Comparison between Occlusal Errors of Single Posterior Crowns Adjusted Using Patient Specific Motion or Conventional Methods
Abstract
1. Introduction
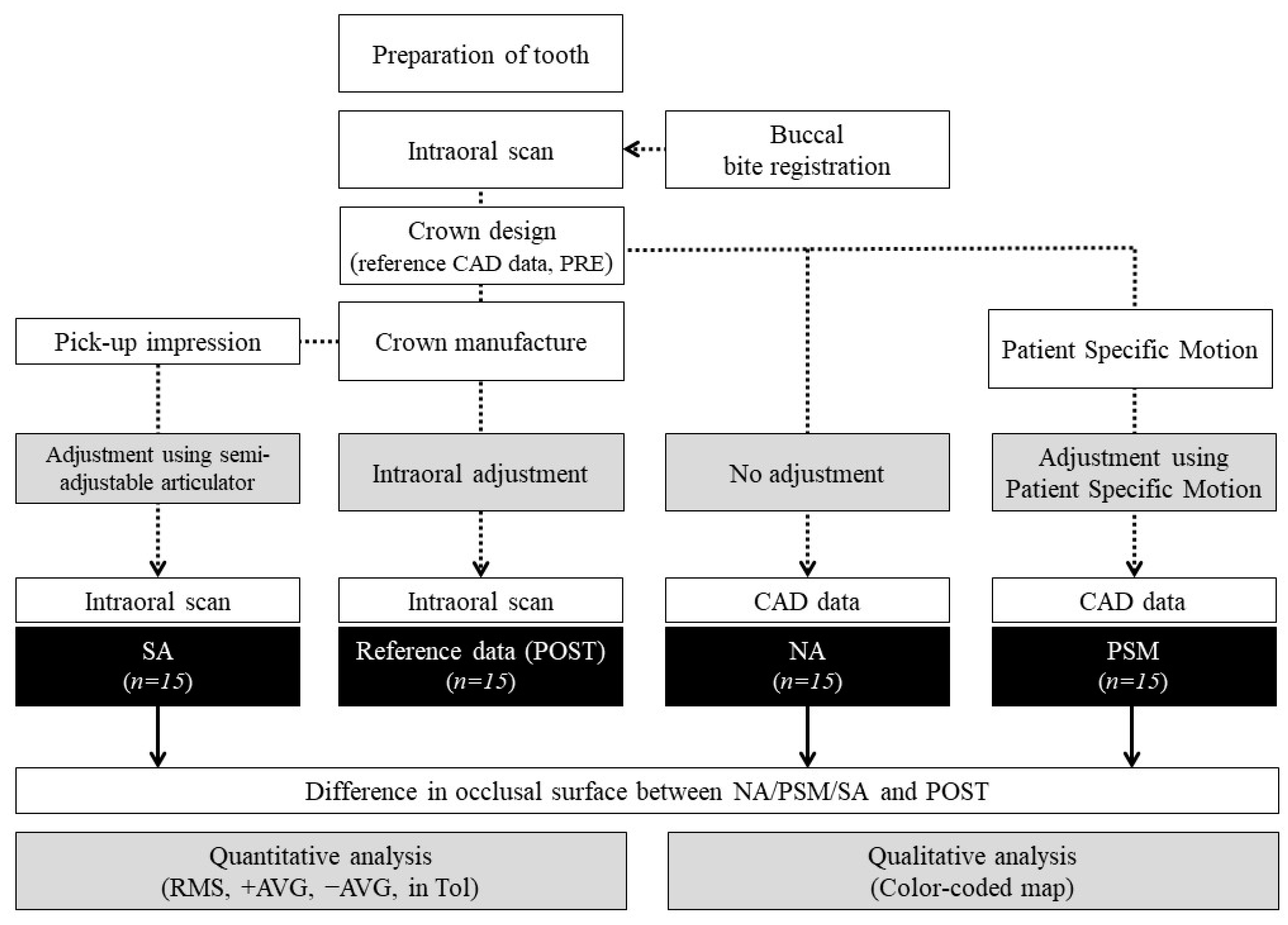
2. Materials & Methods
2.1. Participants
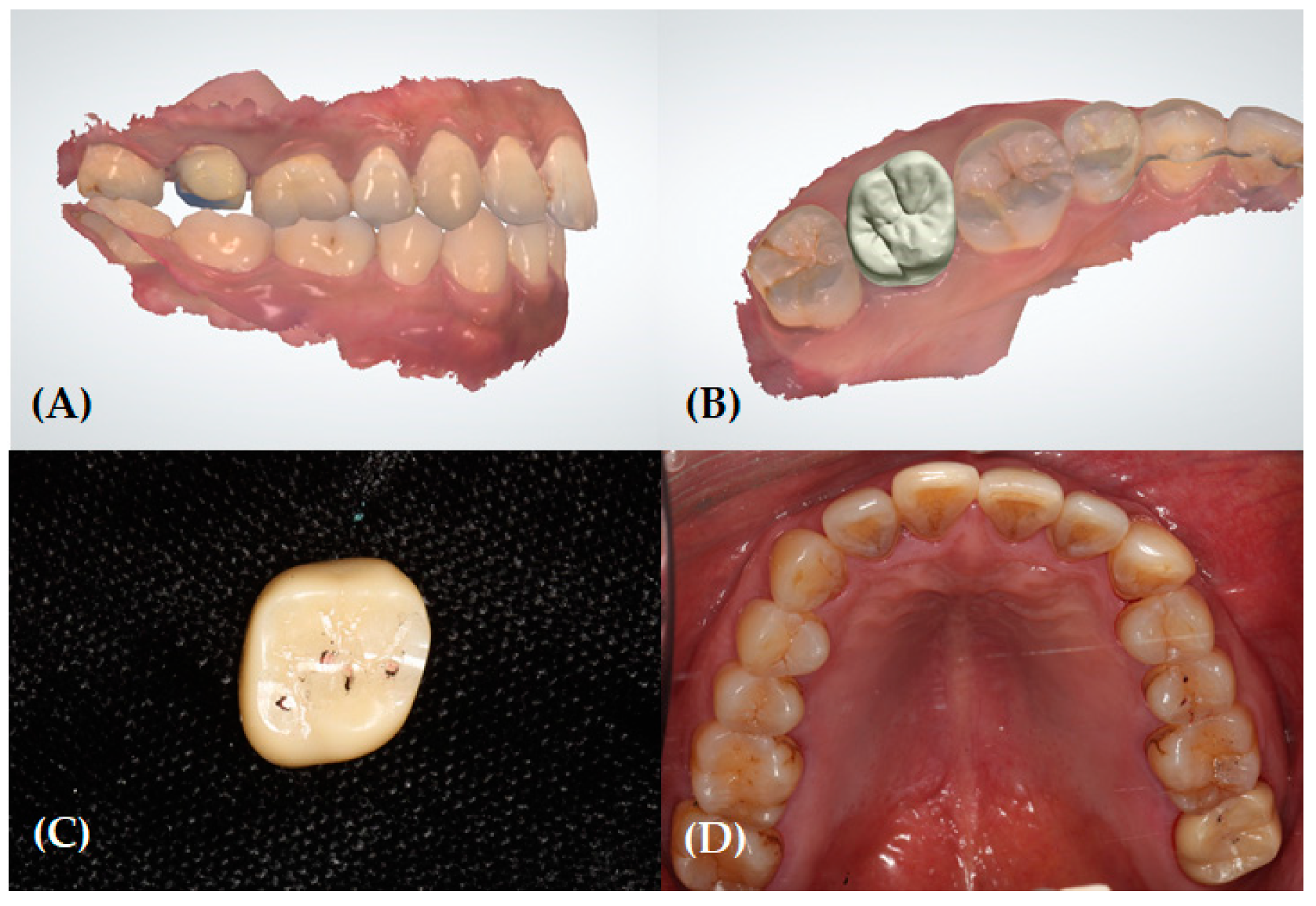
2.2. Clinical and Laboratory Procedures for Reference Data (Pre- and Post-Treatment)
