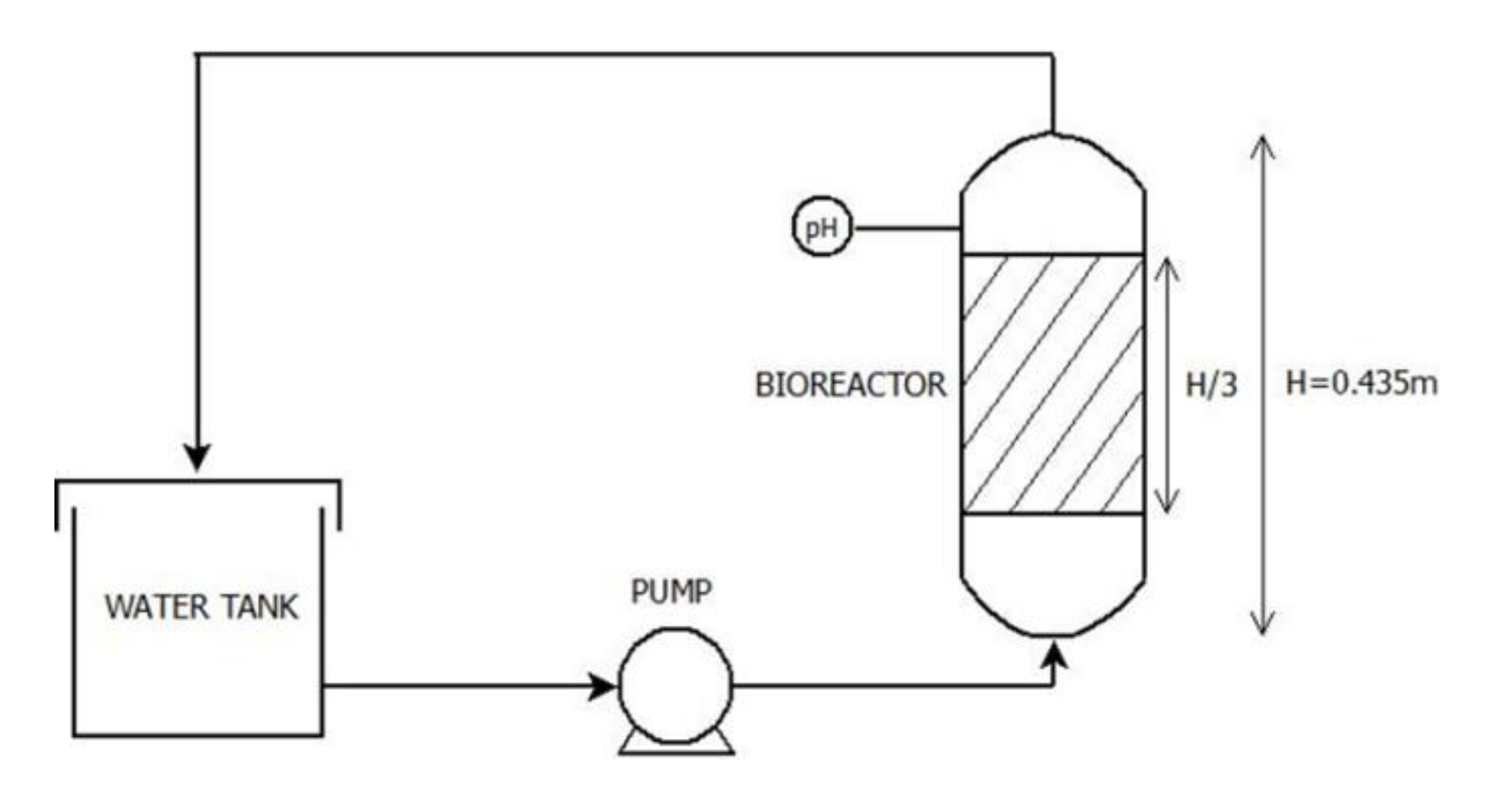
2.1. Description of Lab-Scale Plants
2.2. Filling Materials
2.3. Sampling for Analytical Determination
2.4. Quantification of Culturable Bacteria
2.5. Isolation and Identification of Bacterial Strains
2.6. Hydrocarbon Analysis
2.7. Ecotoxicity Test
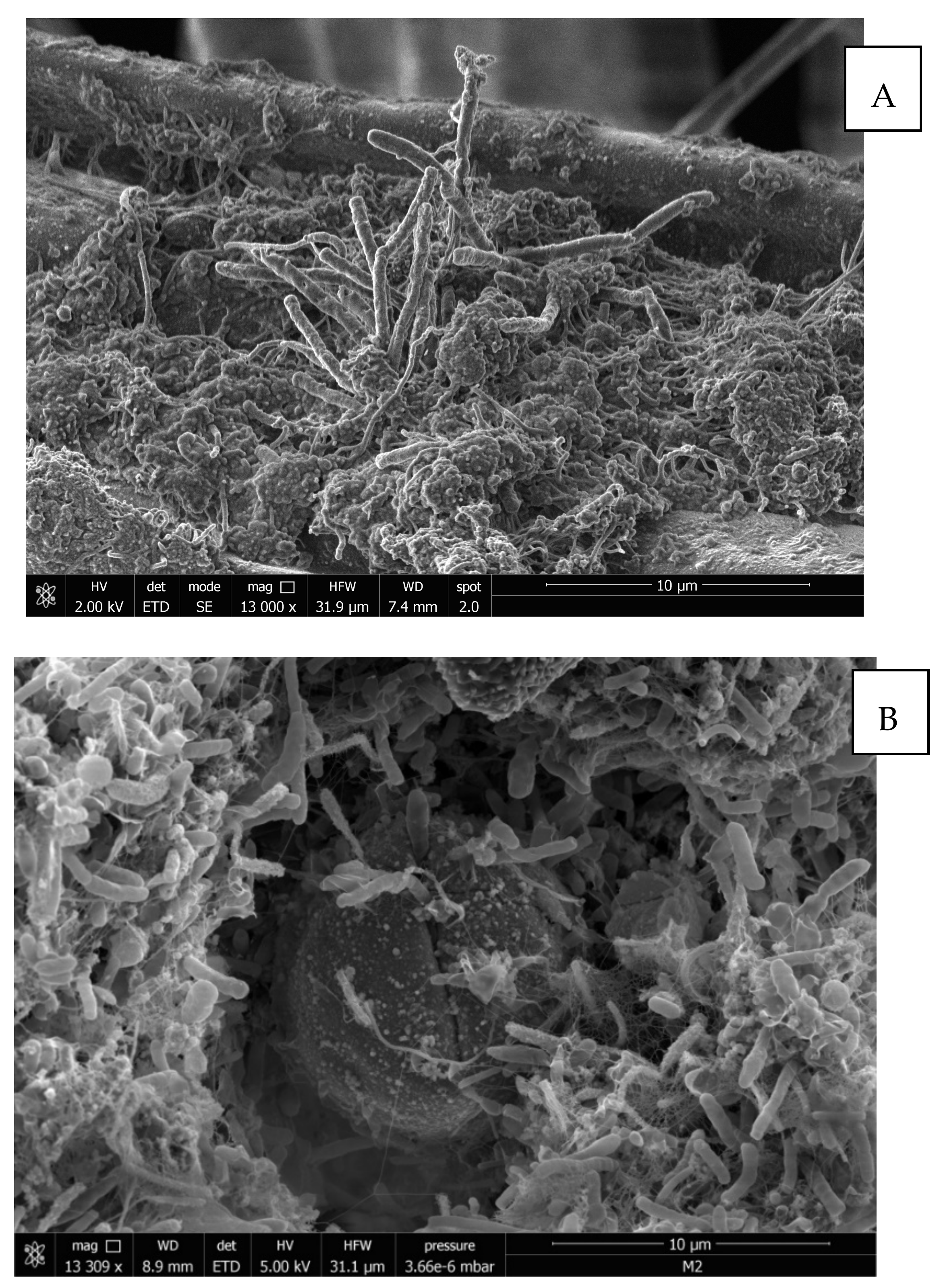
2.8. Scanning Electron Microscopy (SEM)
2.9. Statistical Analysis
3. Results and Discussion
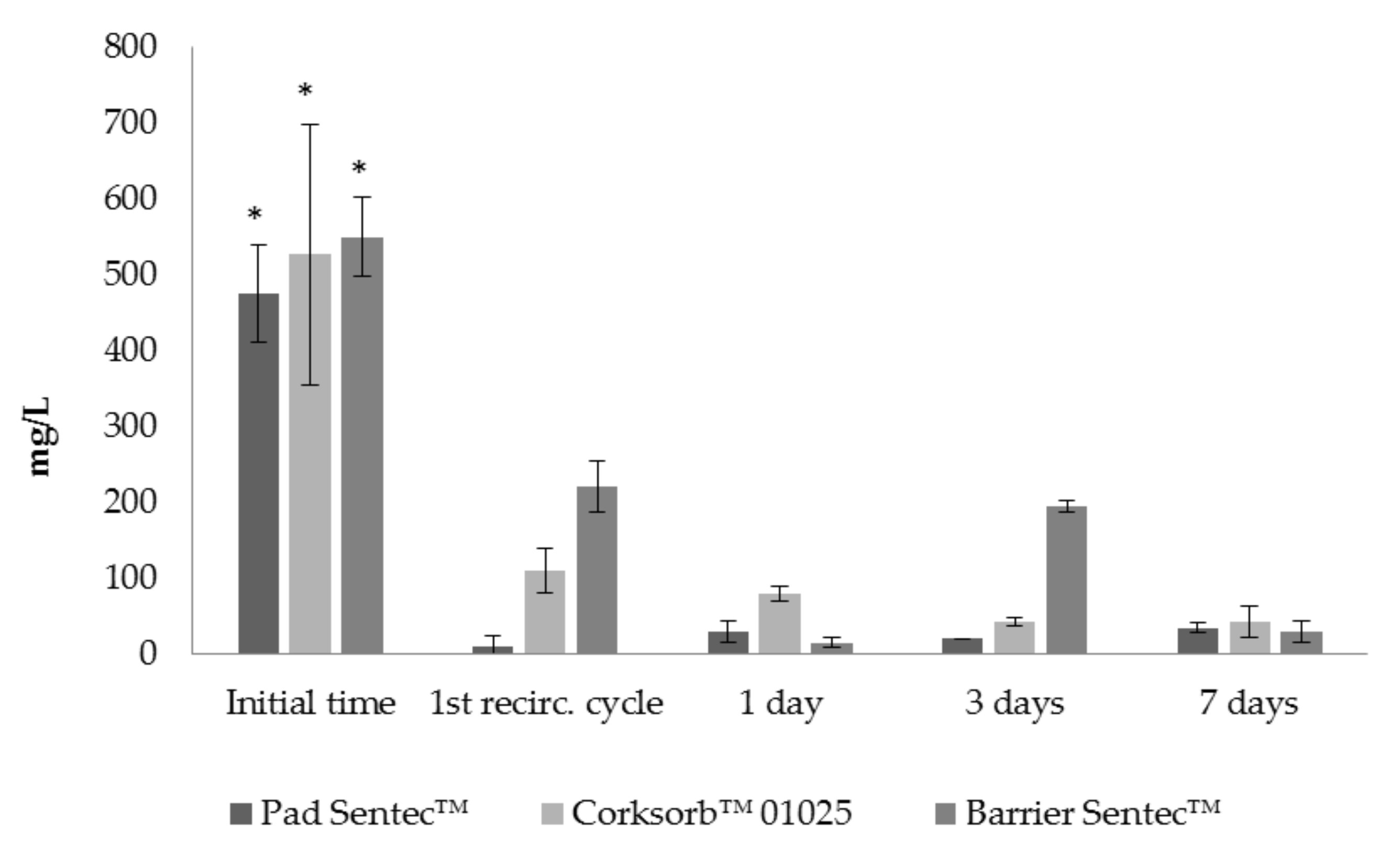
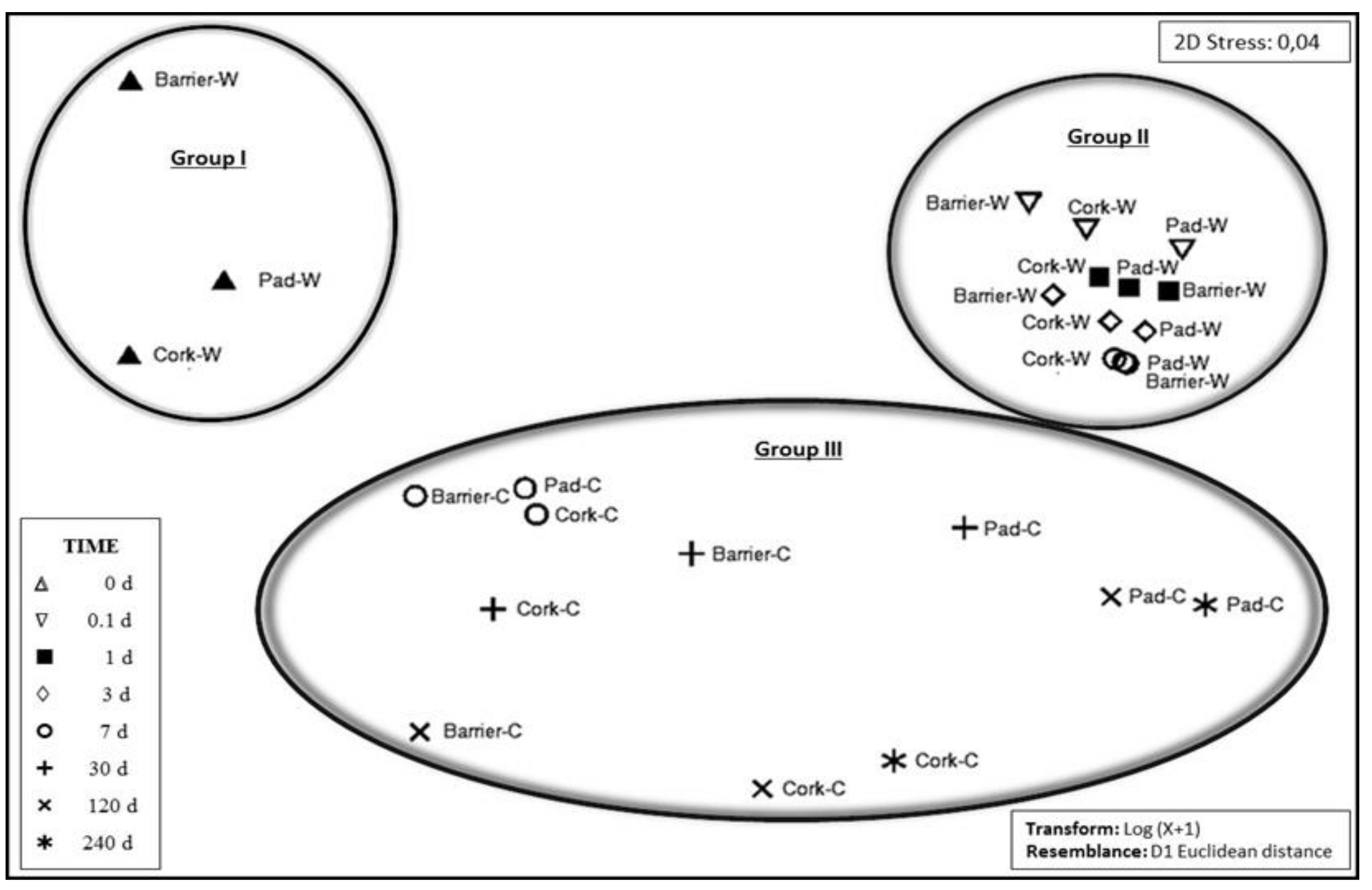
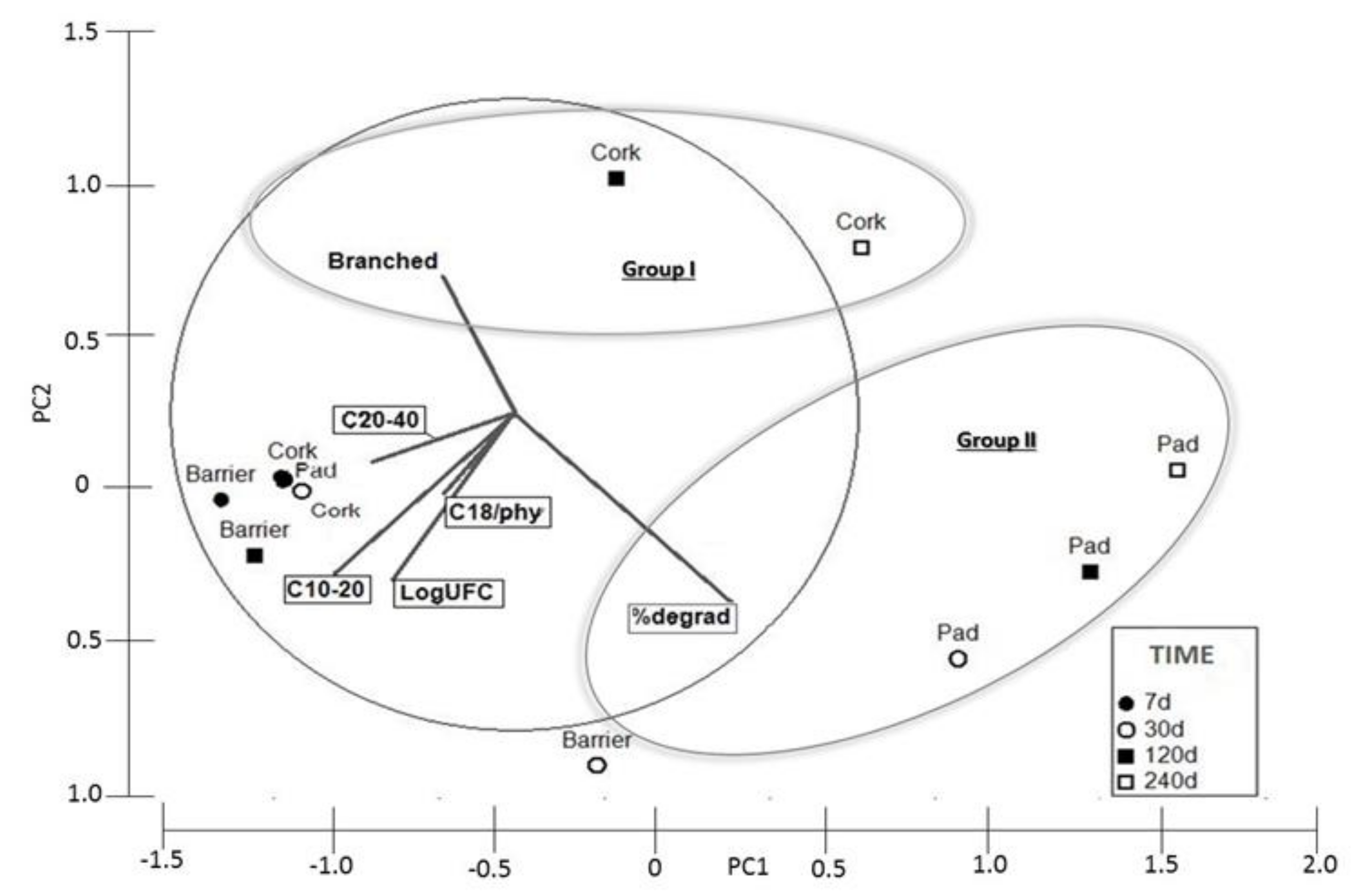
3.1. Bioreactor Experiments
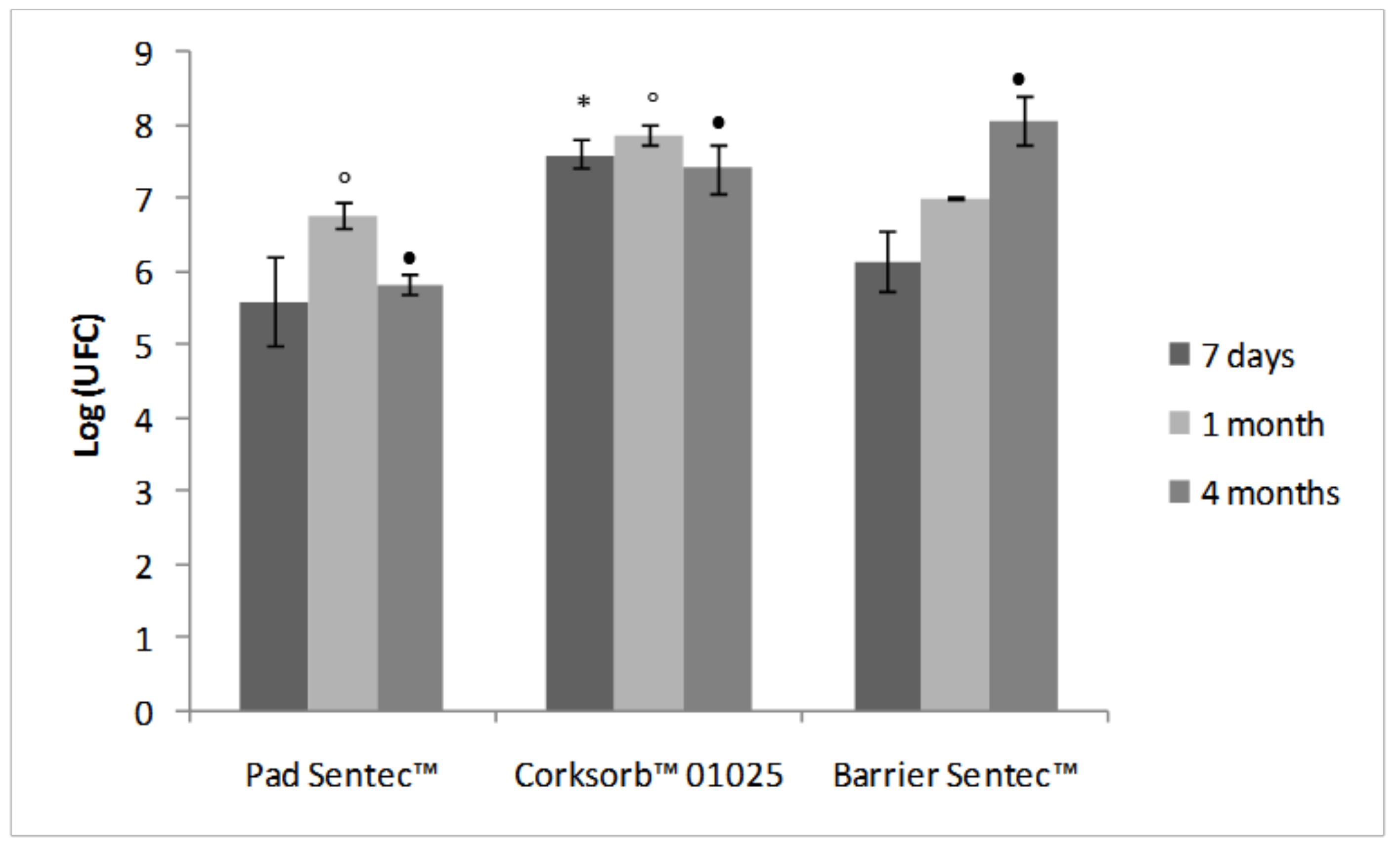
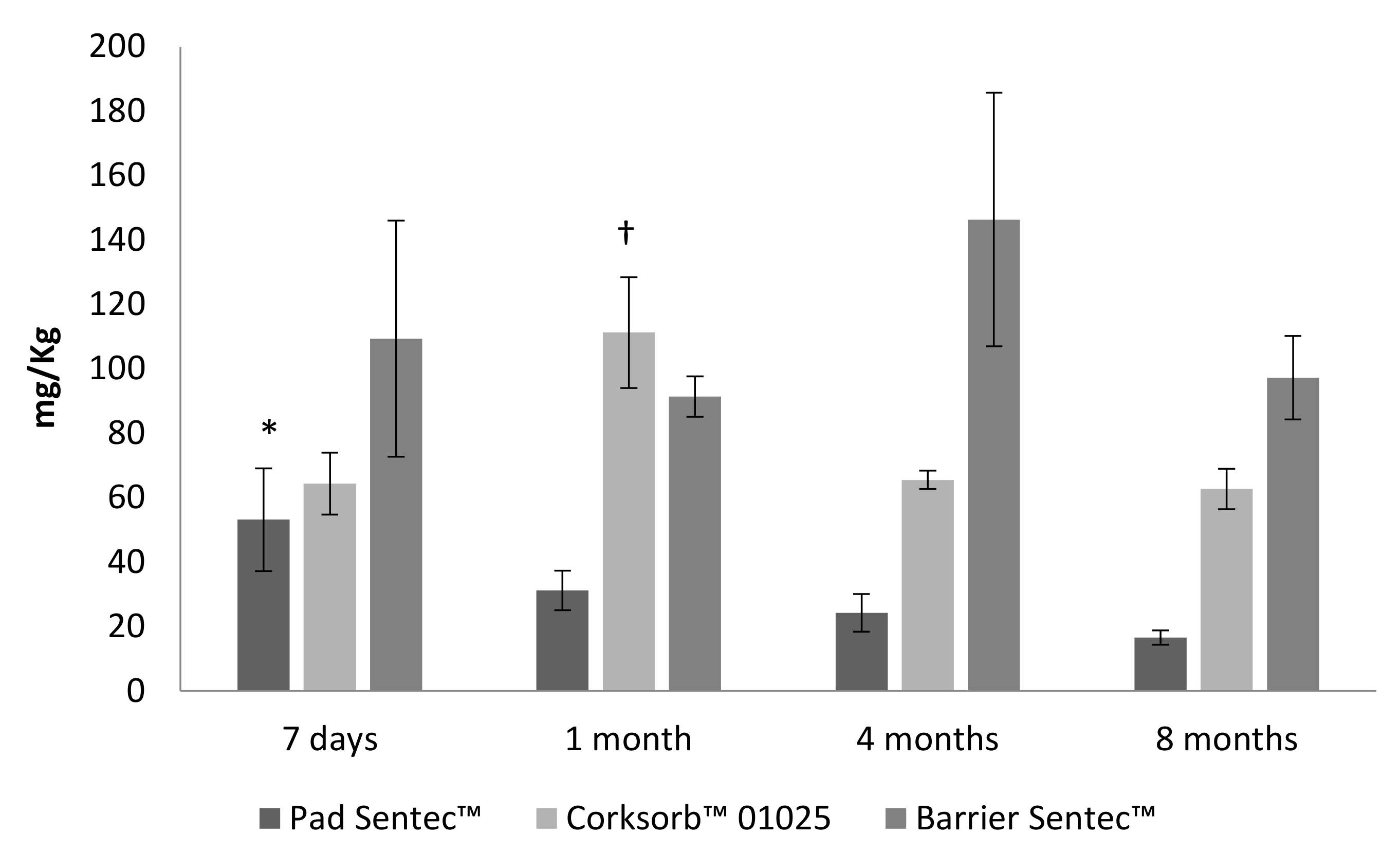
3.2. Biotreatment of Support Materials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef]
- Pandey, P.; Pathak, H.; Dave, S. Microbial Ecology of Hydrocarbon Degradation in the Soil: A Review. Res. J. Environ. Toxicol. 2016, 10, 1–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Gu, C.; Wang, F.; Bian, Y.; Song, Y.; Wang, D.; Jiang, X. A novel bioaccessibility prediction method for PAHs in soil: Composite extraction with hydroxypropyl-β-cyclodextrin and extracellular polymer substances. Sci. Total Environ. 2016, 569, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Soliman, R.M.; El-Gendy, N.S.; Deriase, S.F.; Farahat, L.A.; Mohamed, A.S. The Evaluation of Different Bioremediation Processes for Egyptian Oily Sludge Polluted Soil on a Microcosm Level. Energy Sources Part A Recovery Utilization Environ. Eff. 2014, 36, 231–241. [Google Scholar] [CrossRef]
- Pintor, A.M.A.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Oil and grease removal from wastewaters: Sorption treatment as an alternative to state-of-the-art technologies. A critical review. Chem. Eng. J. 2016, 297, 229–255. [Google Scholar] [CrossRef]
