Curcumin Innovative Delivery Forms: Paving the ‘Yellow Brick Road’ of Antitumoral Phytotherapy
Abstract
1. Introduction
1.1. Curcumin Overview
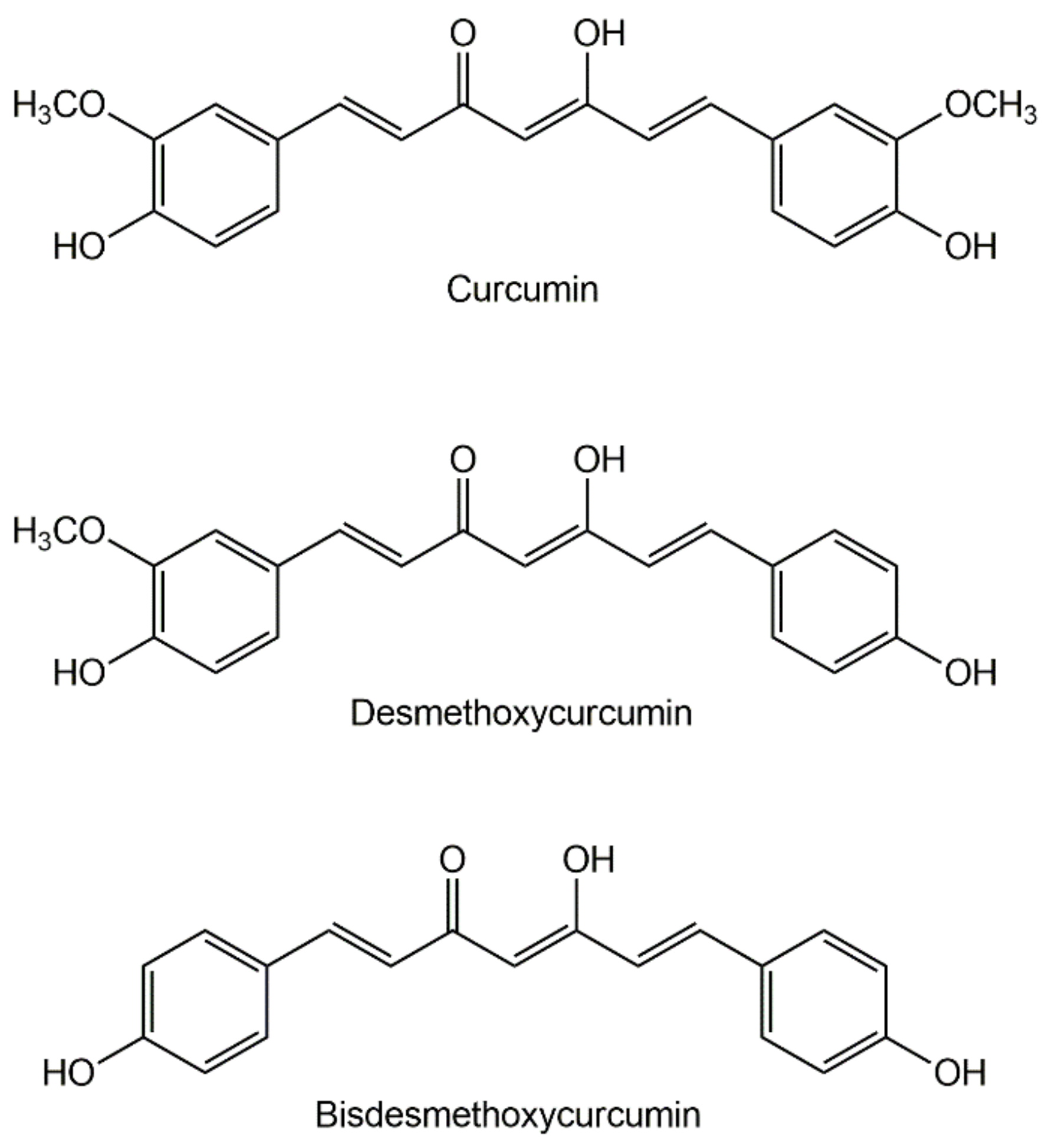
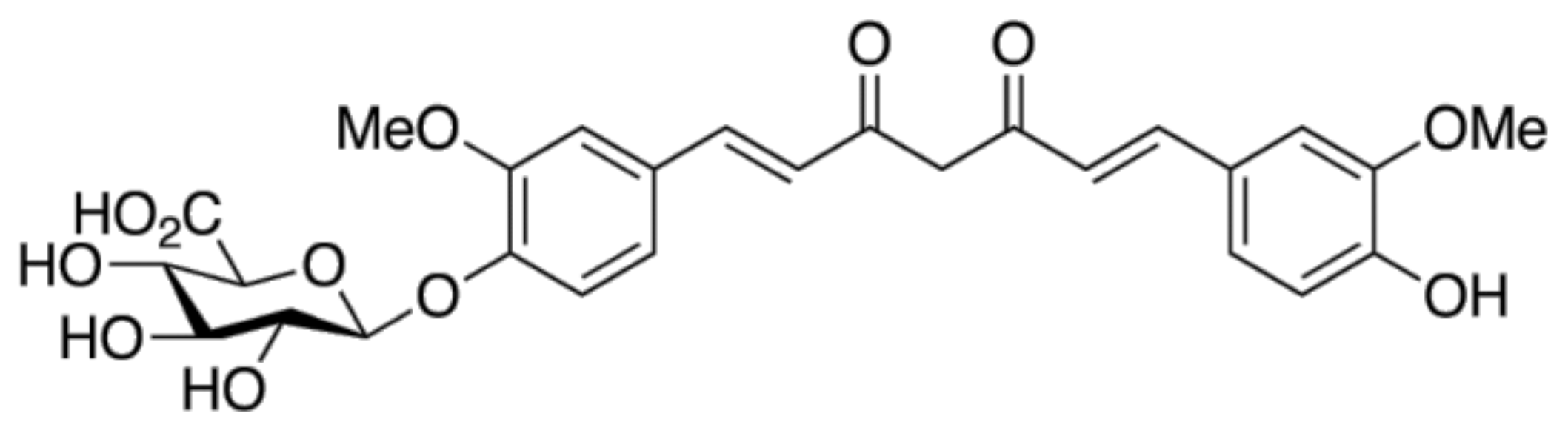
1.2. The Chemistry of Curcumin
2. Biological Actions of Curcumin
2.1. Medicinal Activity in Humans
2.1.1. Curcumin against Inflammation and Oxidative Stress
2.1.2. Antitumoral Action
- In colorectal cancer, curcumin was studied for both tumor prevention and chemotherapy. In cancer prevention, it was demonstrated to reduce by 40% the formation of aberrant crypt foci in smoking patients (intake of 4 g/day for one month) [63]. In a combination study, curcumin, and quercetin (1440 + 60 mg/day for six months) were shown to reduce the number and size of polyps in patients with familial adenomatous polyposis, a hereditary disorder characterized by the development of hundreds of colorectal adenomas which turn malign when left untreated [64]. In chemotherapy, 1 g/day curcumin for up to one month (prior to surgical removal of the tumor) was shown to improve the patient’s body weight and to increase the apoptosis rates of the patient’s tumor cells [65].
