Radiation-Induced Structural Changes of Miscanthus Biomass
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. 60Co γ-Ray Irradiation Pretreatment of Miscanthus
2.3. Chemical Compositions Analyses
2.4. Structural Properties of the Irradiated Miscanthus
2.4.1. Gel Permeation Chromatography (GPC)
2.4.2. X-Ray Diffraction Analysis (XRD)
2.4.3. Solid State 1H and 13C nuclear magnetic resonance (NMR)
2.4.4. Fourier Transform Infrared spectroscopy (FTIR)
2.4.5. Particle size distribution and specific surface Area (SSA)
2.4.6. Scanning electron microscope (SEM)
2.4.7. Atomic force microscope (AFM)
2.4.8. Thermogravimetric snalysis (TGA)
2.4.9. Degree of Polymerization (DP)
2.5. Enzymatic Hydrolysis of the Irradiated Miscanthus
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of Irradiation Treatment on Chemical Compositions of Miscanthus Biomass
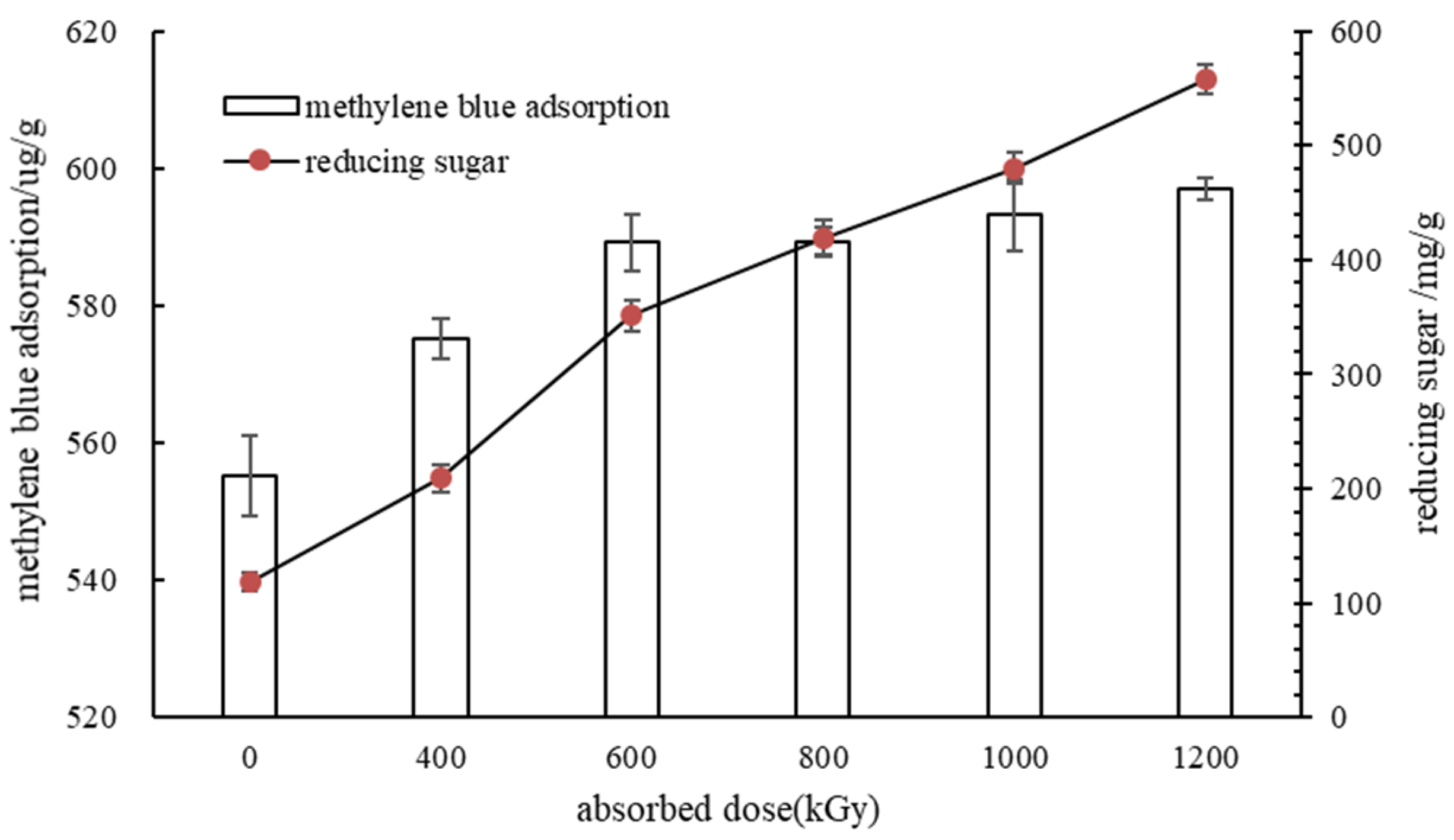
3.2. Effect of Irradiation Treatment on Enzymatic Hydrolysis of Miscanthus
3.3. Influence of Irradiation Treatment on Structural Properties of Miscanthus Biomass
3.3.1. DP, CrI, and Molecular Weight Distribution
3.3.2. TGA Measurement
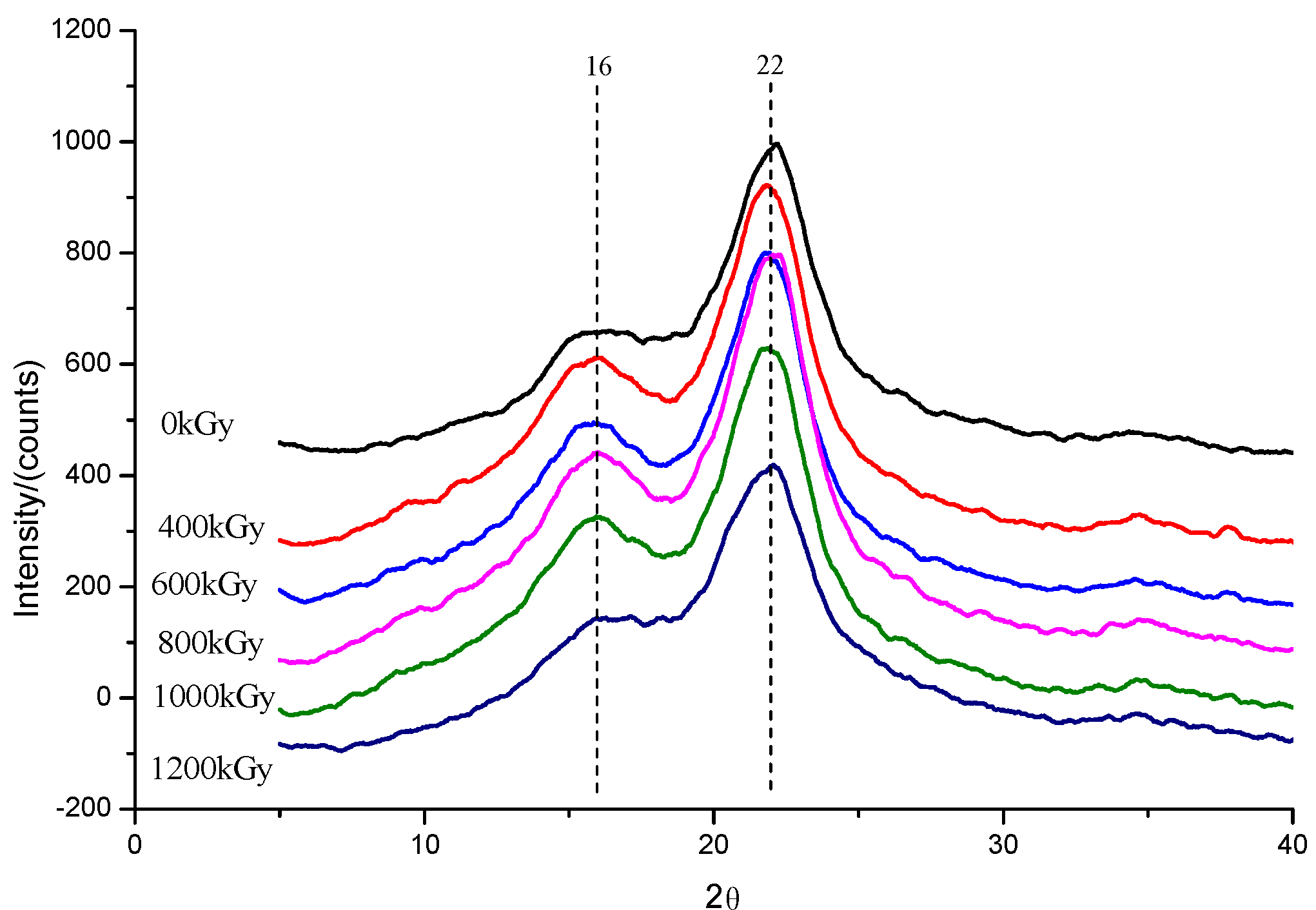
3.3.3. XRD Analysis
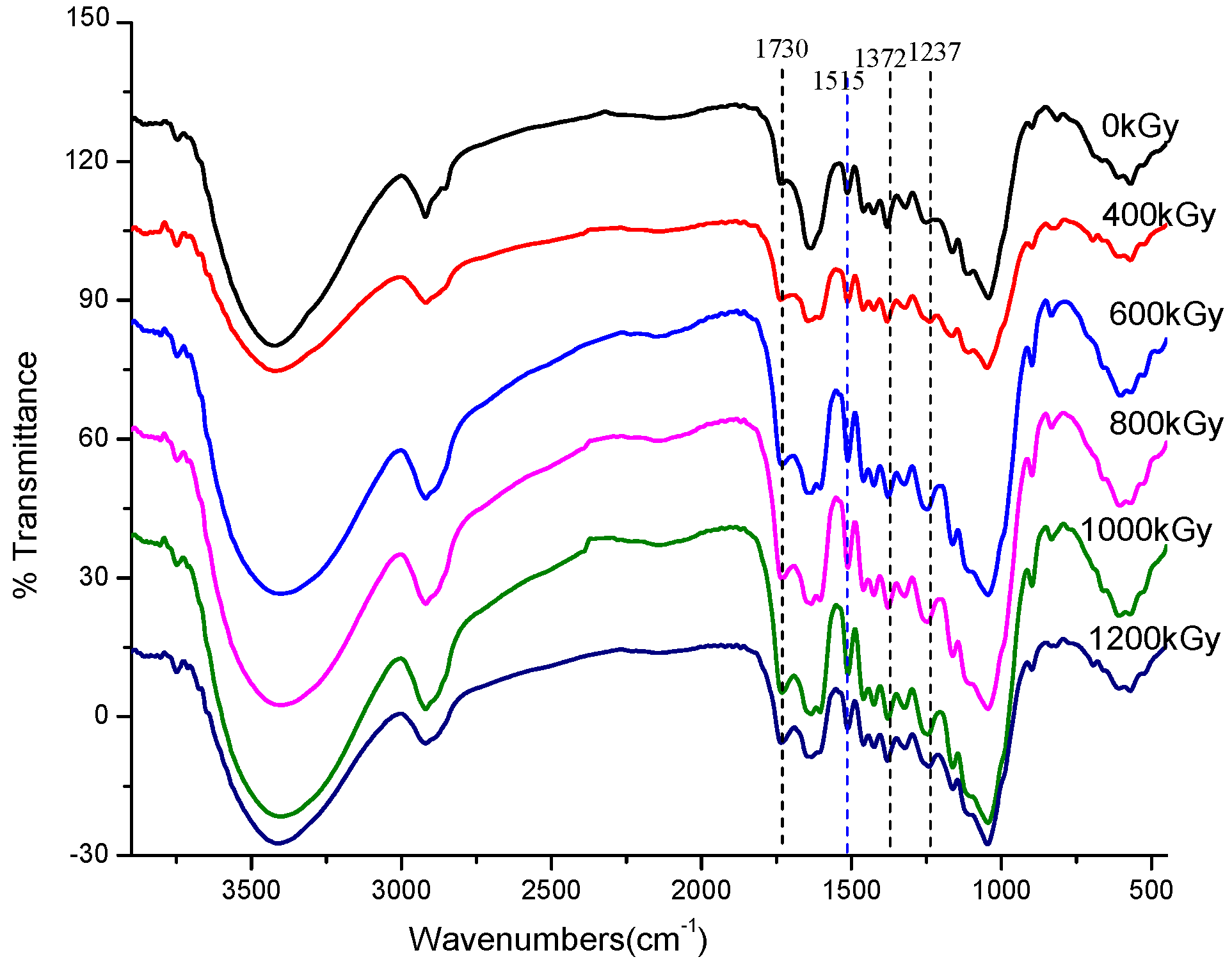
3.3.4. FTIR Analysis
3.3.5. 1H and 13C NMR Analysis
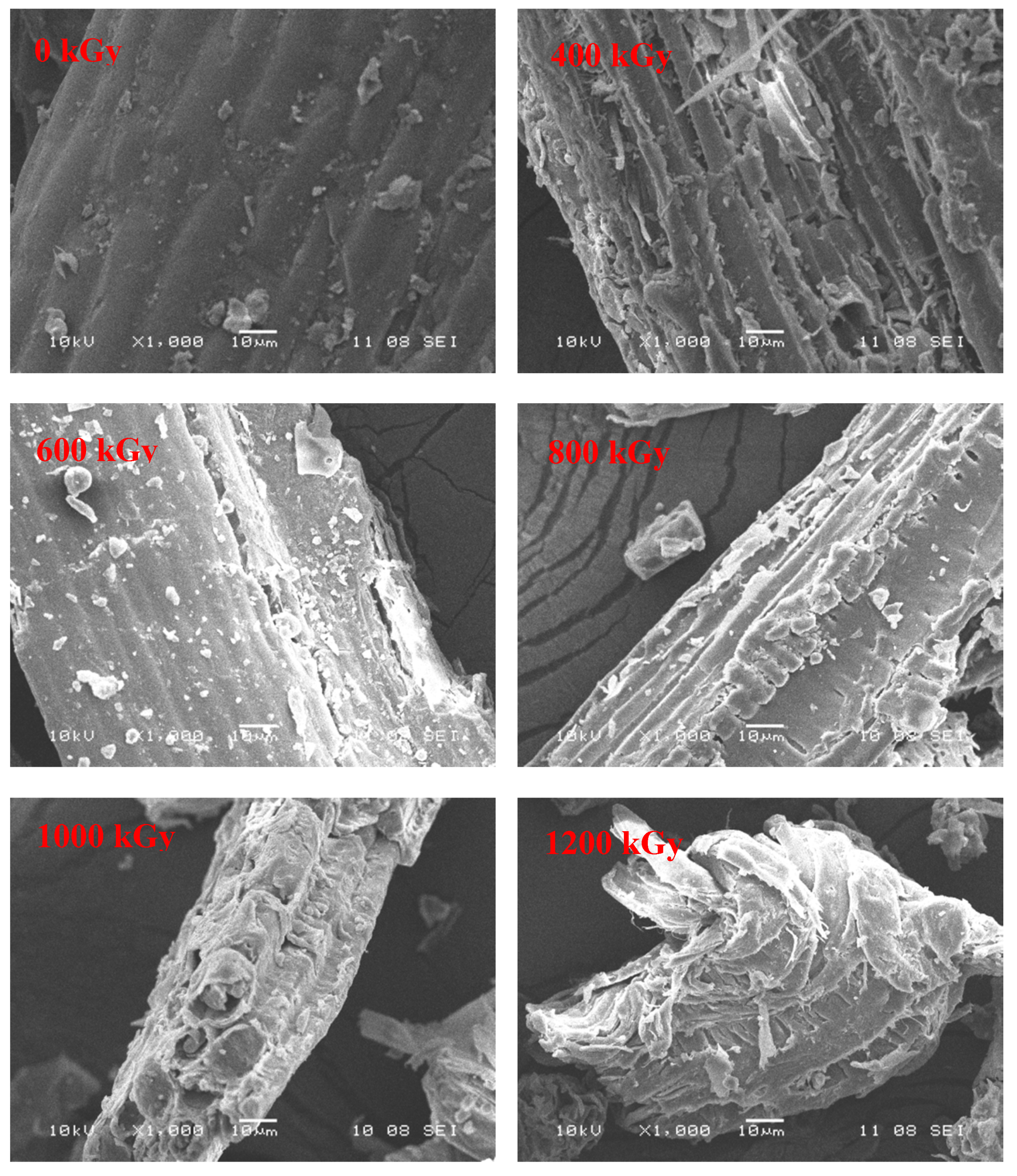
3.3.6. SEM Analysis
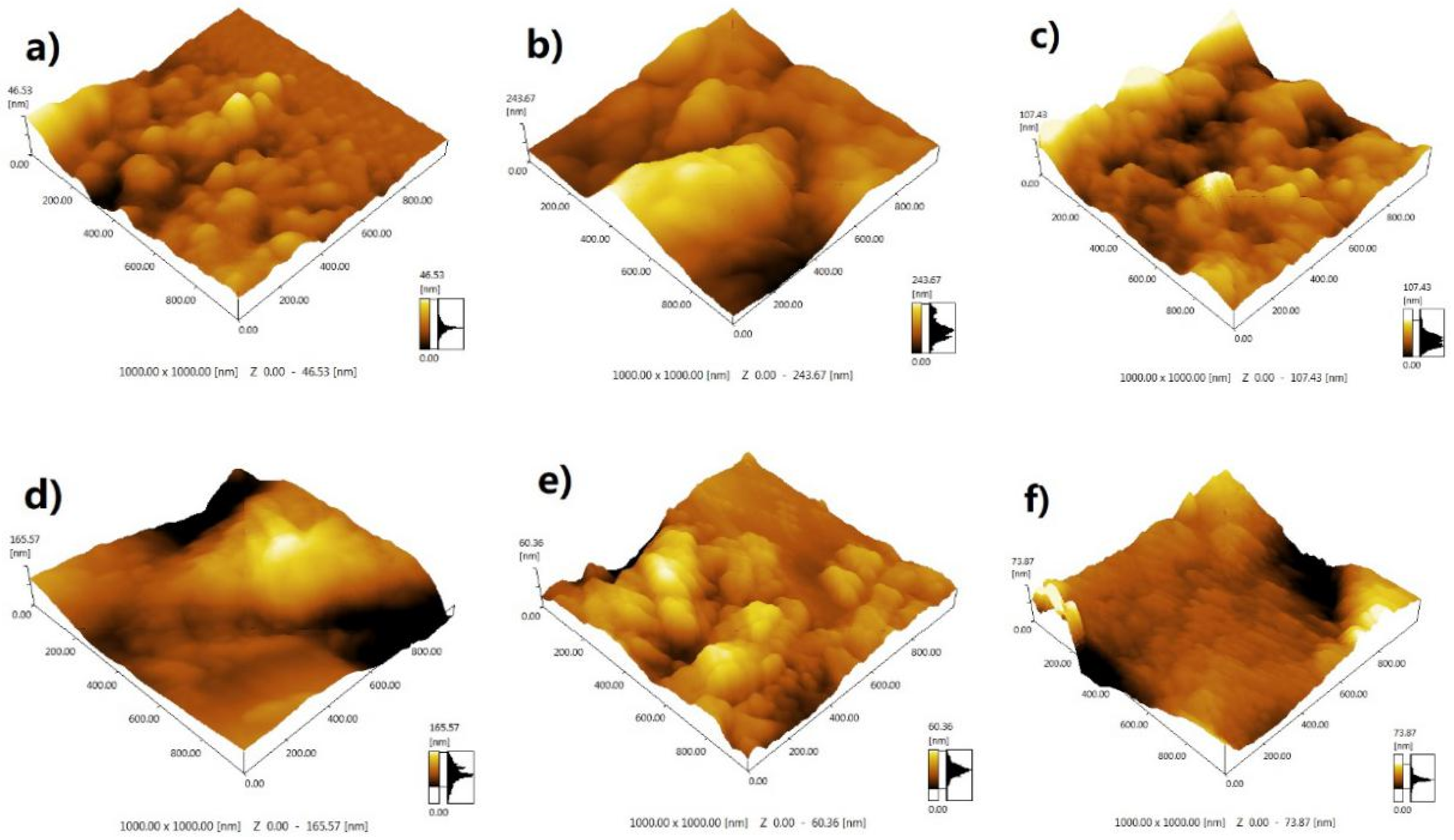