- Saleem, J.; Adil Riaz, M.; Gordon, M. Oil sorbents from plastic wastes and polymers: A review. J. Hazard. Mater. 2018, 341, 424–437. [Google Scholar] [CrossRef]
- Röling, W.F.M.; Milner, M.G.; Jones, D.M.; Lee, K.; Daniel, F.; Swannell, R.J.P.; Head, I.M. Robust Hydrocarbon Degradation and Dynamics of Bacterial Communities during Nutrient-Enhanced Oil Spill Bioremediation. Appl. Environ. Microbiol. 2002, 68, 5537–5548. [Google Scholar] [CrossRef]
- Vieira, P.A.; Vieira, R.B.; de França, F.P.; Cardoso, V.L. Biodegradation of effluent contaminated with diesel fuel and gasoline. J. Hazard. Mater. 2007, 140, 52–59. [Google Scholar] [CrossRef]
- Pintor, A.M.A.; Ferreira, C.I.A.; Pereira, J.C.; Correia, P.; Silva, S.P.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Use of cork powder and granules for the adsorption of pollutants: A review. Water Res. 2012, 46, 3152–3166. [Google Scholar] [CrossRef]
- Vilar, V.; Ferreira, C.I.D.A.; Pereira, J.; Pintor, A.; Botelho, C.; Martins, R.; Órfão, J.; Boaventura, R.; Correia, P.; Silva, S. Utilização de resíduos ou subprodutos de cortiça para a eliminação de óleos e gorduras de águas. In Encontro Brasileiro sobre Adsorção (EBA9) e Congresso Ibero-Americano sobre Adsorção (IBA1); Polytechnic Institute of Bragança: Bragança, Portugal, 2012; Volume 1, pp. 2–6. [Google Scholar]
- Wei, Q.F.; Mather, R.R.; Fotheringham, A.F.; Yang, R.D. Evaluation of nonwoven polypropylene oil sorbents in marine oil-spill recovery. Mar. Pollut. Bull. 2003, 46, 780–783. [Google Scholar] [CrossRef]
- Margesin, R.; Schinner, F. Biodegradation and bioremediation of hydrocarbons in extreme environments. Appl. Microbiol. Biotechnol. 2001, 56, 650–663. [Google Scholar] [CrossRef] [PubMed]