2.3. Data Acquisition for the Experimental Groups
2.3.1. No Adjustment Group (Control)
2.3.2. Patient Specific Motion Group
2.3.3. Semi-Adjustable Articulator Group
2.4. Data Processing
2.5. Statistical Analysis
3. Results
3.1. Quantitative Analysis
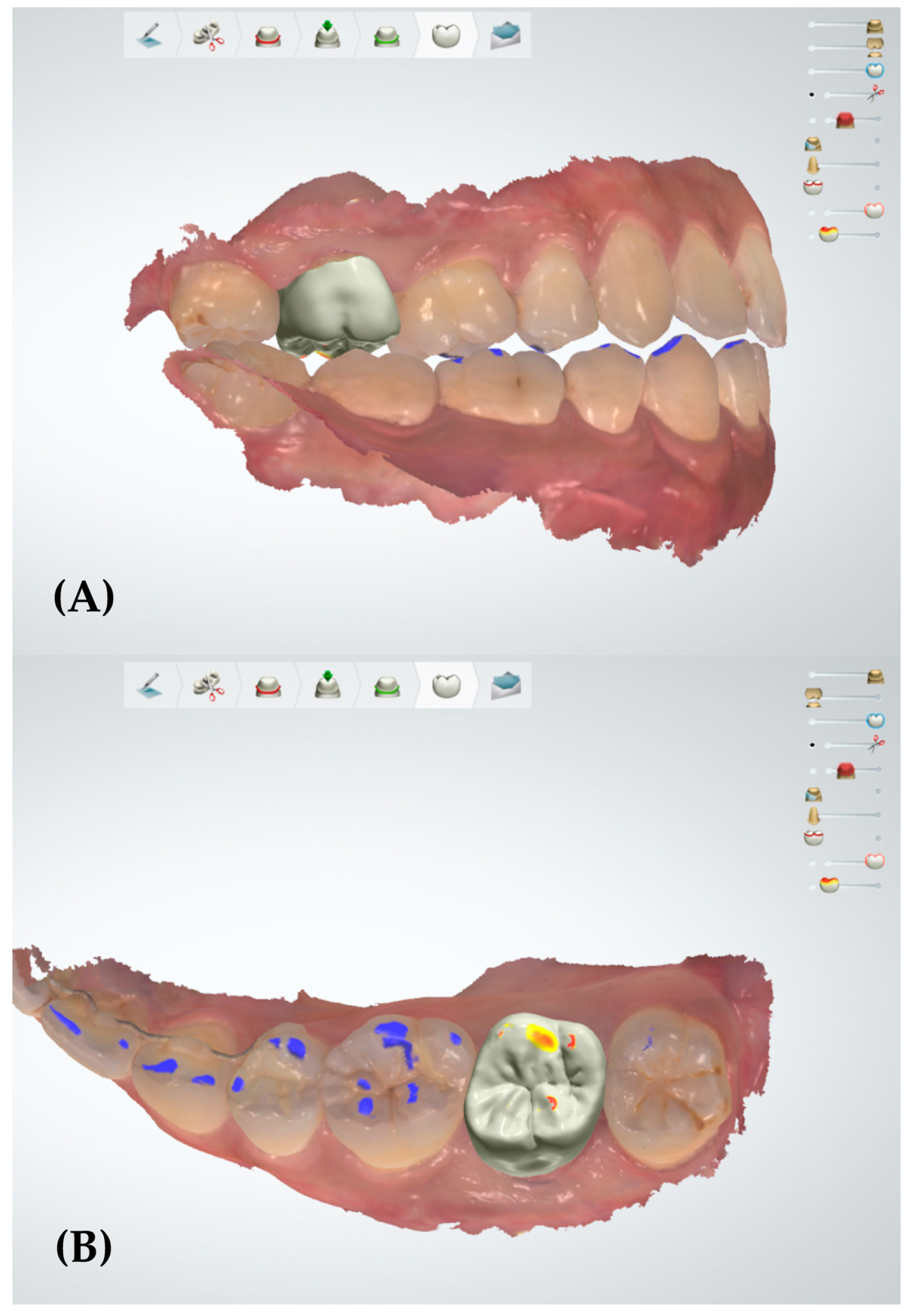
3.2. Qualitative Analysis
4. Discussion
5. Conclusions
- In the quantitative analysis, there was a statistically reduced occlusal error in the group with adjustments using PSM on the out of tolerance area compared to the group design based on static occlusion. However, no significant difference in the amount of occlusal error was revealed in the entire and subdivided occlusal surface level between the PSM and conventional methods.