3.3.7. AFM Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Huang, J.; Li, Y.; Xiong, K.; Wang, Y.; Li, F.; Liu, M.; Wu, Z.; Tu, Y.; Peng, L. Ammonium Oxalate-Extractable Uronic Acids Positively Affect Biomass Enzymatic Digestibility by Reducing Lignocellulose Crystallinity in Miscanthus. Bioresour. Technol. 2015, 196, 391–398. [Google Scholar] [CrossRef]
- Morandi, F.; Perrin, A.; Østergård, H. Miscanthus as Energy Crop: Environmental Assessment of a Miscanthus Biomass Production Case Study in France. J. Clean. Prod. 2016, 137, 313–321. [Google Scholar] [CrossRef]
- Adams, J.M.M.; Winters, A.L.; Hodgson, E.M.; Gallagher, J.A. What Cell Wall Components are the Best Indicators for Miscanthus Digestibility and Conversion to Ethanol Following Variable Pretreatments. Biotechnol. Biofuels 2018, 11, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Zhang, W.; Ren, S.; Liu, F.; Zhao, C.; Liao, H.; Xu, Z.; Huang, J.; Li, Q.; Tu, Y.; et al. Hemicelluloses negatively affect lignocellulose crystallinity for high biomass digestibility under NaOH and H2SO4 pretreatments in Miscanthus. Biotechnol. Biofuels 2012, 5, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Van der Weijde, T.; Kamei, C.L.A.; Severing, E.I.; Torres, A.F.; Gomez, L.D.; Dolstra, O.; Maliepaard, C.A.; McQueen-Mason, S.J.; Visser, R.G.F.; Trindade, L.M. Genetic Complexity of Miscanthus Cell Wall Composition and Biomass Quality for Biofuels. BMC Genom. 2017, 18, 406–421. [Google Scholar] [CrossRef]