- McGenity, T.J.; Folwell, B.D.; McKew, B.A.; Sanni, G.O. Marine crude-oil biodegradation: A central role for interspecies interactions. Aquat. Biosyst. 2012, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Dellagnezze, B.M.; de Sousa, G.V.; Martins, L.L.; Domingos, D.F.; Limache, E.E.G.; de Vasconcellos, S.P.; da Cruz, G.F.; de Oliveira, V.M. Bioremediation potential of microorganisms derived from petroleum reservoirs. Mar. Pollut. Bull. 2014, 89, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Guibert, L.M.; Loviso, C.L.; Marcos, M.S.; Commendatore, M.G.; Dionisi, H.M.; Lozada, M. Alkane Biodegradation Genes from Chronically Polluted Subantarctic Coastal Sediments and Their Shifts in Response to Oil Exposure. Microb. Ecol. 2012, 64, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Radwan, S.S.; Al-Hasan, R.H.; Salamah, S.; Al-Dabbous, S. Bioremediation of oily sea water by bacteria immobilized in biofilms coating macroalgae. Int. Biodeterior. Biodegrad. 2002, 50, 55–59. [Google Scholar] [CrossRef]
- Singh, R.; Paul, D.; Jain, R.K. Biofilms: Implications in bioremediation. Trends Microbiol. 2006, 14, 389–397. [Google Scholar] [CrossRef]
- Chandran, P.; Das, N. Degradation of diesel oil by immobilized Candida tropicalis and biofilm formed on gravels. Biodegradation 2011, 22, 1181–1189. [Google Scholar] [CrossRef]
- Al-Kharusi, S.; Abed, R.M.M.; Dobretsov, S. Changes in respiration activities and bacterial communities in a bioaugmented oil-polluted soil in response to the addition of acyl homoserine lactones. Int. Biodeterior. Biodegrad. 2016, 107, 165–173. [Google Scholar] [CrossRef]
- Rodríguez-Calvo, A.; Silva-Castro, G.A.; Robledo-Mahón, T.; González-López, J.; Calvo, C. Capacity of Hydrophobic Carriers to Form Biofilm for Removing Hydrocarbons from Polluted Industrial Wastewater: Assay in Microcosms. Water Air Soil Pollut. 2018, 229, 175. [Google Scholar] [CrossRef]
- APHA (American Public Health Association) Standard Methods for the Examination of Water and Waste Water; American Public Health Association: Washington, DC, USA, 2005.
