New Bioadsorbent Derived from Winemaking Waste Cluster Stalks: Application to the Removal of Toxic Cr(VI) from Liquid Effluents
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Activated Carbon
2.2. Adsorption Experiments
2.3. Desorption Experiments
3. Results and Discussion
3.1. Cr(VI) Adsorption Experiments
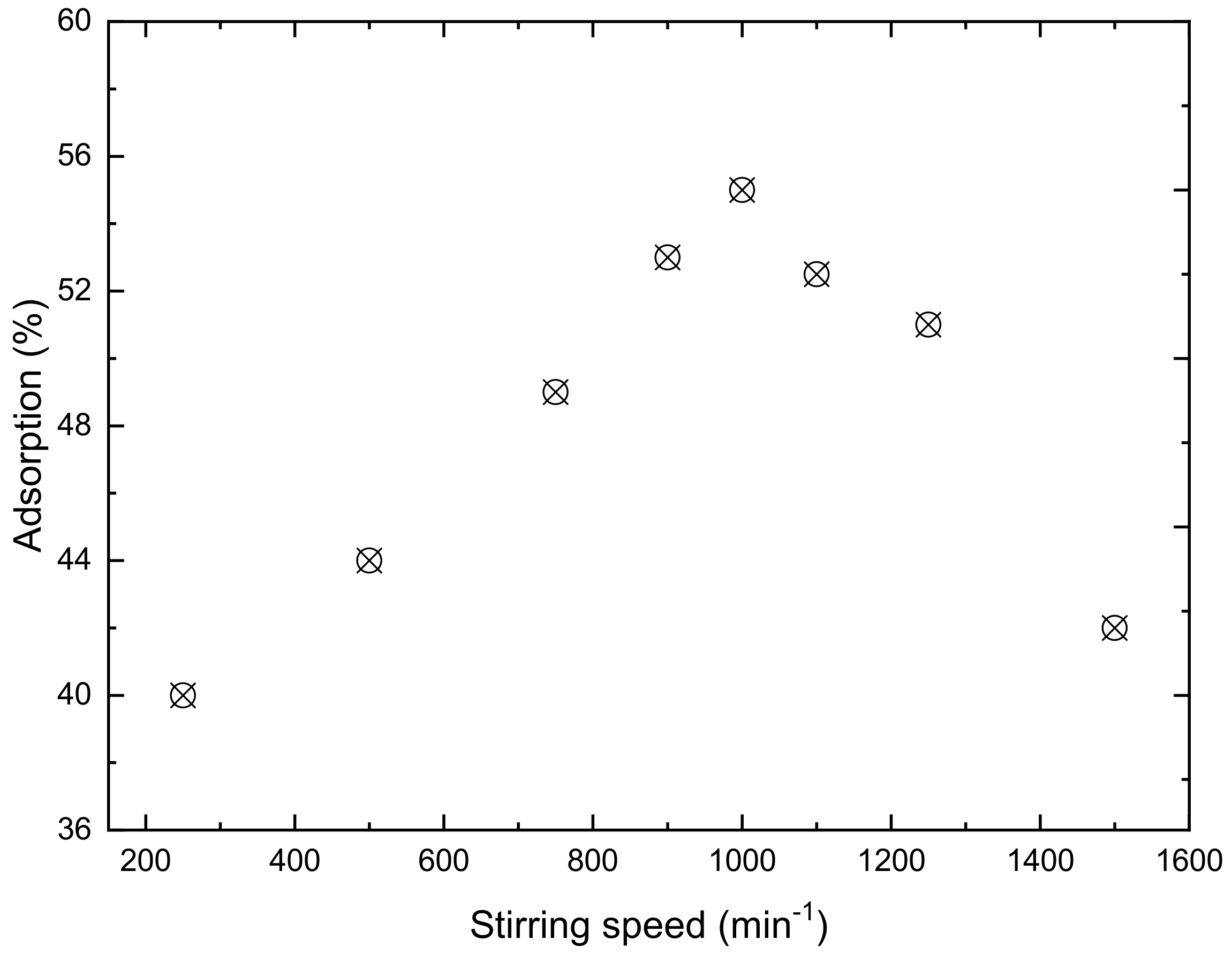