- Cianchetta, S.; Bregoli, L.; Galletti, S. Microplate-Based Evaluation of the Sugar Yield from Giant Reed, Giant Miscanthus and Switchgrass after Mild Chemical Pre-Treatments and Hydrolysis with Tailored Trichoderma Enzymatic Blends. Appl. Biochem. Biotechnol. 2017, 183, 876–892. [Google Scholar] [CrossRef]
- Alvira, P.; Tomas-Pejo, E.; Ballesteros, M.; Negro, M.J. Pretreatment Technologies for an Efficient Bioethanol Production Process Based on Enzymatic Hydrolysis: A Review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, Q.; Cheng, G. Deconstruction of Corncob by Steam Explosion Pretreatment: Correlations between Sugar Conversion and Recalcitrant Structures. Carbohydr. Polym. 2017, 156, 351–356. [Google Scholar] [CrossRef]
- Zhang, T.; Wyman, C.E.; Jakob, K.; Yang, B. Rapid Selection and Identification of Miscanthus Genotypes with Enhanced Glucan and Xylan Yields from Hydrothermal Pretreatment Followed by Enzymatic Hydrolysis. Biotechnol. Biofuels 2012, 5, 56–69. [Google Scholar] [CrossRef]
- Li, W.; Wang, W.; Xu, P.; Xu, P.; Zhao, X.; Wang, Y. Pretreatment of Miscanthus stalk with organic alkali guanidine and amino-guanidine. Bioresour. Technol. 2015, 179, 606–610. [Google Scholar] [CrossRef]
- Li, M.; Si, S.; Hao, B.; Zha, Y.; Wan, C.; Hong, S.; Kang, Y.; Jia, J.; Zhang, J.; Li, M.; et al. Mild Alkali-Pretreatment Effectively Extracts Guaiacyl-Rich Lignin for High Lignocellulose Digestibility Coupled with Largely Diminishing Yeast Fermentation Inhibitors in Miscanthus. Bioresour. Technol. 2014, 169, 447–454. [Google Scholar] [CrossRef]