- In prostate cancer, a trial has demonstrated that curcumin/flavone association reduces the chances of developing cancer by lowering the levels of prostate-specific antigen (PSA). PSA levels are increased due to the presence of chronic inflammation in the prostate, which is one of the most significant causes of tumorigenesis [66]. Association of curcumin (5.4 g/day for seven days around chemotherapy) with docetaxel/prednisone (75 mg/m2 + 24 mg, once every three weeks, for six cycles) demonstrated encouraging results, with a tumor objective response in 40% and a PSA response in 59% of the patients in a group having castration-resistant prostate cancer [67]. There is also preliminary evidence on the ability to reduce the formation of metastases. An association of polyphenols (pomegranate seed, green tea, broccoli, and turmeric), taken over six months, has lowered PSA by 63.8% (compared to placebo) in prostatectomized patients [68]. Note that, since these men have no prostate, PSA is produced only by neoplastic cells, thus being a good indicator of metastasis growth. Curcumin can confer radioprotective effect in patients with prostate cancer who undergo radiation therapy, reducing the severity of radiotherapy related urinary symptoms. Patients were given 3 g of curcuminoids per day (corresponding to ca. 2 g/day of curcumin) for one week before the onset of radiotherapy and until completion of radiotherapy [69,70].
- In breast cancer, curcumin was used in co-therapy with both chemotherapeutic agents and radiation. A combination therapy with docetaxel and curcumin (in escalating doses of up to 6 g/day) was found to afford better therapeutic results than docetaxel used alone: histological improvements were observed in the fourteen patients under study, all having reduction or elimination of disseminated foci [71]. Curcumin was evaluated in two clinical trials regarding protective action against radiation-induced dermatitis during radiotherapy of breast cancer patients. Despite promising results on a pilot study, with slightly less severe dermatitis in the curcumin group, a second trial on 686 patients showed no significant changes in pain, symptoms, and quality of life of the patients taking curcumin (1.5 g daily) in regard to those taking placebo [72].
- Pancreatic cancer, in the advanced stage, is a condition with very poor prognosis. In a phase II study with twenty-one patients taking curcumin (8 g/day for up to 18 months), partial regression was observed during the treatment period; after treatment, patient responses varied, one of them having become stable and another having shown a strong tumor response [73]. Another trial evaluated the association of curcuminoids (8 g/day, corresponding to 6.14 g/day of curcumin) with a gemcitabine-based chemotherapeutic treatment. A total of 21 patients was divided into two groups: one, with 2 patients, received gemcitabine monotherapy; the other, with 19 patients, received a combination therapy of gemcitabine and S-1. S-1 is a novel oral antitumor formula based on fluorouracil, comprising three pharmacological agents: (i) tegafur, a prodrug of 5-fluorouracil, (ii) 5-chloro-2,4-dihydroxypyridine, which inhibits dihydropyrimidine dehydrogenase activity; and (iii) potassium oxonate, which reduces gastrointestinal toxicity was also evaluated. Eighty-one percent of the patients died during the study period. In the surviving patients, the treatment was able to stabilize the disease [74].
2.2. Molecular Targets of Curcumin
2.2.1. Curcumin Modulates the Activity of Transcription Factors
- the families of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) and of the activated protein-1 (AP-1),
- signal transducers and activators of transcription (STAT), and
- steroid receptors [77].
| Family | Molecular Target | Effect | Ref |
|---|---|---|---|
| Transcription factors | NF-kB | ↓ | [78,79,80,88,95] |
| Nrf2 | ↑ | [96] | |
| AP-1 | ↓ | [83,84,85,86,87,88] | |
| STAT-3 | ↓ | [97,98] | |
| STAT-5 | ↓ | [99,100] | |
| β-catenin | ↓ | [101,102] | |
| EGR-1 | ↓ | [103,104] | |
| HIF-1 | ↓ | [105] | |
| Notch-1 | ↓ | [106] | |
| Growth factors | EGF | ↓ | [107] |
| FGF | ↓ | [108] | |
| PDGF | ↓ | [109] | |
| TGF-β | ↓ | [110,111,112,113,114] | |
| VEGF | ↓ | [115,116,117] | |
| Cytokines, pro-inflammatory | TNF-α | ↓ | [95,118,119,120] |
| IL-1 | ↓ | [121] | |
| IL-2 | ↓ | [122] | |
| IL-5 | ↓ | [123] | |
| IL-6 | ↓ | [118] | |
| IL-8 | ↓ | [121] | |
| IL-12 | ↓ | [124] | |
| IL-18 | ↓ | [125] | |
| Enzymes | COX-2 | ↓ | [72,80,126,127] |
| iNOS | ↓ | [127] | |
| Lipoxygenase | ↓ | [128] | |
| MMP-9 | ↓ | [78,129,130,131] | |
| Kinases | JNK | ↑ | [132] |
| MAPK | ↓ | [133] | |
| PKC | ↓ | [131] | |
| Akt | ↓ | [134] | |
| CDKs | ↓ | [135] | |
| Receptors | AR | ↓ | [86] |
| EGFR | ↓ | [79,119] | |
| Adhesion molecules | ICAM-1 | ↓ | [95] |
| VCAM-1 | ↓ | [95] | |
| ELAM-1 | ↓ | [95] | |
| Antiapoptotic proteins | Bcl-2 | ↓ | [136,137,138,139,140] |
| Bcl-xL | ↓ | [136,137,138,141] | |
| Proapoptotic proteins | Bax | ↑ | [136,137,138,139,140] |
| Bak | ↑ | [140] | |
| Others | Cyclin D1 | ↓ | [142,143] |
| p53 | ↑ | [144,145] |
2.2.2. Curcumin Decreases Tumor Angiogenesis
2.2.3. Curcumin Inhibits Inflammatory Cytokines
2.2.4. Curcumin Regulates the Activity of Enzymes with Roles in Inflammation and Cancer
2.2.5. Curcumin and Cell Cycle Regulation
3. Pharmacokinetics and Bioavailability of Curcumin from Different Administration Routes
3.1. Oral Administration
| Daily Dose | Cmax Mean ± S.D. (nM) | Tmax (h) | Ref. |
|---|---|---|---|
| 0.18 g | n.d. a | — | [150] |