- Aguilera-Vázquez, L.; Soto-Cruz, N.O.; Saucedo-Castañeda, G.; Gutiérrez-Rojas, M. A model system for cocomposting hydrocarbon contaminated soil by using water activity and porosity as response variables. Chem. Eng. J. 2001, 81, 197–202. [Google Scholar] [CrossRef]
- Silva-Castro, G.A.; Rodriguez-Calvo, A.; Laguna, J.; González-López, J.; Calvo, C. Autochthonous microbial responses and hydrocarbons degradation in polluted soil during biostimulating treatments under different soil moisture. Assay in pilot plant. Int. Biodeterior. Biodegrad. 2016, 108, 91–98. [Google Scholar] [CrossRef]
- Zucconi, F.; Pera, A.; Forte, M.; De Bertoldi, M. Evaluating toxicity of immature compost. BioCycle 1981, 22, 54–57. [Google Scholar]
- Bayat, A.; Aghamiri, S.F.; Moheb, A.; Vakili-Nezhaad, G.R. Oil Spill Cleanup from Sea Water by Sorbent Materials. Chem. Eng. Technol. 2005, 28, 1525–1528. [Google Scholar] [CrossRef]
- Bazargan, A.; Hui, C.W.; Mckay, G. Marine residual fuel sorption and desorption kinetics by alkali treated rice husks. Cellulose 2014, 21, 1997–2006. [Google Scholar] [CrossRef]
- Gómez, M.A.; González-López, J.; Hontoria-Garcı́a, E. Influence of carbon source on nitrate removal of contaminated groundwater in a denitrifying submerged filter. J. Hazard. Mater. 2000, 80, 69–80. [Google Scholar] [CrossRef]
- Gómez-Villalba, B.; Calvo, C.; Vilchez, R.; González-López, J.; Rodelas, B. TGGE analysis of the diversity of ammonia-oxidizing and denitrifying bacteria in submerged filter biofilms for the treatment of urban wastewater. Appl. Microbiol. Biotechnol. 2006, 72, 393–400. [Google Scholar] [CrossRef]
- Moreno, B.; Gómez, M.A.; González-López, J.; Hontoria, E. Inoculation of a submerged filter for biological denitrification of nitrate polluted groundwater: A comparative study. J. Hazard. Mater. 2005, 117, 141–147. [Google Scholar] [CrossRef]