- Vasco Correa, J.; Ge, X.; Li, Y. Fungal Pretreatment of Non-Sterile Miscanthus for Enhanced Enzymatic Hydrolysis. Bioresour. Technol. 2016, 203, 118–123. [Google Scholar] [CrossRef]
- Tissot, C.; Grdanovska, S.; Barkatt, A.; Silverman, J.; Al-Sheikhly, M. On the mechanisms of the radiation-induced degradation of cellulosic substances. Rad. Phys. Chem. 2013, 84, 185–190. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Wang, S.; Wang, K.; Su, X. Comparison of Gamma-Irradiation with other Pretreatments Followed with Simultaneous Saccharification and Fermentation on Bioconversion of Microcrystalline Cellulose for Bioethanol Production. Bioresour. Technol. 2015, 182, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, L.; Su, X.; Wang, K.; Wu, X.; Chen, L.; Xiong, X.; Zhou, H.; Liu, Y. Structure, Morphology, Thermostability and Irradiation Mediated Degradation Fractions of Hemicellulose Treated with γ-Irradiation. Waste Biomass Valorization 2016, 7, 1415–1425. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Wu, X.; Wang, K.; Su, X.; Chen, L.; Zhou, H.; Xiong, X. Insights into the effects of γ-irradiation on the microstructure, thermal stability and irradiation-derived degradation components of microcrystalline cellulose (MCC). RSC Adv. 2015, 5, 34353–34363. [Google Scholar] [CrossRef]