| 3.6 g | 11.1 ± 0.6 | 1 | [152] |
| 4.0 g | 510 ± 110 | 1.67 | [153] |
| 6.0 g | 640 ± 60 | 2 | [153] |
| 8.0 g | 1770 ± 1870 b | 1.75 | [153] |
| 10.0 g | 138 c | 4 | [154] |
| 12.0 g | 157 c | 2 | [154] |
| 0.03 g/kg | 4.9 ± 7.6 | 6 | [151] |
| 2.0 g/kg | 16.3 ± 13.5 | 1 | [157] |
3.2. Parenteral Administration
4. Innovative Curcumin Formulations and High-Performance Delivery Forms
4.1. Oral Delivery Forms
4.1.1. Marketed Products
| Trade Name | Formulation Strategy | Dosage Form | Product Composition | Ref. |
|---|---|---|---|---|
| CurcuminRich | Colloidal NP w/curcuminoids (theracurmin) | 30 mg capsules | Theracurmin: polysacharide NPs loaded with curcuminoids Excipients: MCC, magnesium stearate, silica | [151] |
| Biocurcumax | Curcuminoids w/turmeric essential oil | 350 mg capsules | Curcuminoids (titrated 95% curcumin) Excipients: Maltodextrin, magnesium stearate, HPMC | [164] |
| Biomor | Curcuminoids w/turmeric essential oil | 500 mg capsules | Curcuminoids (titrated 95% curcumin) in a vegetable capsule | [14] |
| Cavacurmin | Cyclodextrin inclusion complex w/curcumin | Dry powder | Cavacurmin: curcumin, γ-cyclodextrin | [179] |
| Meriva | Liposome | 500 mg capsules (100 mg curcumin) | Liposome: Turmeric root extract, phosphatidylcholine, phospholipids Excipients: HPMC, leucine, silicon dioxide and MCC | [165] |
| Liposomal curcumin mango | Liposome | Liquid, 20 g/L | Liposome: curcumin, phospholipids from sunflower Excipients: water, xylitol, mango aroma, ascorbic acid, Preservative: seabuckthorn extract | [177] |
| Liposomal curcumin | Liposome | Liquid, 41.7 g/L | Liposome: Turmetic root extract, phospholipids from sunflower Excipients: water, glucosylsteviosides, flavors, glycerin, xanthan/acacia gums Preservative: potassium sorbate | [178] |
| Dr. Mercola Curcumin Advanced | Micronized curcuminoids (microactive) | 500 mg capsules | Micronized curcumin Excipients: HPMC, MCC, polyglycerol oleate, silicon dioxide, metolose, medium chain triglycerides and sodium alginate | [167] |
| Curcumin C3 Complex | Combined therapy of curcuminoids & piperidine | 500 + 5 mg capsules | Complex: Turmeric root extract (73% curcumin), piperine Excipients: rice flour, cellulose and ascorbyl palmitate | [166,168] |
4.1.2. Emerging Solutions for Oral Delivery
4.1.3. Buccal Delivery
4.2. New Injectable forms of Curcumin
4.3. Inhalable Curcumin Formulations
4.3.1. Dry Powder Inhaler for Pulmonary Delivery
4.3.2. Intranasal Aerosol for Delivery to the Brain
4.4. Transdermal Administration
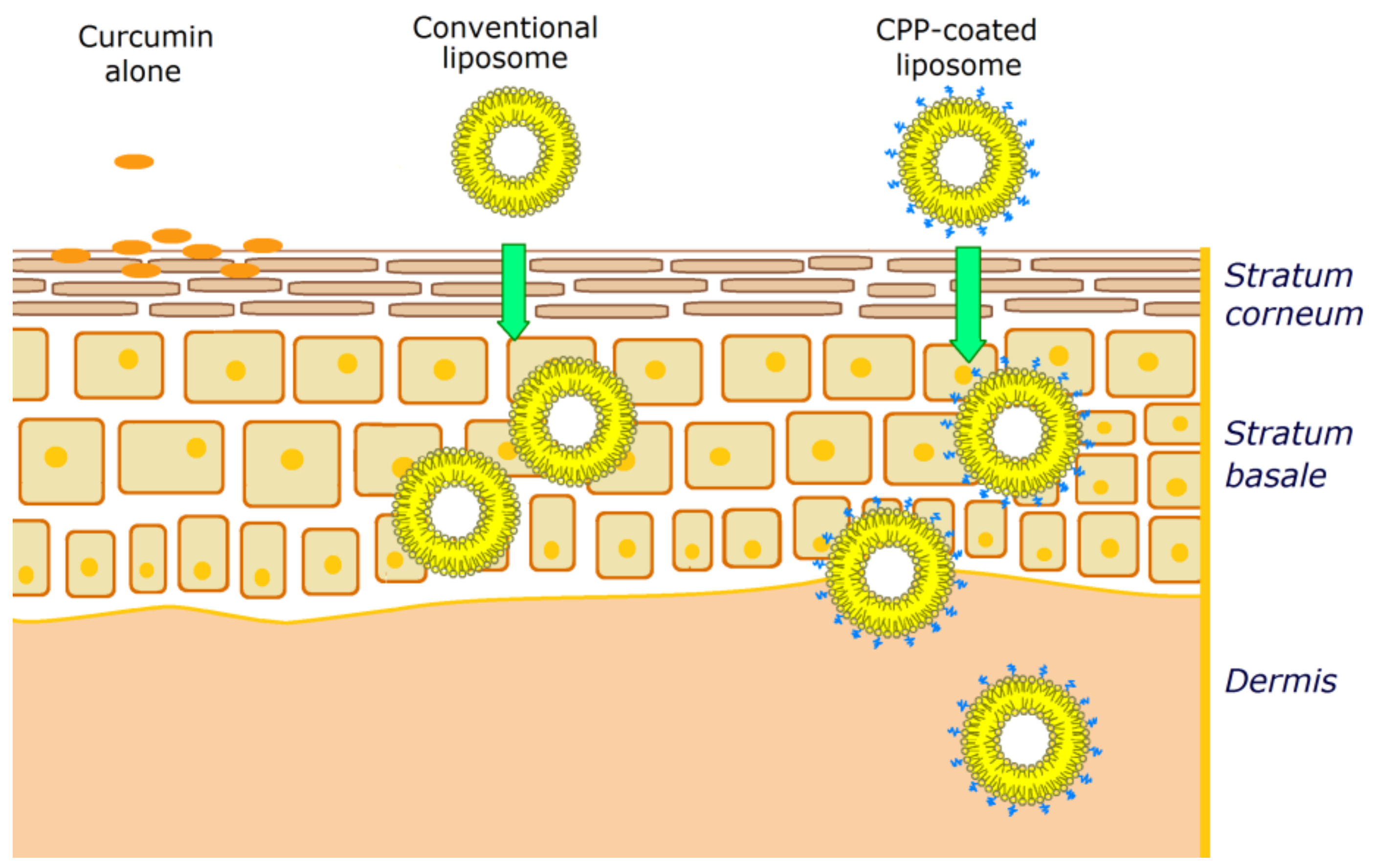
4.4.1. Curcumin Topical Formulations with Liposomes, Microemulsions, and Polymeric Nanoparticles
4.4.2. Dermal Compositions Containing Silver and Curcumin for Combined Therapy
4.4.3. Iontophoresis Techniques
4.4.4. Photodynamic Therapy for Increased Antitumoral Action on Skin
4.5. Curcumin Prodrugs and Metal Complexes
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akram, M.; Uddin, S.; Ahmed, A.; Khan, U.; Hannan, A.; Mohihuddin, E.; Asif, M. Curcuma Longa and Curcumin. Rom. J. Biol. Plant. Biol. 2010, 55, 65–70. [Google Scholar]
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Daglia, M.; Moghaddam, A.H.; Habtemariam, S. Curcumin and Liver Disease: From Chemistry to Medicine. Compr. Rev. Food Sci. Food Saf. 2013, 13, 62–77. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Wahile, A. Integrated approaches towards drug development from Ayurveda and other Indian system of medicines. J. Ethnopharmacol. 2006, 103, 25–35. [Google Scholar] [CrossRef]
- de Orta, G. Colóquios Dos Simples, E Drogas E Coisas Medicinais Da India E Assim de Algumas Frutas Achadas Nela Onde Se Tratam Algumas Coisas Tocantes a Medicina Prática, E Outras Coisas Boas Para Saber; Ioannes de Endem: Goa, India, 1563. [Google Scholar]
- Govindarajan, V.; Stahl, W.H. Turmeric—chemistry, technology, and quality. CRC Crit. Rev. Food Sci. Nutr. 1980, 12, 199–301. [Google Scholar] [CrossRef]
- Prasad, S.; Aggarwal, B.B. Turmeric, the Golden Spice: From Traditional Medicine to Modern Medicine. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Kita, T.; Imai, S.; Sawada, H.; Kumagai, H.; Seto, H. The Biosynthetic Pathway of Curcuminoid in Turmeric (Curcuma longa) as Revealed by13C-Labeled Precursors. Biosci. Biotechnol. Biochem. 2008, 72, 1789–1798. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian Solid Gold. In Results and Problems in Cell Differentiation; Springer Science and Business Media LLC: Berlin, Germany, 2007; Volume 595, pp. 1–75. [Google Scholar]
- Toda, S.; Miyase, T.; Arichi, H.; Tanizawa, H.; Takino, Y. Natural antioxidants. III. Antioxidative components isolated from rhizome of Curcuma longa L. Chem. Pharm. Bull. 1985, 33, 1725–1728. [Google Scholar] [CrossRef]
- Osawa, T.; Sugiyama, Y.; Inayoshi, M.; Kawakishi, S. Antioxidative Activity of Tetrahydrocurcuminoids. Biosci. Biotechnol. Biochem. 1995, 59, 1609–1612. [Google Scholar] [CrossRef]
- FDA. Inventory of GRAS Notices, Number 460. Curcuminoids Purified from Turmeric (Curcuma longa L.); Food and Drugs Administration: Silver Spring, MD, USA, 2013. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=460&sort=GRN_No&order=DESC&startrow=1&type=basic&search=460 (accessed on 15 December 2020).
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Turmeric and curcumin (WHO Food Additives Series 6). Available online: http://www.inchem.org/documents/jecfa/jecmono/v06je29.htm (accessed on 16 November 2020).
- Anna’s Tuin & Ruigte. Available online: http://annastuinenruigte.nl/ (accessed on 24 March 2020).