- Lamichhane, S.; Bal Krishna, K.C.; Sarukkalige, R. Polycyclic aromatic hydrocarbons (PAHs) removal by sorption: A review. Chemosphere 2016, 148, 336–353. [Google Scholar] [CrossRef]
- Albareda, M.; Rodríguez-Navarro, D.N.; Camacho, M.; Temprano, F.J. Alternatives to peat as a carrier for rhizobia inoculants: Solid and liquid formulations. Soil Biol. Biochem. 2008, 40, 2771–2779. [Google Scholar] [CrossRef]
- Ferreira, E.M.; Castro, I.E. Residues of the Cork Industry as Carriers for the Production of Legume Inoculants. Silva. Lusit. 2005, 13, 159–167. [Google Scholar]
- Bartowsky, E.J.; Henschke, P.A. Acetic acid bacteria spoilage of bottled red wine—A review. Int. J. Food Microbiol. 2008, 125, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Jurecska, L.; Barkács, K.; Kiss, É.; Gyulai, G.; Felföldi, T.; Törő, B.; Kovács, R.; Záray, G. Intensification of wastewater treatment with polymer fiber-based biofilm carriers. Microchem. J. 2013, 107, 108–114. [Google Scholar] [CrossRef]
- Krivorot, M.; Kushmaro, A.; Oren, Y.; Gilron, J. Factors affecting biofilm formation and biofouling in membrane distillation of seawater. J. Membr. Sci. 2011, 376, 15–24. [Google Scholar] [CrossRef]
- Zhou, D.; Hai, R.; Wang, W. Novel Complex Fiber Biofilm Carrier in an Anaerobic/Anoxic/Oxic Reactor for Sewage Mixture Treatment. Asian J. Chem. 2013, 25, 6943–6947. [Google Scholar] [CrossRef]
- Rosenberg, M. Microbial adhesion to hydrocarbons: Twenty-five years of doing MATH. FEMS Microbiol. Lett. 2006, 262, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Abarian, M.; Hassanshahian, M.; Badoei-Dalfard, A. Isolation, Screening, and Characterization of Naphthalene-Degrading Bacteria from Zarand Mine, Iran. Polycycl. Aromat. Compd. 2018, 38, 410–419. [Google Scholar] [CrossRef]