- Su, X.; Hu, Q.; Wang, K.; Li, Q.; Cai, L.; Hu, T.; Jiang, Y.; Xiong, X. Enzymatic Hydrolysis and Structural Characterization of Miscanthus Straw Exposed to 60Co γ- Ray Irradiation Pretreatment. J. Biobased Mater. Bioenergy 2015, 9, 55–61. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Jiang, Y.; Xiong, X.; Hu, Q.; Tan, X.; Wang, K.; Su, X. Analysis of degradation products and structural characterization of giant reed and Chinese silvergrass pretreated by 60Co-γ irradiation. Ind. Crop. Prod. 2016, 83, 307–315. [Google Scholar] [CrossRef]
- Zhang, C.; Su, X.; Xiong, X.; Hu, Q.; Amartey, S.; Tan, X.T.; Qin, W. 60Co-γ Radiation-Induced Changes in the Physical and Chemical Properties of Rapeseed Straw. Biomass Bioenergy 2016, 85, 207–214. [Google Scholar] [CrossRef]
- Chandel, A.K.; Antunes, F.F.A.; Anjos, V.; Bell, M.J.V.; Rodrigues, L.N.; Singh, O.V.; Rosa, C.A.; Pagnocca, F.C.; Silva, S.S.D. Ultra-Structural Mapping of Sugarcane Bagasse after Oxalic Acid Fiber Expansion (OAFEX) and Ethanol Production by Candida Shehatae and Saccharomyces Cerevisiae. Biotechnol. Biofuels 2013, 6, 4. [Google Scholar] [CrossRef]
- Yang, C.; Shen, Z.; Yu, G.; Wang, J. Effect and Aftereffect of γ Radiation Pretreatment on Enzymatic Hydrolysis of Wheat Straw. Bioresour. Technol. 2008, 99, 6240–6245. [Google Scholar] [CrossRef] [PubMed]
- Beardmore, D.H.; Fan, L.T.; Lee, Y.-H. Gamma-Ray Irradiation as a Pretreatment for the Enzymatic Hydrolysis of Cellulose. Biotechnol. Lett. 1980, 2, 435–438. [Google Scholar] [CrossRef]