- The State of the Curcumin Market. Available online: http://www.naturalproductsinsider.com/articles/2015/12/the-state-of-the-curcumin-market.aspx (accessed on 16 November 2020).
- Curcumin Market. Size, Share & Trends Analysis Report by Application (Pharmaceutical, Food, Cosmetics), By Region (North America, Europe, Asia Pacific, Central & South America, Middle East & Africa), And Segment Forecasts, 2020–2027. Available online: http://www.grandviewresearch.com/industry-analysis/turmeric-extract-curcumin-market (accessed on 16 November 2020).
- Pelletier, J.; Vogel, A. Examen Chimique de La Racine de Curcuma. J. Pharm. 1815, 1, 289–300. [Google Scholar]
- Daube, F.W. Ueber Curcumin, den Farbstoff der Curcumawurzel. Eur. J. Inorg. Chem. 1870, 3, 609–613. [Google Scholar] [CrossRef]
- Lampe, V.; Milobedzka, J. Studien Über Curcumin. Berichte der Dtsch. Chem. Gesellschaft 1913, 46, 2235–2240. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.D.M.; Takeuchi, K.P.; Geraldine, R.M.; De Moura, C.J.; Torres, M.C.L. Production, solubility and antioxidant activity of curcumin nanosuspension. Food Sci. Technol. 2015, 35, 115–119. [Google Scholar] [CrossRef]
- Chavda, H.; Patel, C.; Anand, I. Biopharmaceutics classification system. Syst. Rev. Pharm. 2010, 1, 62. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Tortia, F.M.; Tortic, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Bernabé-Pineda, M.; Ramírez-Silva, M.T.; Romero-Romo, M.; González-Vergara, E.; Rojas-Hernández, A. Determination of acidity constants of curcumin in aqueous solution and apparent rate constant of its decomposition. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2004, 60, 1091–1097. [Google Scholar] [CrossRef]
- Chignell, C.F.; Bilskj, P.; Reszka, K.J.; Motten, A.G.; Sik, R.H.; Dahl, T.A. spectral and photochemical properties of curcumin. Photochem. Photobiol. 1994, 59, 295–302. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Photophysics, photochemistry and photobiology of curcumin: Studies from organic solutions, bio-mimetics and living cells. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 81–95. [Google Scholar] [CrossRef]
- Khurana, A.; Ho, C.-T. High Performance Liquid Chromatographic Analysis of Curcuminoids and Their Photo-oxidative Decomposition Compounds in Curcuma Longa L. J. Liq. Chromatogr. 1988, 11, 2295–2304. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J.; van Henegouwen, G.B. Studies on Curcumin and Curcuminoids VIII. Photochemical Stability of Curcumin. Z. Lebensm. Unters. Forsch. 1986, 183, 116–122. [Google Scholar] [CrossRef]
- De Jager, P. Turmeric: The Ayurvedic Spice of Life, 2nd ed.; Pioneer imprints: Maui, HI, USA, 2010. [Google Scholar]
- De Oliveira, M.R.; Jardim, F.R.; Setzer, W.N.; Nabavi, S.M. Curcumin, mitochondrial biogenesis, and mitophagy: Exploring recent data and indicating future needs. Biotechnol. Adv. 2016, 34, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.R.; Katz, S.; Kirshoff, R. Curcumin Therapy in Inflammatory Bowel Disease: A Pilot Study. Dig. Dis. Sci. 2005, 50, 2191–2193. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Sethi, G.; Aggarwal, B.B. Curcumin: Getting Back to the Roots. Ann. N. Y. Acad. Sci. 2005, 1056, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, M.; Sreejayan; Devasagayam, T.P.; Singh, B. Diminution of singlet oxygen-induced DNA damage by curcmin and related antioxidants. Mutat. Res. Mol. Mech. Mutagen. 1994, 311, 249–255. [Google Scholar] [CrossRef]
- Iqbal, M.; Sharma, S.D.; Okazaki, Y.; Fujisawa, M.; Okada, S. Dietary Supplementation of Curcumin Enhances Antioxidant and Phase II Metabolizing Enzymes in ddY Male Mice: Possible Role in Protection against Chemical Carcinogenesis and Toxicity. Pharmacol. Toxicol. 2003, 92, 33–38. [Google Scholar] [CrossRef]
- Kuo, M.-L.; Huang, T.-S.; Lin, J.-K. Curcumin, an antioxidant and anti-tumor promoter, induces apoptosis in human leukemia cells. Biochim. et Biophys. Acta (BBA) Mol. Basis Dis. 1996, 1317, 95–100. [Google Scholar] [CrossRef]
- Sreejayan; Rao, M.N.A. Curcuminoids as Potent Inhibitors of Lipid Peroxidation. J. Pharm. Pharmacol. 1994, 46, 1013–1016. [Google Scholar] [CrossRef]
- Busquets, S.; Carbó, N.; Almendro, V.; Quiles, M.T.; López-Soriano, F.J.; Argilés, J.M. Curcumin, a natural product present in turmeric, decreases tumor growth but does not behave as an anticachectic compound in a rat model. Cancer Lett. 2001, 167, 33–38. [Google Scholar] [CrossRef]
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005, 223, 181–190. [Google Scholar] [CrossRef]
- Garcea, G.; Berry, D.P.; Jones, D.J.L.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the putative chemopreventive agent curcumin by cancer patients: Assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidem. Biomarkers Prev. 2005, 14, 120–125. [Google Scholar]
- Bentham Science Publisher Tzeng-Horng Leu; Bentham Science Publisher Ming-Chei Maa. The Molecular Mechanisms for the Antitumorigenic Effect of Curcumin. Curr. Med. Chem. Agents 2002, 2, 357–370. [Google Scholar] [CrossRef]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by Curcumin as Revealed by Molecular Interaction Studies. Nat. Prod. Rep. 2011, 28, 1937. [Google Scholar] [CrossRef]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and Cancer Cells: How Many Ways Can Curry Kill Tumor Cells Selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Strimpakos, A.S.; Sharma, R.A. Curcumin: Preventive and Therapeutic Properties in Laboratory Studies and Clinical Trials. Antioxidants Redox Signal. 2008, 10, 511–546. [Google Scholar] [CrossRef] [PubMed]