- Admon, S.; Green, M.; Avnimelech, Y. Biodegradation Kinetics of Hydrocarbons in Soil during Land Treatment of Oily Sludge. Bioremed. J. 2001, 5, 193–209. [Google Scholar] [CrossRef]
- Leahy, J.G.; Colwell, R.R. Microbial degradation of hydrocarbons in the environment. Microbiol. Mol. Biol. Rev. 1990, 54, 305–315. [Google Scholar] [CrossRef]
- Mishra, S.; Jyot, J.; Kuhad, R.C.; Lal, B. Evaluation of Inoculum Addition to Stimulate in Situ Bioremediation of Oily-Sludge-Contaminated Soil. Appl. Environ. Microbiol. 2001, 67, 1675–1681. [Google Scholar] [CrossRef]
- Abbasian, F.; Lockington, R.; Mallavarapu, M.; Naidu, R. A Comprehensive Review of Aliphatic Hydrocarbon Biodegradation by Bacteria. Appl. Biochem. Biotechnol. 2015, 176, 670–699. [Google Scholar] [CrossRef]
- Asif, M.; Grice, K.; Fazeelat, T. Assessment of petroleum biodegradation using stable hydrogen isotopes of individual saturated hydrocarbon and polycyclic aromatic hydrocarbon distributions in oils from the Upper Indus Basin, Pakistan. Org. Geochem. 2009, 40, 301–311. [Google Scholar] [CrossRef]
- Shelton, J.L.; McIntosh, J.C.; Warwick, P.D.; McCray, J.E. Impact of formation water geochemistry and crude oil biodegradation on microbial methanogenesis. Org. Geochem. 2016, 98, 105–117. [Google Scholar] [CrossRef]
- Varjani, S.J.; Upasani, V.N. A new look on factors affecting microbial degradation of petroleum hydrocarbon pollutants. Int. Biodeterior. Biodegrad. 2017, 120, 71–83. [Google Scholar] [CrossRef]
- Xue, J.; Yu, Y.; Bai, Y.; Wang, L.; Wu, Y. Marine Oil-Degrading Microorganisms and Biodegradation Process of Petroleum Hydrocarbon in Marine Environments: A Review. Curr. Microbiol. 2015, 71, 220–228. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Liu, M.; Bu, Y.; Zhang, J.; Chen, J.; Zhao, J. Adsorption–synergic biodegradation of diesel oil in synthetic seawater by acclimated strains immobilized on multifunctional materials. Mar. Pollut. Bull. 2015, 92, 195–200. [Google Scholar] [CrossRef]