- Takács, E.; Wojnárovits, L.; Borsa, J.; Földváry, C.; Zöld, O. Effect of γ-Irradiation on Cotton-Cellulose. Radiat. Phys. Chem. 1999, 55, 663–666. [Google Scholar] [CrossRef]
- Jiang, W.; Chang, S.; Qu, Y.; Zhang, Z.; Xu, J. Changes on structural properties of biomass pretreated by combined deacetylation with liquid hot water and its effect on enzymatic hydrolysis. Bioresour. Technol. 2016, 220, 448–456. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, L.; Wang, D.; Zhang, R.; Liu, G.; Cheng, G. Thermogravimetric analysis of lignocellulosic biomass with ionic liquid pretreatment. Bioresour. Technol. 2014, 153, 379–382. [Google Scholar] [CrossRef]
- Huang, C.; Lin, W.; Lai, C.; Li, X.; Jin, Y.; Yong, Q. Coupling the post-extraction process to remove residual lignin and alter the recalcitrant structures for improving the enzymatic digestibility of acid-pretreated bamboo residues. Bioresour. Technol. 2019, 285, 121355. [Google Scholar] [CrossRef]
- Sun, J.; Xu, L.; Ge, M.; Zhai, M. Radiation degradation of microcrystalline cellulose in solid status. J. Appl. Polym. Sci. 2013, 127, 1630–1636. [Google Scholar] [CrossRef]
- Hong, S.H.; Lee, J.T.; Lee, S.; Wi, S.G.; Cho, E.J.; Singh, S.; Lee, S.S.; Chung, B.Y. Improved enzymatic hydrolysis of wheat straw by combined use of gamma ray and dilute acid for bioethanol production. Radiat. Phys. Chem. 2014, 94, 231–235. [Google Scholar] [CrossRef]