- Chainani-Wu, N. Safety and Anti-Inflammatory Activity of Curcumin: A Component of Tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Perrone, D.; Ardito, F.; Giannatempo, G.; Dioguardi, M.; Troiano, G.; Russo, L.L.; De Lillo, A.; Laino, L.; Muzio, L.L. Biological and therapeutic activities, and anticancer properties of curcumin. Exp. Ther. Med. 2015, 10, 1615–1623. [Google Scholar] [CrossRef]
- Satoskar, R.R.; Shah, S.J.; Shenoy, S.G. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986, 24, 651–654. [Google Scholar] [PubMed]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a golden spice with a low bioavailability. J. Herb. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Chandran, B.; Goel, A. A Randomized, Pilot Study to Assess the Efficacy and Safety of Curcumin in Patients with Active Rheumatoid Arthritis. Phytotherapy Res. 2012, 26, 1719–1725. [Google Scholar] [CrossRef]
- Heng, M.; Song, M.; Harker, J. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br. J. Dermatol. 2000, 143, 937–949. [Google Scholar] [CrossRef]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Maruyama, Y.; Andoh, A.; Tsujikawa, T.; Fujiyama, Y.; Mitsuyama, K.; Sata, M.; et al. Curcumin Maintenance Therapy for Ulcerative Colitis: Randomized, Multicenter, Double-Blind, Placebo-Controlled Trial. Clin. Gastroenterol. Hepatol. 2006, 4, 1502–1506. [Google Scholar] [CrossRef] [PubMed]
- Lahiff, C.; Moss, A.C. Curcumin for clinical and endoscopic remission in ulcerative colitis. Inflamm. Bowel Dis. 2011, 17, E66. [Google Scholar] [CrossRef]
- Epstein, J.; Docena, G.; MacDonald, T.T.; Sanderson, I.R. Curcumin Suppresses p38 Mitogen-Activated Protein Kinase Activation, Reduces IL-1beta and Matrix Metalloproteinase-3 and Enhances IL-10 in the Mucosa of Children and Adults with Inflammatory Bowel Disease. Br. J. Nutr. 2010, 103, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Bundy, R.; Walker, A.F.; Middleton, R.W.; Booth, J. Turmeric Extract May Improve Irritable Bowel Syndrome Symptomology in Otherwise Healthy Adults: A Pilot Study. J. Altern. Complement. Med. 2004, 10, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Lal, B.; Kapoor, A.K.; Agrawal, P.K.; Asthana, O.P.; Srimal, R.C. Role of curcumin in idiopathic inflammatory orbital pseudotumours. Phytotherapy Res. 2000, 14, 443–447. [Google Scholar] [CrossRef]
- Lal, B.; Kapoor, A.K.; Asthana, O.P.; Agrawal, P.K.; Prasad, R.; Kumar, P.; Srimal, R.C. Efficacy of Curcumin in the Management of Chronic Anterior Uveitis. Phytother. Res. 1999, 13, 318–322. [Google Scholar] [CrossRef]
- Itokawa, H.; Shi, Q.; Akiyama, T.; Morris-Natschke, S.L.; Lee, K.-H. Recent advances in the investigation of curcuminoids. Chin. Med. 2008, 3. [Google Scholar] [CrossRef]
- Reddy, A.P.; Lokesh, B. Studies on spice principles as antioxidants in the inhibition of lipid peroxidation of rat liver microsomes. Mol. Cell. Biochem. 1992, 111, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Noorafshan, A.; Ashkani-Esfahani, S. A Review of Therapeutic Effects of Curcumin. Curr. Pharm. Des. 2013, 19, 2032–2046. [Google Scholar]
- Balamurugan, A.; Akhov, L.; Selvaraj, G.; Pugazhenthi, S. Induction of Antioxidant Enzymes by Curcumin and Its Analogues in Human Islets. Pancreas 2009, 38, 454–460. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and Type 2 Diabetes Mellitus: Prevention and Treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef]
- Hatcher, H.C.; Torti, F.M.; Torti, S.V. Curcumin, Oxidative Stress, and Cancer Therapy. In Oxidative Stress in Cancer Biology and Therapy; Springer Science and Business Media LLC: Berlin, Germany, 2012; pp. 233–256. [Google Scholar]
- Kuttan, R.; Sudheeran, P.; Josph, C. Turmeric and Curcumin as Topical Agents in Cancer Therapy. Tumori J. 1987, 73, 29–31. [Google Scholar] [CrossRef]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L.; et al. Phase IIa Clinical Trial of Curcumin for the Prevention of Colorectal Neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Cruz–Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination Treatment With Curcumin and Quercetin of Adenomas in Familial Adenomatous Polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Shi, C.-B.; Wen, H.; Li, F.-L.; Wang, B.-L.; Wang, J. Upregulation of p53 Expression in Patients with Colorectal Cancer by Administration of Curcumin. Cancer Investig. 2011, 29, 208–213. [Google Scholar] [CrossRef]
- Ide, H.; Tokiwa, S.; Sakamaki, K.; Nishio, K.; Isotani, S.; Muto, S.; Hama, T.; Masuda, H.; Horie, S. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate 2010, 70, 1127–1133. [Google Scholar] [CrossRef]
- Mahammedi, H.; Planchat, E.; Pouget, M.; Durando, X.; Curé, H.; Guy, L.; Van-Praagh, I.; Savareux, L.; Atger, M.; Bayet-Robert, M.; et al. The New Combination Docetaxel, Prednisone and Curcumin in Patients with Castration-Resistant Prostate Cancer: A Pilot Phase II Study. Oncology 2016, 90, 69–78. [Google Scholar] [CrossRef]
- Thomas, R.J.; Williams, M.M.A.; Sharma, H.; Chaudry, A.; Bellamy, P. A double-blind, placebo RCT evaluating the effect of a polyphenol-rich whole food supplement on PSA progression in men with prostate cancer: The U.K. National Cancer Research Network (NCRN) Pomi-T study. J. Clin. Oncol. 2013, 31. [Google Scholar] [CrossRef]
- Taleban, R.R.F.-A.; Hejazi, J. A Pilot Clinical Trial of Radioprotective Effects of Curcumin Supplementation in Patients with Prostate Cancer. J. Cancer Sci. Ther. 2013, 5, 320–324. [Google Scholar] [CrossRef]