- Naeem, A.H.; Mumtaz, S.; Haleem, A.; Qazi, M.A.; Malik, Z.A.; Dasti, J.I.; Ahmed, S. Isolation and Molecular Characterization of Biosurfactant-Producing Bacterial Diversity of Fimkassar Oil Field, Pakistan. Arab. J. Sci. Eng. 2017, 42, 2349–2359. [Google Scholar] [CrossRef]
- Mahjoubi, M.; Jaouani, A.; Guesmi, A.; Ben Amor, S.; Jouini, A.; Cherif, H.; Najjari, A.; Boudabous, A.; Koubaa, N.; Cherif, A. Hydrocarbonoclastic bacteria isolated from petroleum contaminated sites in Tunisia: Isolation, identification and characterization of the biotechnological potential. New Biotechnol. 2013, 30, 723–733. [Google Scholar] [CrossRef]
- Guermouche M’rassi, A.; Bensalah, F.; Gury, J.; Duran, R. Isolation and characterization of different bacterial strains for bioremediation of n-alkanes and polycyclic aromatic hydrocarbons. Environ. Sci. Pollut. Res. 2015, 22, 15332–15346. [Google Scholar] [CrossRef]
- Olivella, M.À.; Jové, P.; Bianchi, A.; Bazzicalupi, C.; Cano, L. An integrated approach to understanding the sorption mechanism of phenanthrene by cork. Chemosphere 2013, 90, 1939–1944. [Google Scholar] [CrossRef]
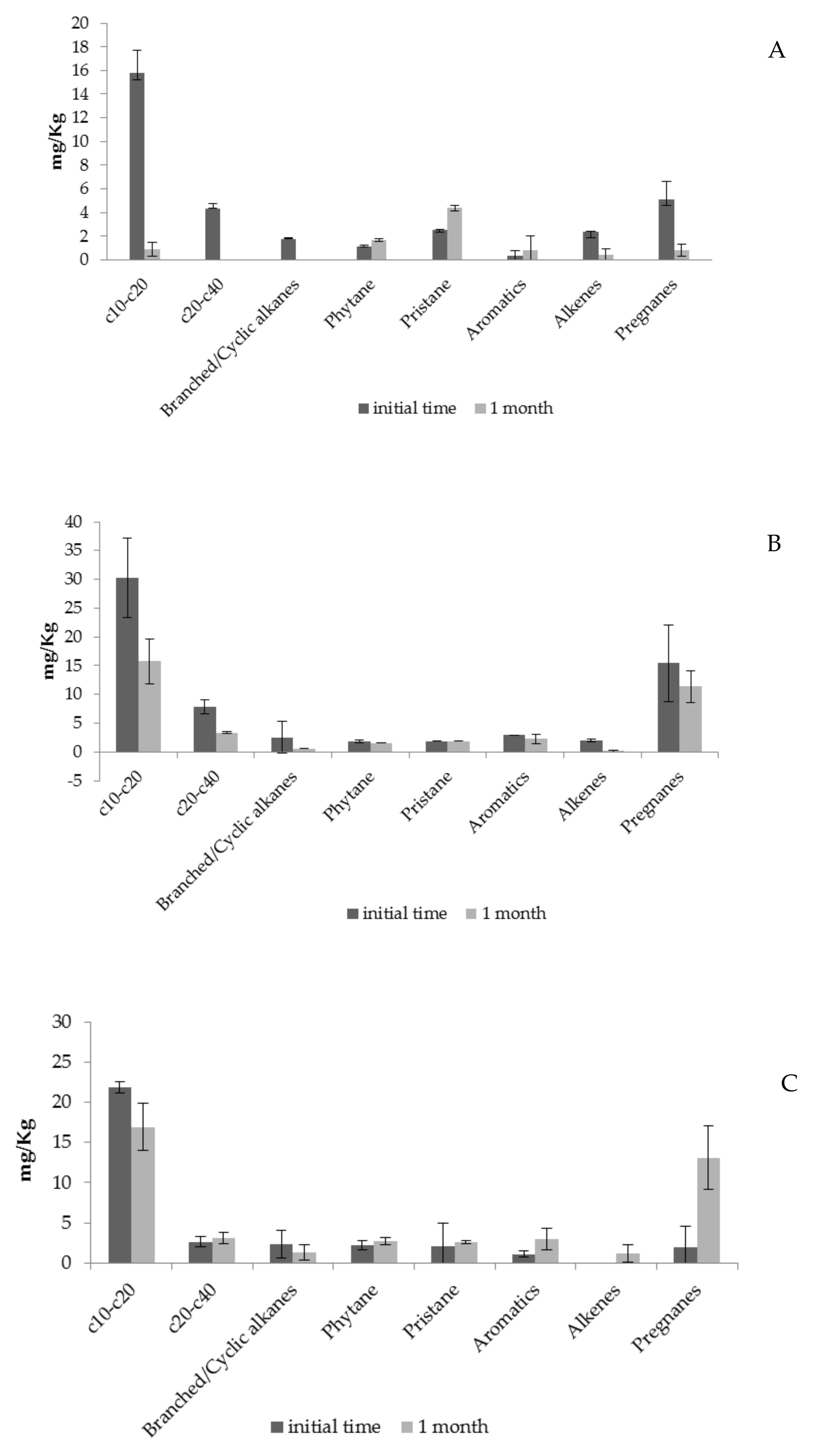

| Filling Material | Composition | Absorption Capacity |
|---|---|---|
| CorkSorb™ 01025 | Granulated hydrophobic cork (Granulometry: 0.5−3 mm). | 9.43 L oil/kg sorbent. |
| Pad Sentec™ | Meltblown polypropylene 99.7%, Blue pigment 0.3%. | 150−200 L/1 pad (Depending on the pollutant). |
| Barrier Sentec™ | Meltblown polypropylene 90% Other no harmful fibers 10%. | 30−40 L/1 barrier (Depending on the pollutant). |
| Hydrocarbon Fraction | Average Concentration (ppm) |
|---|---|
| c10–c20 | 145.6 ± 45.1 |
| c20–c40 | 14.6 ± 5.1 |