- Wang, K.Q.; Xiong, X.Y.; Chen, J.P.; Chen, L.; Su, X.J.; Liu, Y. Comparison of gamma irradiation and steam explosion pretreatment for ethanol production from agricultural residues. Biomass Bioenergy 2012, 46, 301–308. [Google Scholar] [CrossRef]
- Li, C.; Liu, G.J.; Nges, I.A.; Liu, J. Enhanced biomethane production from Miscanthus lutarioriparius using steam explosion pretreatment. Fuel 2016, 179, 267–273. [Google Scholar] [CrossRef]
- Chundawat, S.P.S.; Donohoe, B.S.; Sousa, L.D.C.; Elder, T.; Agarwal, U.P.; Lu, F.; Ralph, J.; Himmel, M.E.; Balan, V.; Dale, B.E. Multi-Scale Visualization and Characterization of Lignocellulosic Plant Cell Wall Deconstruction during Thermochemical Pretreatment. Energy Environ. Sci. 2011, 4, 973–984. [Google Scholar] [CrossRef]


| Absorbed Dose (kGy) | 0 (Untreated) | 400 | 600 | 800 | 1000 | 1200 |
|---|---|---|---|---|---|---|
| Cellulose (%) | 36.60 | 37.20 | 33.90 | 33.10 | 31.00 | 23.00 |
| Hemicellulose (%) | 31.80 | 17.80 | 11.60 | 10.50 | 7.40 | 4.90 |
| Lignin (%) | 21.30 | 23.10 | 21.70 | 22.00 | 23.00 | 19.70 |
| Total (%) | 89.70 | 78.10 | 67.20 | 65.60 | 61.40 | 47.60 |
| Absorbed Dose (kGy) | SSA (m2/g) | D [3, 2] (μm) | D [4, 3] (μm) | d (0.1) (μm) | d (0.5) (μm) | d (0.9) (μm) |
|---|---|---|---|---|---|---|
| 0 (Untreated) | 0.252 | 23.808 | 221.005 | 18.026 | 153.465 | 547.317 |
| 400 | 0.256 | 23.432 | 203.056 | 14.963 | 131.620 | 496.540 |
| 600 | 0.315 | 19.053 | 169.749 | 4.397 | 107.316 | 462.257 |
| 800 | 0.319 | 18.806 | 169.307 | 4.641 | 105.537 | 461.163 |