- Hejazi, J.; Rastmanesh, R.; Taleban, F.-A.; Molana, S.-H.; Hejazi, E.; Ehtejab, G.; Hara, N. Effect of Curcumin Supplementation During Radiotherapy on Oxidative Status of Patients with Prostate Cancer: A Double Blinded, Randomized, Placebo-Controlled Study. Nutr. Cancer 2016, 68, 77–85. [Google Scholar] [CrossRef]
- Bayet-Robert, M.; Kwiatkowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. Ther. 2010, 9, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.L.; Heckler, C.E.; Guido, J.J.; Peoples, A.R.; Gewandter, J.S.; Ling, M.; Vinciguerra, V.P.; Anderson, T.; Evans, L.; Wade, J.; et al. Oral curcumin for radiation dermatitis: A URCC NCORP study of 686 breast cancer patients. Support. Care Cancer 2017, 26, 1543–1552. [Google Scholar] [CrossRef]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II Trial of Curcumin in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y.; et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2010, 68, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Tamilselvam, R.; Skalicka-Woźniak, K.; Nabavi, S.M.; Daglia, M.; Bishayee, A.; Pazoki-Toroudi, H. Molecular targets of curcumin for cancer therapy: An updated review. Tumor Biol. 2016, 37, 13017–13028. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef]
- Libermann, T.A.; Zerbini, L.F. Targeting Transcription Factors for Cancer Gene Therapy. Curr. Gene Ther. 2006, 6, 17–33. [Google Scholar] [CrossRef]
- Xiang, L.; He, B.; Liu, Q.; Hu, D.; Liao, W.; Li, R.; Peng, X.; Wang, Q.; Zhao, G. Antitumor effects of curcumin on the proliferation, migration and apoptosis of human colorectal carcinoma HCT-116 cells. Oncol. Rep. 2020, 44, 1997–2008. [Google Scholar] [CrossRef]
- Juturu, V.; Sahin, K.; Pala, R.; Tuzcu, M.; Ozdemir, O.; Orhan, C.; Sahin, N. Curcumin prevents muscle damage by regulating NF-kB and Nrf2 pathways and improves performance: An in vivo model. J. Inflamm. Res. 2016, 9, 147–154. [Google Scholar] [CrossRef]
- Vadhan-Raj, S.; Weber, D.M.; Wang, M.; Giralt, S.A.; Thomas, S.K.; Alexanian, R.; Zhou, X.; Patel, P.; Bueso-Ramos, C.E.; Newman, R.A.; et al. Curcumin Downregulates NF-kB and Related Genes in Patients with Multiple Myeloma: Results of a Phase I/II Study. Blood 2015, 110, 1177. [Google Scholar] [CrossRef]
- Wagner, E.F. Functions of AP1 (Fos/Jun) in bone development. Ann. Rheum. Dis. 2002, 61, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Dorai, T.; Aggarwal, B.B. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004, 215, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Zhang, Y.; Quehenberger, P.; Luther, T.; Haase, M.; Müller, M.; Mackman, N.; Ziegler, R.; Nawroth, P.P. The dietary pigment curcumin reduces endothelial tissue factor gene expression by inhibiting binding of AP-1 to the DNA and activation of NF-kappa B. Thromb. Haemost. 1997, 77, 772–782. [Google Scholar] [CrossRef]
- Han, S.-S.; Keum, Y.-S.; Seo, H.-J.; Surh, Y.-J. Curcumin Suppresses Activation of NF-kappaB and AP-1 Induced by Phorbol Ester in Cultured Human Promyelocytic Leukemia Cells. J. Biochem. Mol. Biol. 2002, 35, 337–342. [Google Scholar] [PubMed]
- Balasubramanian, S.; Eckert, R.L. Curcumin Suppresses AP1 Transcription Factor-dependent Differentiation and Activates Apoptosis in Human Epidermal Keratinocytes. J. Biol. Chem. 2007, 282, 6707–6715. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yasunaga, Y.; Segawa, T.; Ko, D.; Moul, J.W.; Srivastava, S.; Rhim, J.S. Curcumin down-regulates AR gene expression and activation in prostate cancer cell lines. Int. J. Oncol. 2002, 21, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Teiten, M.-H.; Gaascht, F.; Eifes, S.; Dicato, M.; Han, B.W. Chemopreventive potential of curcumin in prostate cancer. Genes Nutr. 2010, 5, 61–74. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, R.; Tyagi, A.; Kohaar, I.; Hedau, S.; Bharti, A.C.; Sarker, S.; Dey, D.; Saluja, D.; Das, B. Curcumin modulates cellular AP-1, NF-kB, and HPV16 E6 proteins in oral cancer. Ecancermedicalscience 2015, 9. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Schwartz, D.M.; Villarino, A.V.; Gadina, M.; McInnes, I.B.; Laurence, A. The JAK-STAT Pathway: Impact on Human Disease and Therapeutic Intervention. Annu. Rev. Med. 2015, 66, 311–328. [Google Scholar] [CrossRef]
- Yang, C.-L.; Liu, Y.; Ma, Y.-G.; Xue, Y.-X.; Liu, D.-G.; Ren, Y.; Liu, X.-B.; Li, Y.; Li, Z. Curcumin Blocks Small Cell Lung Cancer Cells Migration, Invasion, Angiogenesis, Cell Cycle and Neoplasia through Janus Kinase-STAT3 Signalling Pathway. PLoS ONE 2012, 7, e37960. [Google Scholar] [CrossRef]
- Kroon, P.; Berry, P.A.; Stower, M.J.; Rodrigues, G.; Mann, V.M.; Simms, M.; Bhasin, D.; Chettiar, S.; Li, C.; Li, P.-K.; et al. JAK-STAT Blockade Inhibits Tumor Initiation and Clonogenic Recovery of Prostate Cancer Stem-like Cells. Cancer Res. 2013, 73, 5288–5298. [Google Scholar] [CrossRef] [PubMed]
- Weissenberger, J.; Priester, M.; Bernreuther, C.; Rakel, S.; Glatzel, M.; Seifert, V.; Kögel, D. Dietary Curcumin Attenuates Glioma Growth in a Syngeneic Mouse Model by Inhibition of the JAK1,2/STAT3 Signaling Pathway. Clin. Cancer Res. 2010, 16, 5781–5795. [Google Scholar] [CrossRef] [PubMed]
- Blasius, R.; Reuter, S.; Henry, E.; Dicato, M.; Diederich, M. Curcumin regulates signal transducer and activator of transcription (STAT) expression in K562 cells. Biochem. Pharmacol. 2006, 72, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Saydmohammed, M.; Joseph, D.; Syed, V. Curcumin suppresses constitutive activation of STAT-3 by up-regulating protein inhibitor of activated STAT-3 (PIAS-3) in ovarian and endometrial cancer cells. J. Cell. Biochem. 2010, 110, 447–456. [Google Scholar] [CrossRef]