| Branched/Cyclic alkanes | 21.9 ± 6.4 |
| Phytane | 15.5 ± 4.0 |
| Pristane | 19.8 ± 7.3 |
| Aromatics | 10.4 ± 3.0 |
| Alkenes | 6.7 ± 3.8 |
| Pregnanes | 20.9 ± 6.8 |
| Hahnfett | 0.0 ± 0.0 |
| Sample | PGR | CRR | GI |
|---|---|---|---|
| Influent wastewater | 92.3 ± 8.2 | 95.5 ± 6.8 | 88.3 ± 11.3 |
| Effluent (Pad Sentec™) | 82.3 ± 5.8 | 94.4 ± 6.6 | 77.8 ± 5.4 |
| Effluent (CorkSorb™ 01025) | 77.8 ± 5.2 | 97.2 ± 6.5 | 75.6 ± 5.5 |
| Effluent (Barrier Sentec™) | 83.3 ± 6.0 | 90.0 ± 6.3 | 75.0 ± 5.2 |
| n-C18/Ph | 7 Days | 1 Month | 4 Months | 8 Months |
|---|---|---|---|---|
| Pad Sentec™ | 1.3 ± 0.3 | 0.43 ± 0.1 | ND | ND |
| Corksorb™ 01025 | 1.1 ± 0.2 | 0.86 ± 0.01 | ND | ND |
| Barrier Sentec™ | 1.4 ± 0,2 | 1.1 ± 0.2 | 0.7 ± 0.3 | ND |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Calvo, A.; Silva-Castro, G.A.; Olicón-Hernández, D.R.; González-López, J.; Calvo, C. Biodegradation and Absorption Technology for Hydrocarbon-Polluted Water Treatment. Appl. Sci. 2020, 10, 841. https://doi.org/10.3390/app10030841
Rodríguez-Calvo A, Silva-Castro GA, Olicón-Hernández DR, González-López J, Calvo C. Biodegradation and Absorption Technology for Hydrocarbon-Polluted Water Treatment. Applied Sciences. 2020; 10(3):841. https://doi.org/10.3390/app10030841
Chicago/Turabian StyleRodríguez-Calvo, Alfonso, Gloria Andrea Silva-Castro, Darío Rafael Olicón-Hernández, Jesús González-López, and Concepción Calvo. 2020. "Biodegradation and Absorption Technology for Hydrocarbon-Polluted Water Treatment" Applied Sciences 10, no. 3: 841. https://doi.org/10.3390/app10030841
APA StyleRodríguez-Calvo, A., Silva-Castro, G. A., Olicón-Hernández, D. R., González-López, J., & Calvo, C. (2020). Biodegradation and Absorption Technology for Hydrocarbon-Polluted Water Treatment. Applied Sciences, 10(3), 841. https://doi.org/10.3390/app10030841