- In the qualitative analysis, a decrease of the occlusal error areas was mainly displayed in the inclined plane of the cusp and triangular ridge on the occlusal surface in a single posterior crown adjusted with the PSM.
- The PSM may be a simple and effective alternative tool that shows clinically acceptable results in the occlusal adjustment of a single posterior crown.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thompson, J.R. Concepts regarding function of the stomatognathic system. J. Am. Dent. Assoc. 1954, 48, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.J. Is occlusion becoming more confusing? A plea for simplicity. J. Am. Dent. Assoc. 2004, 135, 767–768. [Google Scholar] [CrossRef] [PubMed]
- Turp, J.C.; Greene, C.S.; Strub, J.R. Dental occlusion: A critical reflection on past, present and future concepts. J. Oral Rehabil. 2008, 35, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Parmar, A.; Choukse, V.; Palekar, U.; Srivastava, R. An Appraisal on Occlusal Philosophies in Full-mouth Rehabilitation: A Literature Review. Eur. J. Prosthodont. 2016, 6, 89–92. [Google Scholar]
- Fiore, A.D.; Monaco, C.; Brunello, G.; Granata, S.; Stellini, E.; Yilmaz, B. Automatic Digital Design of the Occlusal Anatomy of Monolithic Zirconia Crowns Compared to Dental Technicians’ Digital Waxing: A Controlled Clinical Trial. J. Prosthodont. 2020. [Google Scholar] [CrossRef]
- Olthoff, L.; Meijer, I.; de Ruiter, W.; Bosman, F.; van der Zel, J. Effect of virtual articulator settings on occlusal morphology of CAD/CAM restorations. Int. J. Comput. Dent. 2007, 10, 171–185. [Google Scholar]
- Fang, J.-J.; Kuo, T.-H. Tracked motion-based dental occlusion surface estimation for crown restoration. Comput. Aided Des. 2009, 41, 315–323. [Google Scholar] [CrossRef]
- Gracis, S. Clinical considerations and rationale for the use of simplified instrumentation in occlusal rehabilitation. Part 1: Mounting of the models on the articulator. Int. J. Periodontics Restor. Dent. 2003, 23, 57–67. [Google Scholar]
- Pröschel, P.; Morneburg, T.; Hugger, A.; Kordass, B.; Ottl, P.; Niedermeier, W.; Wichmann, M. Articulator-related registration—A simple concept for minimizing eccentric occlusal errors in the articulator. Int. J. Prosthodont. 2002, 15, 289–294. [Google Scholar]
- Schulte, J.K.; Wang, S.H.; Erdman, A.G.; Anderson, G.C. Three-dimensional analysis of cusp travel during a nonworking mandibular movement. J. Prosthet. Dent. 1985, 53, 839–843. [Google Scholar] [CrossRef]
- Schulte, J.K.; Wang, S.H.; Erdman, A.G.; Anderson, G.C. Working condylar movement and its effects on posterior occlusal morphology. J. Prosthet. Dent. 1985, 54, 118–121. [Google Scholar] [CrossRef]
- Weinberg, L.A. An evaluation of basic articulators and their concepts: Part I. Basic concepts. J. Prosthet. Dent. 1963, 13, 622–644. [Google Scholar] [CrossRef]
- Hobo, S.; Shillingburg, H.T.; Whitsett, L.D. Articulator selection for restorative dentistry. J. Prosthet. Dent. 1976, 36, 35–43. [Google Scholar] [CrossRef]
- Kim, J.-E.; Park, J.-H.; Moon, H.-S.; Shim, J.-S. Complete assessment of occlusal dynamics and establishment of a digital workflow by using target tracking with a three-dimensional facial scanner. J. Prosthodont. Res. 2019, 63, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Ak, V.; Ali, M.; Chaturvedi, S.; Naeem, A.; Amrit, R. Articulators—A review article. Int. J. Appl. Res. 2014, 1, 6–8. [Google Scholar]