3.1.1. Stirring Speed
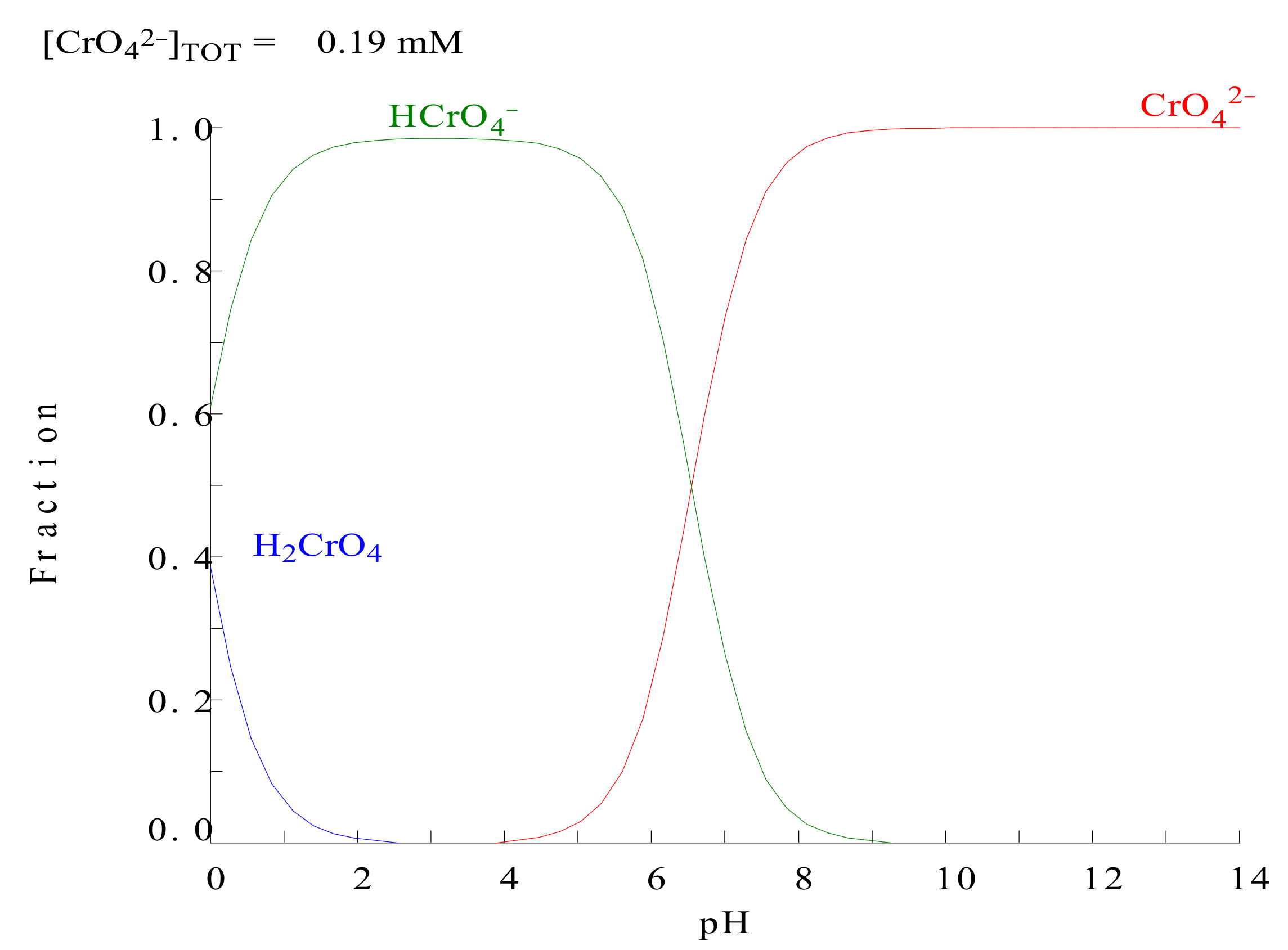
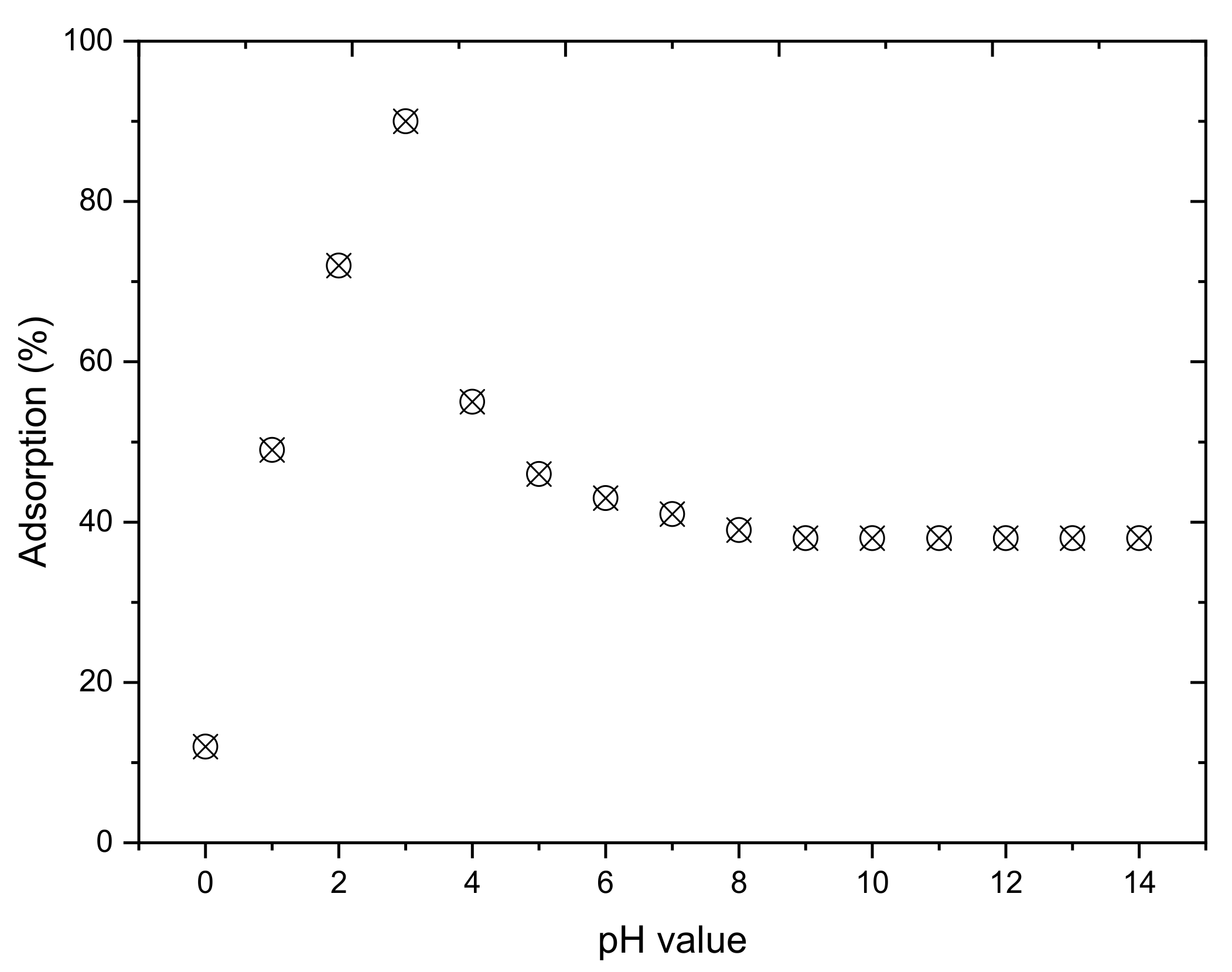
3.1.2. pH of the Solution
3.1.3. Effect of the Temperature
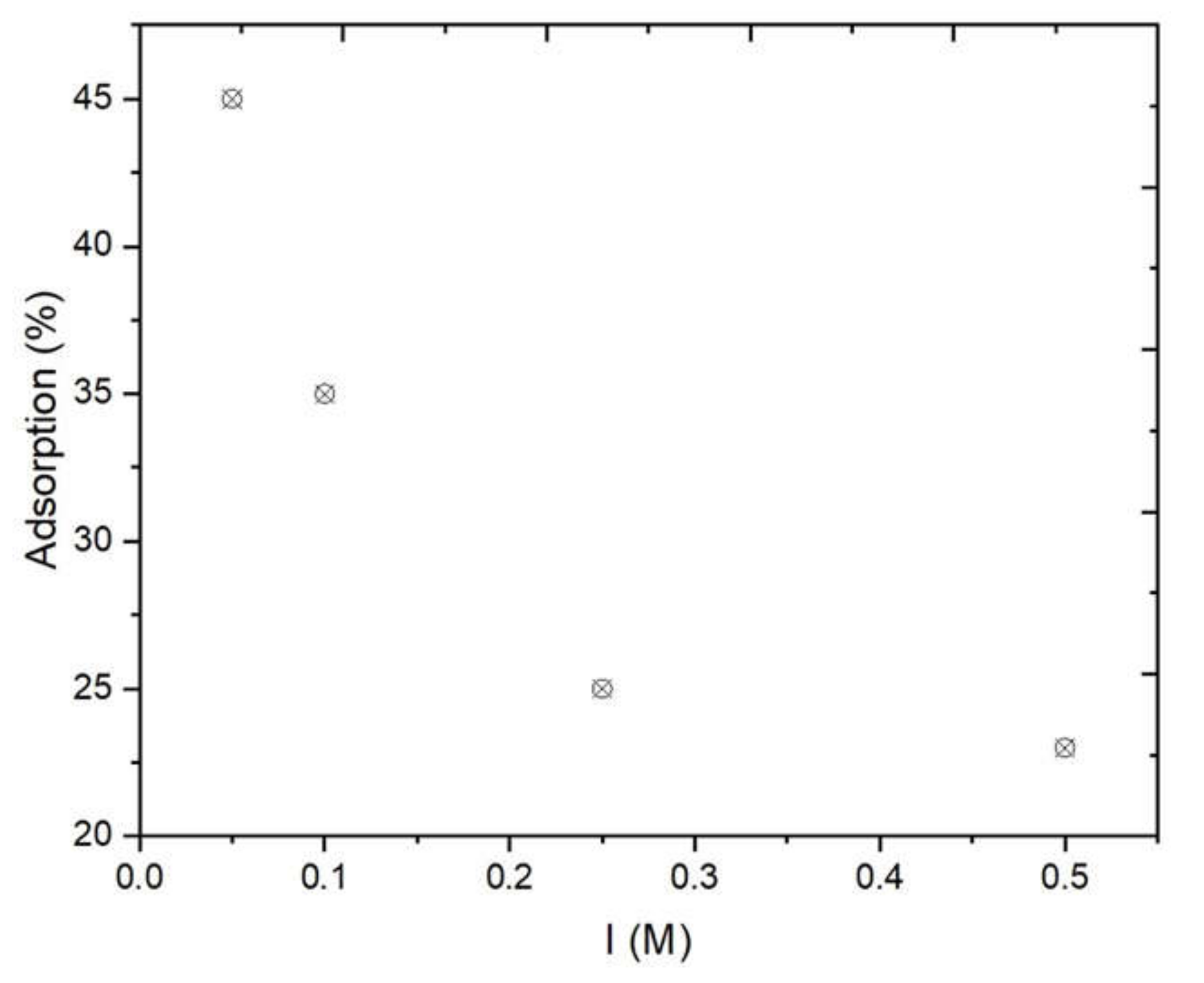
3.1.4. Effect of the Ionic Strength
3.1.5. Influence of the Adsorbent Dosage
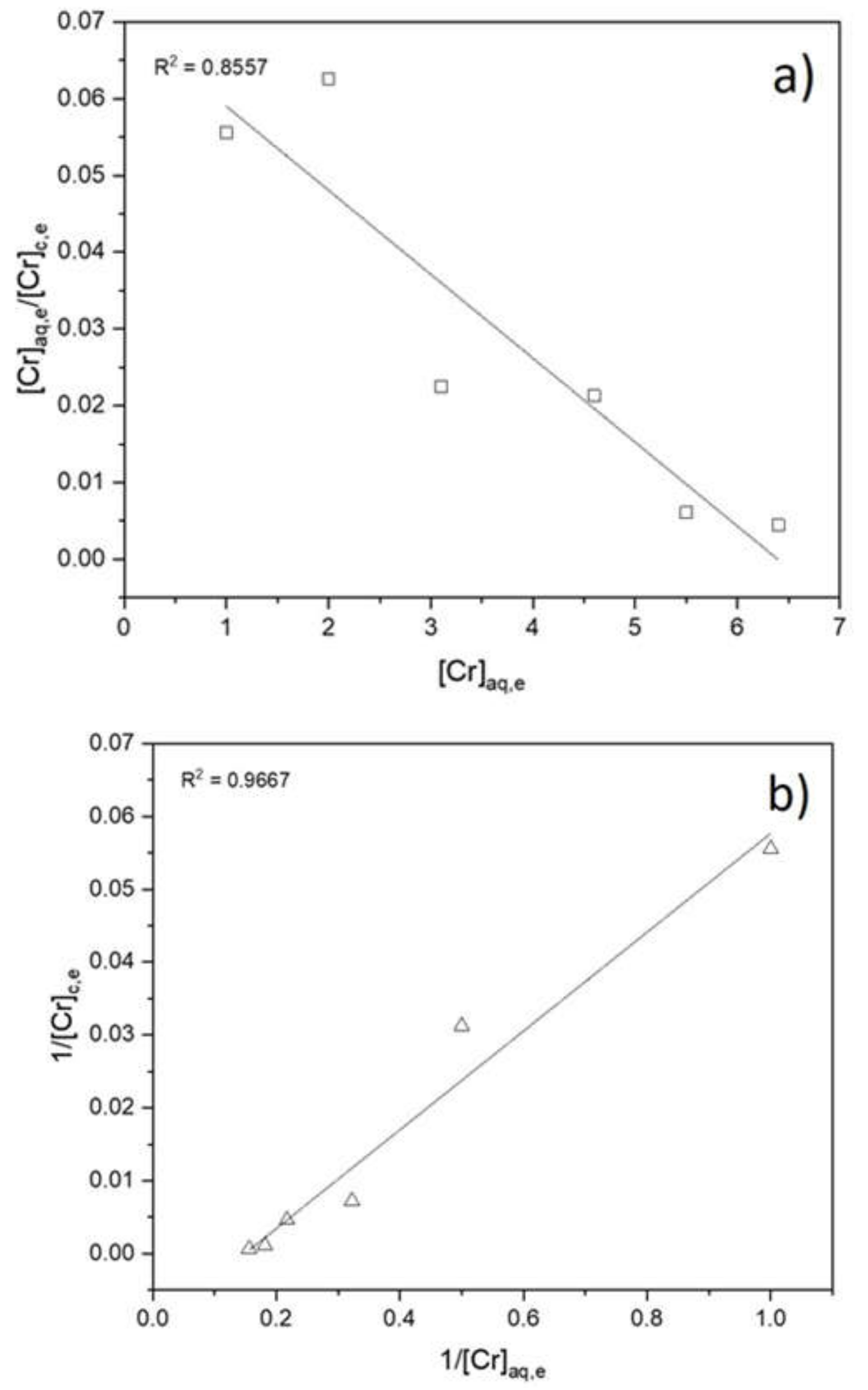
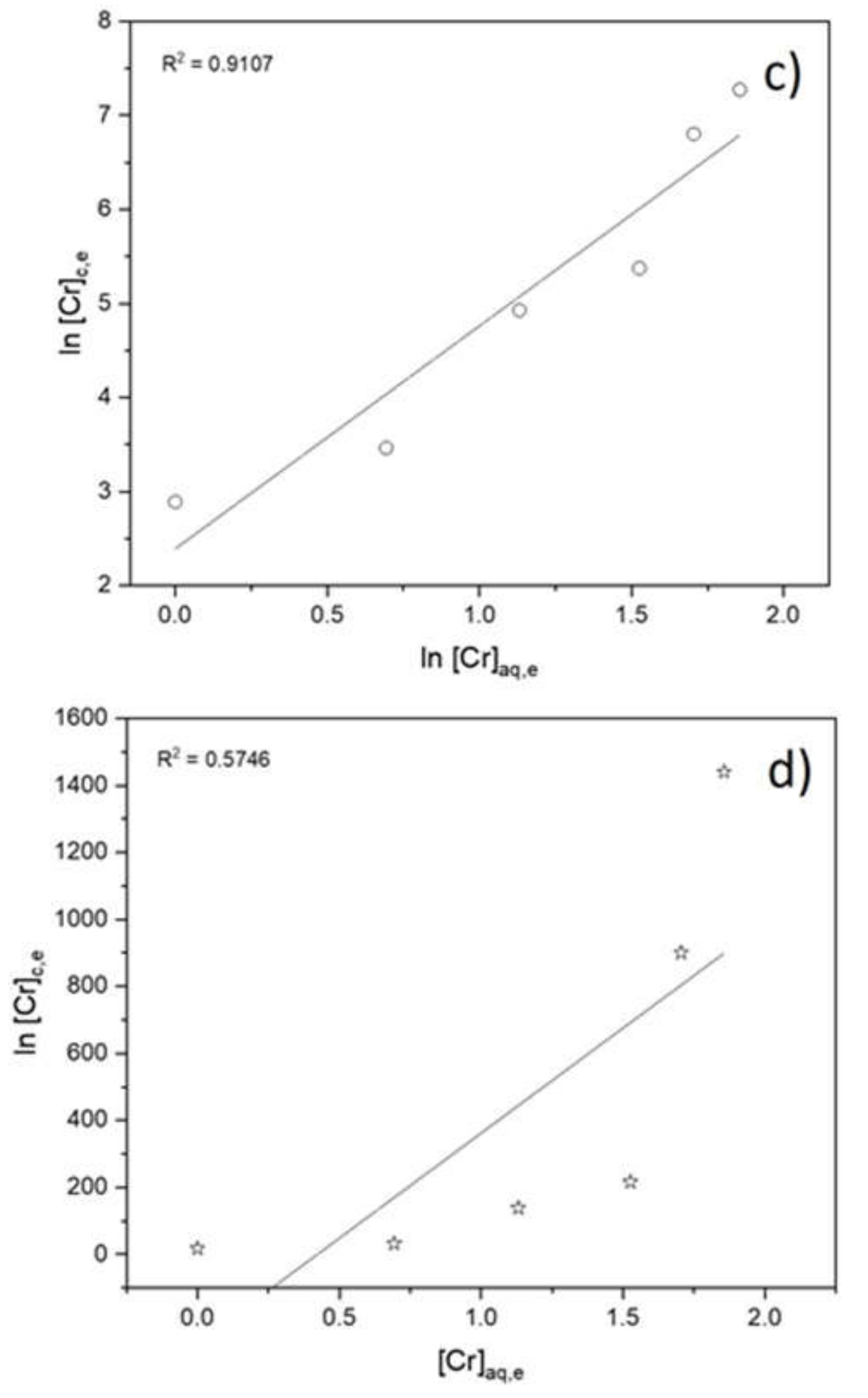