- Kumar, A.; Dhawan, S.; Hardegen, N.J.; Aggarwal, B.B. Curcumin (Diferuloylmethane) Inhibition of Tumor Necrosis Factor (TNF)-Mediated Adhesion of Monocytes to Endothelial Cells by Suppression of Cell Surface Expression of Adhesion Molecules and of Nuclear Factor-kappaB Activation. Biochem. Pharmacol. 1998, 55, 775–783. [Google Scholar] [CrossRef]
- Kang, E.S.; Woo, I.S.; Kim, H.J.; Eun, S.Y.; Paek, K.S.; Chang, K.C.; Lee, J.H.; Lee, H.T.; Kim, J.-H.; Kim, H.J.; et al. Up-regulation of aldose reductase expression mediated by phosphatidylinositol 3-kinase/Akt and Nrf2 is involved in the protective effect of curcumin against oxidative damage. Free. Radic. Biol. Med. 2007, 43, 535–545. [Google Scholar] [CrossRef]
- Kahl, G. Nuclear Factor. In The Dictionary of Genomics, Transcriptomics and Proteomics; Wiley: Hoboken, NJ, USA, 2015; Volume 103, p. 1. [Google Scholar]
- Bharti, A.C.; Donato, N.; Aggarwal, B.B. Curcumin (Diferuloylmethane) Inhibits Constitutive and IL-6-Inducible STAT3 Phosphorylation in Human Multiple Myeloma Cells. J. Immunol. 2003, 171, 3863–3871. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Cui, G.-H.; Gu, J.; Hu, N.; Li, X.-G. Effect of curcumin on STAT5 signaling pathway in primary CML cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2004, 12, 572–576. [Google Scholar]
- Chen, W.; Chen, Y.; Gu, J.; He, J. Effect of curcumin on STAT5 signaling molecule in K562 cells. Zhonghua Xue Ye Xue Za Zhi 2004, 25, 151–153. [Google Scholar]
- Jaiswal, A.S.; Marlow, B.P.; Gupta, N.; Narayan, S. Beta-Catenin-Mediated Transactivation and Cell-Cell Adhesion Pathways Are Important in Curcumin (Diferuylmethane)-Induced Growth Arrest and Apoptosis in Colon Cancer Cells. Oncogene 2002, 21, 8414–8427. [Google Scholar] [CrossRef]
- Park, C.H.; Hahm, E.R.; Park, S.; Kim, H.-K.; Yang, C.H. The inhibitory mechanism of curcumin and its derivative against β-catenin/Tcf signaling. FEBS Lett. 2005, 579, 2965–2971. [Google Scholar] [CrossRef] [PubMed]
- Pendurthi, U.R.; Rao, L.M. Suppression of Transcription Factor Egr-1 by Curcumin. Thromb. Res. 2000, 97, 179–189. [Google Scholar] [CrossRef]
- Chen, A.; Xu, J.; Johnson, A.C. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene 2006, 25, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.-K.; Kim, S.-H.; Jeong, J.-W.; Lee, Y.M.; Kim, H.-S.; Kim, S.-R.; Yun, I.; Bae, S.-K.; Kim, K.-W. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol. Rep. 2006, 15, 1557–1562. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Banerjee, S.; Li, Y.; Sarkar, F.H. Retracted: Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer 2006, 106, 2503–2513. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, S.; Lin, J.; Zhang, Q.-J.; Chen, A. The interruption of the PDGF and EGF signaling pathways by curcumin stimulates gene expression of PPARγ in rat activated hepatic stellate cell in vitro. Lab. Investig. 2007, 87, 488–498. [Google Scholar] [CrossRef][Green Version]
- Mohan, R.; Sivak, J.; Ashton, P.; Russo, L.A.; Pham, B.Q.; Kasahara, N.; Raizman, M.B.; Fini, M.E. Curcuminoids Inhibit the Angiogenic Response Stimulated by Fibroblast Growth Factor-2, Including Expression of Matrix Metalloproteinase Gelatinase B. J. Biol. Chem. 2000, 275, 10405–10412. [Google Scholar] [CrossRef]
- Yang, X.; Thomas, D.P.; Zhang, X.; Culver, B.W.; Alexander, B.M.; Murdoch, W.J.; Rao, M.N.; Tulis, D.A.; Ren, J.; Sreejayan, N. Curcumin Inhibits Platelet-Derived Growth Factor–Stimulated Vascular Smooth Muscle Cell Function and Injury-Induced Neointima Formation. Arter. Thromb. Vasc. Biol. 2006, 26, 85–90. [Google Scholar] [CrossRef]
- Santibanez, J.F.; Quintanilla, M.; Martínez, J. Genistein and Curcumin Block TGF-β1-Induced u-PA Expression and Migratory and Invasive Phenotype in Mouse Epidermal Keratinocytes. Nutr. Cancer 2000, 37, 49–54. [Google Scholar] [CrossRef]
- Gaedeke, J.; Noble, N.A.; Border, W.A. Curcumin blocks multiple sites of the TGF-β signaling cascade in renal cells. Kidney Int. 2004, 66, 112–120. [Google Scholar] [CrossRef]
- Hu, Y.; Liang, H.; Du, Y.; Zhu, Y.; Wang, X. Curcumin Inhibits Transforming Growth Factor-β Activity via Inhibition of Smad Signaling in HK-2 Cells. Am. J. Nephrol. 2010, 31, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Peng, S.; Sun, Z.; Li, H.; Yang, R. Curcumin suppresses TGF-β signaling by inhibition of TGIF degradation in scleroderma fibroblasts. Biochem. Biophys. Res. Commun. 2011, 411, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, X.; Gao, Y.; Zhang, C.; Bao, J.; Guan, H.; Yu, H.; Lu, R.; Xu, Q.; Sun, Y. Curcumin Inhibits Metastasis in Human Papillary Thyroid Carcinoma BCPAP Cells via down-Regulation of the TGF-β/Smad2/3 Signaling Pathway. Exp. Cell Res. 2016, 341, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, G.; Jain, S.; Kale, S.; Raja, R.; Kumar, S.; Mishra, R.; Kundu, G.C. Curcumin suppresses breast tumor angiogenesis by abrogating osteopontin-induced VEGF expression. Mol. Med. Rep. 2008, 1, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Yoysungnoen, P.; Wirachwong, P.; Changtam, C.; Suksamrarn, A.; Patumraj, S. Anti-cancer and anti-angiogenic effects of curcumin and tetrahydrocurcumin on implanted hepatocellular carcinoma in nude mice. World J. Gastroenterol. 2008, 14, 2003–2009. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Arbab, A.S.; Jardim-Perassi, B.V.; Borin, T.F.; Gonçalves, N.N.; Nadimpalli, R.S.V.; Zuccari, D.A.P.D.C. Abstract A02: Effect of curcumin on the tumor growth and angiogenesis of breast cancer. Tumor-Assoc. Blood Vessels Lymph. 2015, 75, A02. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Anand, P.; Aggarwal, B.B. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008, 269, 199–225. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Bhatt, I.D.; Ichikawa, H. Curcumin-Biological and Medicinal Properties. In Tumeric: The Genus Curcuma; CRC Press: Boca Raton, FL, USA, 2006; pp. 297–368. [Google Scholar]