- Meyer, F.S. The generated path technique in reconstruction dentistry: Part II. Fixed partial dentures. J. Prosthet. Dent. 1959, 9, 432–440. [Google Scholar] [CrossRef]
- Pankey, L.D.; Mann, A.W. Oral rehabilitation: Part II. Reconstruction of the upper teeth using a functionally generated path technique. J. Prosthet. Dent. 1960, 10, 151–162. [Google Scholar] [CrossRef]
- Curtis, S.R. Functionally generated paths for ceramometal restorations. J. Prosthet. Dent. 1999, 81, 33–36. [Google Scholar] [CrossRef]
- Mehl, A. A new concept for the integration of dynamic occlusion in the digital construction process. Int. J. Comput. Dent. 2012, 15, 109–123. [Google Scholar]
- Di Fiore, A.; Meneghello, R.; Graiff, L.; Savio, G.; Vigolo, P.; Monaco, C.; Stellini, E. Full arch digital scanning systems performances for implant-supported fixed dental prostheses: A comparative study of 8 intraoral scanners. J. Prosthodont. Res. 2019, 63, 396–403. [Google Scholar] [CrossRef]
- Bando, E.; Nishigawa, K.; Nakano, M.; Takeuchi, H.; Shigemoto, S.; Okura, K.; Satsuma, T.; Yamamoto, T. Current status of researches on jaw movement and occlusion for clinical application. Jpn. Dent. Sci. Rev. 2009, 45, 83–97. [Google Scholar] [CrossRef]
- Ender, A.; Mörmann, W.H.; Mehl, A. Efficiency of a mathematical model in generating CAD/CAM-partial crowns with natural tooth morphology. Clin. Oral Investig. 2011, 15, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Baroudi, K.; Ibraheem, S.N. Assessment of Chair-side Computer-Aided Design and Computer-Aided Manufacturing Restorations: A Review of the Literature. J. Int. Oral Health 2015, 7, 96–104. [Google Scholar] [PubMed]
- Ender, A.; Zimmermann, M.; Attin, T.; Mehl, A. In vivo precision of conventional and digital methods for obtaining quadrant dental impressions. Clin. Oral Investig. 2016, 20, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Sornsuwan, T.; Swain, M.V. Influence of occlusal geometry on ceramic crown fracture; role of cusp angle and fissure radius. J. Mech. Behav. Biomed. 2011, 4, 1057–1066. [Google Scholar] [CrossRef]
- Valenti, M.; Schmitz, J.H. A reverse digital workflow by using an interim restoration scan and patient-specific motion with an intraoral scanner. J. Prosthet. Dent. 2020, in press. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Kollmuss, M.; Jakob, F.-M.; Kirchner, H.-G.; Ilie, N.; Hickel, R.; Huth, K.C. Comparison of biogenerically reconstructed and waxed-up complete occlusal surfaces with respect to the original tooth morphology. Clin. Oral Investig. 2013, 17, 851–857. [Google Scholar] [CrossRef]
- Brizuela-Velasco, A.; Álvarez-Arenal, Á.; Ellakuria-Echevarria, J.; del Río-Highsmith, J.; Santamaría-Arrieta, G.; Martín-Blanco, N. Influence of Articulating Paper Thickness on Occlusal Contacts Registration: A Preliminary Report. Int. J. Prosthodont. 2015, 28, 360–362. [Google Scholar] [CrossRef]
- Hobo, S.; Takayama, H. Twin-stage procedure. Part 1: A new method to reproduce precise eccentric occlusal relations. Int. J. Periodontics Restor. Dent. 1997, 17, 112–123. [Google Scholar]