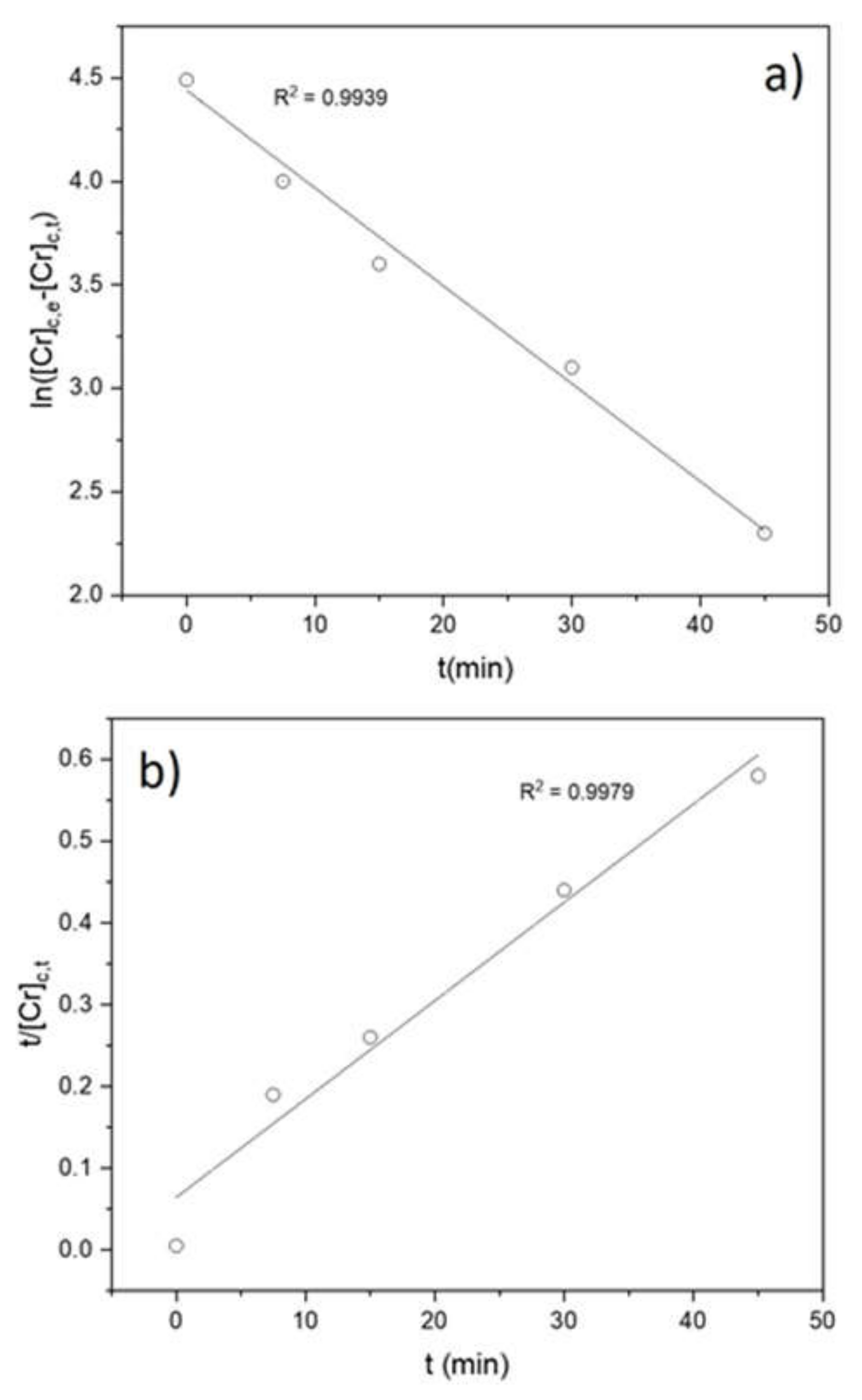
3.2. Adsorption Isotherms, Kinetic Study and Rate Law
3.3. Desorption Process
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, H.; Ye, S.; Zeng, Z.; Zeng, G.; Tan, X.; Xiao, R.; Wang, J.; Song, B.; Du, L.; Qin, M.; et al. Utilization of biochar for resource recovery from water: A review. Chem. Eng. J. 2020, 397, 125502. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.M.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef]
- Brdar, M.; Šćiban, M.; Takači, A.; Došenović, T. Comparison of two and three parameters adsorption isotherm for Cr(VI) onto Kraft lignin. Chem. Eng. J. 2012, 183, 108–111. [Google Scholar] [CrossRef]
- Kot, A. The role of speciation in analytical chemistry. TrAC Trends Anal. Chem. 2000, 19, 69–79. [Google Scholar] [CrossRef]
- Dai, J.; Ren, F.L.; Tao, C. Adsorption of Cr(VI) and speciation of Cr(VI) and Cr(III) in aqueous solutions using chemically modified chitosan. Int. J. Environ. Res. Public Health 2012, 9, 1757–1770. [Google Scholar] [CrossRef]
- Cronje, K.J.; Chetty, K.; Carsky, M.; Sahu, J.N.; Meikap, B.C. Optimization of chromium(VI) sorption potential using developed activated carbon from sugarcane bagasse with chemical activation by zinc chloride. Desalination 2011, 275, 276–284. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Toxicological Profile for Chromium. ATSDR Toxicol. Profiles. 2012. Available online: www.atsdr.cdc.gov/toxprofiles (accessed on 17 December 2020).