| 1000 | 0.642 | 9.343 | 33.060 | 2.924 | 21.181 | 90.421 |
| 1200 | 0.815 | 7.357 | 20.099 | 2.772 | 18.423 | 39.082 |
| Dose/kGy | 0 | 400 | 600 | 800 | 1000 | 1200 |
|---|---|---|---|---|---|---|
| Mw (Da) | 542,342 | 228,791 | 168,982 | 160,859 | 151,213 | 150,821 |
| Mn (Da) | 45,544 | 37,981 | 36,273 | 34,793 | 33,565 | 30,237 |
| Mw/Mn | 14.952 | 5.024 | 4.449 | 4.623 | 4.505 | 4.988 |
| DP | 366,225 | 44,230 | 30,947 | 20,569 | 18,200 | 11,354 |
| CrI (%) | 37.86 | 33.26 | 28.92 | 29.03 | 30.02 | 28.43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.-J.; Zhang, C.-Y.; Li, W.-J.; Wang, F.; Wang, K.-Q.; Liu, Y.; Li, Q.-M. Radiation-Induced Structural Changes of Miscanthus Biomass. Appl. Sci. 2020, 10, 1130. https://doi.org/10.3390/app10031130
Su X-J, Zhang C-Y, Li W-J, Wang F, Wang K-Q, Liu Y, Li Q-M. Radiation-Induced Structural Changes of Miscanthus Biomass. Applied Sciences. 2020; 10(3):1130. https://doi.org/10.3390/app10031130
Chicago/Turabian StyleSu, Xiao-Jun, Chun-Yan Zhang, Wen-Jia Li, Feng Wang, Ke-Qin Wang, Yun Liu, and Qing-Ming Li. 2020. "Radiation-Induced Structural Changes of Miscanthus Biomass" Applied Sciences 10, no. 3: 1130. https://doi.org/10.3390/app10031130
APA StyleSu, X.-J., Zhang, C.-Y., Li, W.-J., Wang, F., Wang, K.-Q., Liu, Y., & Li, Q.-M. (2020). Radiation-Induced Structural Changes of Miscanthus Biomass. Applied Sciences, 10(3), 1130. https://doi.org/10.3390/app10031130