- Weinberg, L.A. An evaluation of basic articulators and their concepts: Part II. Arbitrary, positional, semi adjustable articulators. J. Prosthet. Dent. 1963, 13, 645–663. [Google Scholar] [CrossRef]
- Boyarsky, H.P.; Loos, L.G.; Leknius, C. Occlusal refinement of mounted casts before crown fabrication to decrease clinical time required to adjust occlusion. J. Prosthet. Dent. 1999, 82, 591–594. [Google Scholar] [CrossRef]
- Meng, J.C.; Nagy, W.W.; Wirth, C.G.; Buschang, P.H. The effect of equilibrating mounted dental stone casts on the occlusal harmony of cast metal complete crowns. J. Prosthet. Dent. 2010, 104, 122–132. [Google Scholar] [CrossRef]
- O’Toole, S.; Osnes, C.; Bartlett, D.; Keeling, A. Investigation into the accuracy and measurement methods of sequential 3D dental scan alignment. Dent. Mater. 2019, 35, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Al Hamad, K.Q.; Al-Rashdan, R.B.; Al-Rashdan, B.A.; Baba, N.Z. Effect of Milling Protocols on Trueness and Precision of Ceramic Crowns. J. Prosthodont. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bosch, G.; Ender, A.; Mehl, A. A 3-dimensional accuracy analysis of chairside CAD/CAM milling processes. J. Prosthet. Dent. 2014, 112, 1425–1431. [Google Scholar] [CrossRef]
- Mangano, F.G.; Hauschild, U.; Veronesi, G.; Imburgia, M.; Mangano, C.; Admakin, O. Trueness and precision of 5 intraoral scanners in the impressions of single and multiple implants: A comparative in vitro study. BMC Oral Health 2019, 19, 101. [Google Scholar] [CrossRef]
- Wang, W.; Yu, H.; Liu, Y.; Jiang, X.; Gao, B. Trueness analysis of zirconia crowns fabricated with 3-dimensional printing. J. Prosthet. Dent. 2019, 121, 285–291. [Google Scholar] [CrossRef]
- Yang, X.; Lv, P.; Liu, Y.; Si, W.; Feng, H. Accuracy of Digital Impressions and Fitness of Single Crowns Based on Digital Impressions. Materials 2015, 8, 3945–3957. [Google Scholar] [CrossRef]
- Schaefer, O.; Watts, D.C.; Sigusch, B.W.; Kuepper, H.; Guentsch, A. Marginal and internal fit of pressed lithium disilicate partial crowns in vitro: A three-dimensional analysis of accuracy and reproducibility. Dent. Mater. 2012, 28, 320–326. [Google Scholar] [CrossRef]
- Kim, C.-M.; Kim, S.-R.; Kim, J.-H.; Kim, H.-Y.; Kim, W.-C. Trueness of milled prostheses according to number of ball-end mill burs. J. Prosthet. Dent. 2016, 115, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Beuer, F.; Schweiger, J.; Edelhoff, D. Digital dentistry: An overview of recent developments for CAD/CAM generated restorations. Br. Dent. J. 2008, 204, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Strub, J.R.; Rekow, E.D.; Witkowski, S. Computer-aided design and fabrication of dental restorations: Current systems and future possibilities. J. Am. Dent. 2006, 137, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tang, Q.; Xi, S.; Liu, R.; Niu, L. Comparison and evaluation of the morphology of crowns generated by biogeneric design technique with CEREC chairside system. PLoS ONE 2020, 15, e0227050. [Google Scholar] [CrossRef] [PubMed]
- Olthoff, L.W.; van der Zel, J.M.; de Ruiter, W.J.; Vlaar, S.T.; Bosman, F. Computer modeling of occlusal surfaces of posterior teeth with the CICERO CAD/CAM system. J. Prosthet. Dent. 2000, 84, 154–162. [Google Scholar] [CrossRef]