- Audin, F.; Soylak, M. A novel multi-element coprecipitation technique for separation and enrichment of metal ions in environmental samples. Talanta 2007, 73, 134–141. [Google Scholar] [CrossRef]
- Kongsricharoern, N.; Polprasert, C. Chromium removal by a bipolar electro-chemical precipitation process. Water Sci. Technol. 1996, 34, 109–116. [Google Scholar] [CrossRef]
- Yasuaki, O.; Yoshitaka, N.; Hiderou, N.; Kazuyuki, I.; Terufumi, F.K.T. High preconcentration of ultra-trace metal ions by liquid–liquid extraction using water/oil/water emulsions as liquid surfactant membranes. Microchem. J. 2000, 65, 341–346. [Google Scholar]
- Tiravanti, G.; Petruzzelli, D.; Passino, R. Pretreatment of tannery wastewaters by an ion exchange process for Cr(III) removal and recovery. Water Sci. Technol. 1997, 36, 197–207. [Google Scholar] [CrossRef]
- Alguacil, F.J.; López, F.A. Removal of Cr(VI) from Waters by Multi-Walled Carbon Nanotubes: Optimization and Kinetic Investigations. In Water and Wastewater Treatment; IntechOpen: London, UK, 2019. [Google Scholar]
- Dönmez, G.; Aksu, Z. Removal of chromium(VI) from saline wastewaters by Dunaliella species. Process Biochem. 2002, 38, 751–762. [Google Scholar] [CrossRef]
- Beltrame, K.K.; Cazetta, A.L.; de Souza, P.S.C.; Spessato, L.; Silva, T.L.; Almeida, V.C. Adsorption of caffeine on mesoporous activated carbon fibers prepared from pineapple plant leaves. Ecotoxicol. Environ. Saf. 2018, 147, 64–71. [Google Scholar] [CrossRef]
- Alcaraz, L.; López Fernández, A.; García-Díaz, I.; López, F.A. Preparation and characterization of activated carbons from winemaking wastes and their adsorption of methylene blue. Adsorpt. Sci. Technol. 2018, 36, 1331–1351. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Escudero, E. Removal of arsenic(V) from aqueous wastes by ion exchange with Lewatit MP64 resin. Desalin. WATER Treat. 2018, 133, 257–261. [Google Scholar] [CrossRef]
- Al-Ghamdi, Y.O.; Alamry, K.A.; Hussein, M.A.; Marwani, H.M.; Asiri, A.M. Sulfone-modified chitosan as selective adsorbent for the extraction of toxic Hg(II) metal ions. Adsorpt. Sci. Technol. 2019, 37, 139–159. [Google Scholar] [CrossRef]
- Abd El Salam, H.; Sharara, T. A novel microwave synthesis of manganese based MOF for adsorptive of Cd(II), Pb(II) and Hg(II) ions from aqua medium. Egypt. J. Chem. 2019, 62, 837–851. [Google Scholar] [CrossRef]
- Alguacil, F.J. La eliminación de metales tóxicos presentes en efluentes líquidos mediante resinas de cambio iónico. Parte XIII: Zinc(II)/H+/Lewatit OC-1026. Rev. Metal. 2020, 56, 172. [Google Scholar] [CrossRef]
- Wang, X.; Cui, S.; Yan, B.; Wang, L.; Chen, Y.; Zhang, J. Isothermal Adsorption Characteristics and Kinetics of Cr Ions onto Ettringite. J. Wuhan Univ. Technol. Sci. Ed. 2019, 34, 587–595. [Google Scholar] [CrossRef]
- Elbadawy, H.A. Adsorption and structural study of the chelating resin, 1,8-(3,6-dithiaoctyl)-4-polyvinyl benzenesulphonate (dpvbs) performance towards aqueous Hg(II). J. Mol. Liq. 2019, 277, 584–593. [Google Scholar] [CrossRef]
- Chanda, M.; Rempel, G.L. Quaternized Poly(4-vinylpyridine) Gel-Coated on Silica.Fast Kinetics of Diffusion-Controlled Sorption of Organic Sulfonates. Ind. Eng. Chem. Res. 1994, 33, 623–630. [Google Scholar] [CrossRef]
- Nethaji, S.; Sivasamy, A. Adsorptive removal of an acid dye by lignocellulosic waste biomass activated carbon: Equilibrium and kinetic studies. Chemosphere 2011, 82, 1367–1372. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der sogenannten Adsorption gelöster Stoffe. Zeitschrift für Chemie und Ind. der Kolloide 1907, 2, 15. [Google Scholar]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Chiarizia, R.; Horwitz, E.P.; Alexandratos, S.D. Uptake of metal ions by a new chelating ion-exchange resin. Part 4: Kinetics. Solvent Extr. Ion Exch. 1994, 12, 211–237. [Google Scholar] [CrossRef]
- Saha, B.; Iglesias, M.; Dimming, I.W.; Streat, M. Sorption of tace heavy metals by thiol containing chelating resins. Solvent Extr. Ion Exch. 2000, 18, 133–167. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, X.; Chen, X. Synthesis and utilization of a novel carbon nanotubes supported nanocables for the adsorption of dyes from aqueous solutions. J. Solid State Chem. 2015, 229, 342–349. [Google Scholar] [CrossRef]
- El-Aila, H.J.; Elsousy, K.M.; Hartany, K.A. Kinetics, equilibrium, and isotherm of the adsorption of cyanide by MDFSD. Arab. J. Chem. 2016, 9, S198–S203. [Google Scholar] [CrossRef]
- Sanchez, N.; Benedetti, T.M.; Vazquez, M.; De Torresi, S.I.C.; Torresi, R.M. Kinetic and thermodynamic studies on the adsorption of reactive red 239 by carra sawdust treated with formaldehyde. Adsorpt. Sci. Technol. 2012, 30, 881–899. [Google Scholar] [CrossRef]
- Alcaraz, L.; Alguacil, F.J.; López, F.A. Microporous adsorbent from winemaking waste for the recovery of Mn(VII) in liquid solutions. Can. J. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Lopez, F.A.; Rodriguez, O.; Martinez-Ramirez, S.; Garcia-Diaz, I. Sorption of indium (III) onto carbon nanotubes. Ecotoxicol. Environ. Saf. 2016, 130, 81–86. [Google Scholar] [CrossRef]
- Degefu, D.M.; Dawit, M. Chromium removal from modjo tannery wastewater using moringa stenopetala seed powder as an adsorbent topical collection on remediation of site contamination. Water. Air. Soil Pollut. 2013, 224, 1719. [Google Scholar] [CrossRef]
- Yue, X.; Huang, J.; Jiang, F.; Lin, H.; Chen, Y. Synthesis and characterization of cellulose-based adsorbent for removal of anionic and cationic dyes. J. Eng. Fiber. Fabr. 2019, 14, 155892501982819. [Google Scholar] [CrossRef]
- Puigdomenech, I. Medusa Program. Available online: www.kth.se/che/medusa (accessed on 17 December 2020).