- Lin, P.-t.; Jiao, Y.; Zhao, S.-j.; Wang, F.; Li, L.; Yu, F.; Tian, M.; Yu, H.-h.; Chen, J.-h. Occlusion and Disocclusion Time Changes in Single Unit Crowns Designed by Functional Generated Path Technique: A Randomised Clinical Trial. Sci. Rep. 2017, 7, 388. [Google Scholar] [CrossRef]
- Saini, H.; Wadell, J.N.; Pullan, A.J.; Röhrle, O. Automatically Generating Subject-specific Functional Tooth Surfaces Using Virtual Mastication. Biomed. Eng. 2009, 37, 1646–1653. [Google Scholar] [CrossRef]
- Clark, G.T.; Tsukiyama, Y.; Baba, K.; Watanabe, T. Sixty-eight years of experimental occlusal interference studies: What have we learned? J. Prosthet. Dent. 1999, 82, 704–713. [Google Scholar] [CrossRef]
- Dawson, P.E. Determining the determinants of occlusion. Int. J. Periodont. Rest. 1983, 3, 8–21. [Google Scholar]
- Watamoto, T.; Egusa, H.; Mizumori, T.; Yashiro, K.; Takada, K.; Yatani, H. Restoration of occlusal and proximal contacts by a single molar crown improves the smoothness of the masticatory movement. J. Dent. 2008, 36, 984–992. [Google Scholar] [CrossRef]
- Davies, S.J.; Gray, R.M.J.; Smith, P.W. Good occlusal practice in simple restorative dentistry. Br. Dent. J. 2001, 191, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Altarakemah, Y.; Akbar, J.; Akthar, S.; Qudeimat, M.A.; Omar, R. Evaluation of a Technique for Reducing Chairside Occlusal Adjustment of Crowns. J. Prosthodont. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gracis, S. Clinical considerations and rationale for the use of simplified instrumentation in occlusal rehabilitation. Part 2: Setting of the articulator and occlusal optimization. Int. J. Periodont. Rest. 2003, 23, 139–145. [Google Scholar]
- Dawson, P.E. Functional Occlusion-e-Book: From TMJ to Smile Design; Elsevier Health Sciences: Amsterdam, The Netherlands, 2006. [Google Scholar]
- van Essen, N.L.; Anderson, I.A.; Hunter, P.J.; Carman, J.; Clarke, R.D.; Pullan, A.J. Anatomically based modelling of the human skull and jaw. Cells Tissues Organs 2005, 180, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.; Gandolfi, A.; Luongo, G.; Logozzo, S. Intraoral scanners in dentistry: A review of the current literature. BMC Oral Health 2017, 17, 149. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yamada, T.; Maeda, Y.; Ikebe, K. Markerless three-dimensional tracking of masticatory movement. J. Biomech. 2016, 49, 442–449. [Google Scholar] [CrossRef]
- Zoss, G.; Beeler, T.; Gross, M.; Bradley, D. Accurate markerless jaw tracking for facial performance capture. Acm Trans. Graph. 2019, 38, 1–8. [Google Scholar] [CrossRef]
- Lancellotta, V.; Pagano, S.; Tagliaferri, L.; Piergentini, M.; Ricci, A.; Montecchiani, S.; Saldi, S.; Chierchini, S.; Cianetti, S.; Valentini, V.; et al. Individual 3-dimensional printed mold for treating hard palate carcinoma with brachytherapy: A clinical report. J. Prosthet. Dent. 2019, 121, 690–693. [Google Scholar] [CrossRef]
- Nedelcu, R.; Olsson, P.; Nyström, I.; Thor, A. Finish line distinctness and accuracy in 7 intraoral scanners versus conventional impression: An in vitro descriptive comparison. BMC Oral Health 2018, 18, 27. [Google Scholar] [CrossRef]
- Wulfman, C.; Koenig, V.; Mainjot, A.K. Wear measurement of dental tissues and materials in clinical studies: A systematic review. Dent. Mater 2018, 34, 825–850. [Google Scholar] [CrossRef]