- Alguacil, F.J.; Alcaraz, L.; García-Díaz, I.; López, F.A. Metals Removal of Pb 2+ in Wastewater via Adsorption onto an Activated Carbon Produced from Winemaking Waste. Metals 2018, 8, 697. [Google Scholar] [CrossRef]
- Alcaraz, L.; García-Díaz, I.; Alguacil, F.J.; Lopez, F.A. Removal of Copper Ions in Wastewater by Adsorption onto a Green Adsorbent from Winemaking Wastes. BioResources 2020, 15, 1112–1133. [Google Scholar]
- Javadian, H.; Ruiz, M.; Saleh, T.A.; Sastre, A.M. Ca-alginate/carboxymethyl chitosan/Ni0.2Zn0.2Fe2.6O4 magnetic bionanocomposite: Synthesis, characterization and application for single adsorption of Nd+3, Tb+3, and Dy+3 rare earth elements from aqueous media. J. Mol. Liq. 2020, 306, 112760. [Google Scholar] [CrossRef]
- Yin, R.; Niu, Y.; Zhang, B.; Chen, H.; Yang, Z.; Yang, L.; Cu, Y. Removal of Cr(III) from aqueous solution by silica-gel/PAMAM dendrimer hybrid materials. Environ. Sci. Pollut. Res. 2019, 26, 18098–18112. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Walker, G.M. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigments 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Gao, J.; Qin, Y.; Zhou, T.; Cao, D.; Xu, P.; Hochstetter, D.; Wang, Y. Adsorption of methylene blue onto activated carbon produced from tea (Camellia sinensis L.) seed shells: Kinetics, equilibrium, and thermodynamics studies. J. Zhejiang Univ. Sci. B 2013, 14, 650–658. [Google Scholar] [CrossRef]
- Alguacil, F.J. Facilitated Chromium(VI) Transport across an Ionic Liquid Membrane Impregnated with Cyphos IL102. Molecules 2019, 24, 2437. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, H.; Luo, M.; Luo, K.; Li, P.; Xu, H.; Zhang, Y. Colour performance investigation of a Cr2O3 green pigment prepared via the thermal decomposition of CrOOH. Ceram. Int. 2014, 40, 4367–4373. [Google Scholar] [CrossRef]
- Sangeetha, S.; Basha, R.; Sreeram, K.J.; Narayanan, S.; Nair, B. Functional pigments from chromium(III) oxide nanoparticles. Dyes Pigments 2012, 94, 548–552. [Google Scholar] [CrossRef]



| Volume of the Solution/Carbon Weight | [Cr(III)]aq,t (mg/L) | Desorption (%) |
|---|---|---|
| 2000 | 4 | 49 |
| 1000 | 9 | 50 |
| 500 | 17 | 49 |
| 250 | 34 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcaraz, L.; Alguacil, F.J.; López, F.A. New Bioadsorbent Derived from Winemaking Waste Cluster Stalks: Application to the Removal of Toxic Cr(VI) from Liquid Effluents. Appl. Sci. 2020, 10, 9026. https://doi.org/10.3390/app10249026
Alcaraz L, Alguacil FJ, López FA. New Bioadsorbent Derived from Winemaking Waste Cluster Stalks: Application to the Removal of Toxic Cr(VI) from Liquid Effluents. Applied Sciences. 2020; 10(24):9026. https://doi.org/10.3390/app10249026
Chicago/Turabian StyleAlcaraz, Lorena, Francisco J. Alguacil, and Félix A. López. 2020. "New Bioadsorbent Derived from Winemaking Waste Cluster Stalks: Application to the Removal of Toxic Cr(VI) from Liquid Effluents" Applied Sciences 10, no. 24: 9026. https://doi.org/10.3390/app10249026
APA StyleAlcaraz, L., Alguacil, F. J., & López, F. A. (2020). New Bioadsorbent Derived from Winemaking Waste Cluster Stalks: Application to the Removal of Toxic Cr(VI) from Liquid Effluents. Applied Sciences, 10(24), 9026. https://doi.org/10.3390/app10249026