- Stober, T.; Bermejo, J.L.; Rammelsberg, P.; Schmitter, M. Enamel wear caused by monolithic zirconia crowns after 6 months of clinical use. J. Oral. Rehabil. 2014, 41, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Pagano, S.; Moretti, M.; Marsili, R.; Ricci, A.; Barraco, G.; Cianetti, S. Evaluation of the Accuracy of Four Digital Methods by Linear and Volumetric Analysis of Dental Impressions. Materials 2019, 12, 1958. [Google Scholar] [CrossRef] [PubMed]




| Value | Area | NA | PSM | SA | p-Value |
|---|---|---|---|---|---|
| RMS (μm) | Entire occlusal surface | 168.2 ± 43.3 | 152.1 ± 54.2 | 160.6 ± 84.6 | 0.797 |
| Functional cusp | 170.6 ± 55.6 | 153.3 ± 60.8 | 168.3 ± 16.4 | 0.732 | |
| Central groove | 154.9 ± 47.9 | 140.2 ± 45.8 | 121.9 ± 68.5 | 0.293 | |
| Non-functional cusp | 90.5 ± 6.7 | 92.9 ± 5.9 | 91.5 ± 8.3 | 0.401 | |
| Out of Tolerance | 257.0 ± 73.9 a | 202.3 ± 39.8 b | 222.9 ± 41.9 ab | 0.028 | |
| +AVG (μm) | Entire occlusal surface | 124.5 ± 45.3 | 112.3 ± 46.3 | 135.8 ± 52.7 | 0.443 |
| Functional cusp | 125.0 ± 57.8 | 120.9 ± 58.2 | 138.0 ± 60.6 | 0.728 | |
| Central groove | 103.3 ± 45.0 | 89.2 ± 45.7 | 106.5 ± 57.5 | 0.623 | |
| Non-functional cusp | 122.4 ± 58.9 | 99.9 ± 41.4 | 126.4 ± 51.5 | 0.345 | |
| Out of Tolerance | 210.9 ± 48.6 a | 173.1 ± 31.3 b | 194.7 ± 36.4 ab | 0.040 | |
| −AVG (μm) | Entire occlusal surface | −87.7 ± 73.1 | −93.1 ± 61.5 | −91.5 ± 83.8 | 0.980 |
| Functional cusp | −74.5 ± 65.3 | −73.7 ± 53.3 | −77.7 ± 75.3 | 0.986 | |
| Central groove | −93.8 ± 74.1 | −95.9 ± 67.3 | −76.8 ± 74.2 | 0.744 | |
| Non-functional cusp | −89.6 ± 71.3 | −88.5 ± 54.2 | −81.4 ± 82.5 | 0.946 | |
| Out of Tolerance | −228.9 ± 79.7 | −189.8 ± 40.5 | −190.6 ± 38.6 | 0.106 | |
| In Tol (%) | Entire occlusal surface | 90.5 ± 6.7 | 92.9 ± 5.9 | 91.5 ± 8.3 | 0.658 |
| Functional cusp | 88. 2 ± 11.9 | 90.7 ± 11.5 | 89.0 ± 10.8 | 0.843 | |
| Central groove | 91.9 ± 9.0 | 94.3 ± 5.8 | 94.6 ± 8.0 | 0.607 | |
| Non-functional cusp | 91.1 ± 8.8 | 95.2 ± 5.1 | 91.8 ± 11.9 | 0.459 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-C.; Lee, C.; Shim, J.-S.; Park, J.-M.; Shin, Y.; Kim, J.-E.; Lee, K.-W. Comparison between Occlusal Errors of Single Posterior Crowns Adjusted Using Patient Specific Motion or Conventional Methods. Appl. Sci. 2020, 10, 9140. https://doi.org/10.3390/app10249140
Lee Y-C, Lee C, Shim J-S, Park J-M, Shin Y, Kim J-E, Lee K-W. Comparison between Occlusal Errors of Single Posterior Crowns Adjusted Using Patient Specific Motion or Conventional Methods. Applied Sciences. 2020; 10(24):9140. https://doi.org/10.3390/app10249140
Chicago/Turabian StyleLee, Ye-Chan, Chunui Lee, June-Sung Shim, Ji-Man Park, Yooseok Shin, Jong-Eun Kim, and Keun-Woo Lee. 2020. "Comparison between Occlusal Errors of Single Posterior Crowns Adjusted Using Patient Specific Motion or Conventional Methods" Applied Sciences 10, no. 24: 9140. https://doi.org/10.3390/app10249140
APA StyleLee, Y.-C., Lee, C., Shim, J.-S., Park, J.-M., Shin, Y., Kim, J.-E., & Lee, K.-W. (2020). Comparison between Occlusal Errors of Single Posterior Crowns Adjusted Using Patient Specific Motion or Conventional Methods. Applied Sciences, 10(24), 9140. https://doi.org/10.3390/app10249140