- Aggarwal, B.B. Activation of Transcription Factor NF-kappaB Is Suppressed by Curcumin (Diferuloylmethane). J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar]
- Cho, J.-W.; Lee, K.-S.; Kim, C.-W. Curcumin Attenuates the Expression of IL-1beta, IL-6, and TNF-Alpha as Well as Cyclin E in TNF-Alpha-Treated HaCaT Cells; NF-kappaB and MAPKs as Potential Upstream Targets. Int. J. Mol. Med. 2007, 19, 469–474. [Google Scholar]
- Ranjan, D.; Chen, C.; Johnston, T.D.; Jeon, H.; Nagabhushan, M. Curcumin inhibits mitogen stimulated lymphocyte proliferation, NFκB activation, and IL-2 signaling. J. Surg. Res. 2004, 121, 171–177. [Google Scholar] [CrossRef]
- Kobayashi, T.; Hashimoto, S.; Horie, T. Curcumin inhibition of Dermatophagoides farinea-induced interleukin-5 (IL-5) and granulocyte macrophage-colony stimulating factor (GM-CSF) production by lymphocytes from bronchial asthmatics. Biochem. Pharmacol. 1997, 54, 819–824. [Google Scholar] [CrossRef]
- Fahey, A.J.; Robins, R.A.; Constantinescu, C.S. Curcumin modulation of IFN-β and IL-12 signalling and cytokine induction in human T cells. J. Cell. Mol. Med. 2007, 11, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Grandjean-Laquerriere, A.; Antonicelli, F.; Gangloff, S.C.; Guenounou, M.; Le Naour, R. UVB-Induced IL-18 Production in Human Keratinocyte Cell Line NCTC 2544 through NF-κB Activation. Cytokine 2007, 37, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.-S.; Keum, Y.-S.; Han, S.S.; Song, Y.-S.; Kim, S.-H.; Surh, Y.-J. Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extracellular signal-regulated kinase activity and NF- B activation. Carcinogenesis 2003, 24, 1515–1524. [Google Scholar] [CrossRef]
- Camacho-Barquero, L.; Villegas, I.; Sánchez-Calvo, J.M.; Talero, E.; Sánchez-Fidalgo, S.; Motilva, V.; De La Lastra, C.A. Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int. Immunopharmacol. 2007, 7, 333–342. [Google Scholar] [CrossRef]
- Huang, M.T.; Lysz, T.; Ferraro, T.; Abidi, T.F.; Laskin, J.D.; Conney, A.H. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991, 51, 813–819. [Google Scholar]
- Kunnumakkara, A.B.; Guha, S.; Krishnan, S.; Diagaradjane, P.; Gelovani, J.; Aggarwal, B.B. Curcumin Potentiates Antitumor Activity of Gemcitabine in an Orthotopic Model of Pancreatic Cancer through Suppression of Proliferation, Angiogenesis, and Inhibition of Nuclear Factor-κB–Regulated Gene Products. Cancer Res. 2007, 67, 3853–3861. [Google Scholar] [CrossRef]
- Lin, Y.G.; Kunnumakkara, A.B.; Nair, A.S.; Merritt, W.M.; Han, L.Y.; Armaiz-Pena, G.N.; Kamat, A.A.; Spannuth, W.A.; Gershenson, D.M.; Lutgendorf, S.K.; et al. Curcumin Inhibits Tumor Growth and Angiogenesis in Ovarian Carcinoma by Targeting the Nuclear Factor- B Pathway. Clin. Cancer Res. 2007, 13, 3423–3430. [Google Scholar] [CrossRef]
- Woo, M.-S.; Jung, S.-H.; Kim, S.-Y.; Hyun, J.-W.; Ko, K.-H.; Kim, W.-K.; Kim, H.-S. Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochem. Biophys. Res. Commun. 2005, 335, 1017–1025. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Tan, T.-H. Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene 1998, 17, 173–178. [Google Scholar] [CrossRef]
- Salh, B.; Assi, K.; Templeman, V.; Parhar, K.K.S.; Owen, D.; Gomez-Muñoz, A.; Jacobson, K. Curcumin attenuates DNB-induced murine colitis. Am. J. Physiol. Liver Physiol. 2003, 285, G235–G243. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.R.; Al-Rasheed, M.; Manogaran, P.S.; Al-Hussein, K.A.; Platanias, L.C.; Al Kuraya, K.; Uddin, S. Curcumin induces apoptosis via inhibition of PI3′-kinase/AKT pathway in Acute T cell Leukemias. Apoptosis 2006, 11, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.-J.; Lee, S.-Y.; Huang, Z.; Lim, D.Y.; Chen, H.; Jung, S.K.; Bode, A.M.; Lee, K.W.; Dong, Z. Curcumin Suppresses Proliferation of Colon Cancer Cells by Targeting CDK2. Cancer Prev. Res. 2014, 7, 466–474. [Google Scholar] [CrossRef]
- Bush, J.A.; Cheung, K.-J.J.; Li, G. Curcumin Induces Apoptosis in Human Melanoma Cells through a Fas Receptor/Caspase-8 Pathway Independent of p53. Exp. Cell Res. 2001, 271, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Anto, R.J.; Mukhopadhyay, A.; Denning, K.; Aggarwal, B.B. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: Its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis 2002, 23, 143–150. [Google Scholar] [CrossRef]
- Lin, S.-S.; Huang, H.-P.; Yang, J.-S.; Wu, J.-Y.; Hsai, T.-C.; Lin, C.-C.; Lin, C.-W.; Kuo, C.-L.; Wood, W.G.; Chung, J. DNA damage and endoplasmic reticulum stress mediated curcumin-induced cell cycle arrest and apoptosis in human lung carcinoma A-549 cells through the activation caspases cascade- and mitochondrial-dependent pathway. Cancer Lett. 2008, 272, 77–90. [Google Scholar] [CrossRef]
- Jiang, A.-J.; Jiang, G.; Li, L.-T.; Zheng, J. Curcumin induces apoptosis through mitochondrial pathway and caspases activation in human melanoma cells. Mol. Biol. Rep. 2014, 42, 267–275. [Google Scholar] [CrossRef]
- Li, F.; Chen, X.; Xu, B.; Zhou, H. Curcumin induces p53-independent necrosis in H1299 cells via a mitochondria-associated pathway. Mol. Med. Rep. 2015, 12, 7806–7814. [Google Scholar] [CrossRef]
- Shishodia, S.; Amin, H.M.; Lai, R.; Aggarwal, B.B. Curcumin (Diferuloylmethane) Inhibits Constitutive NF-κB Activation, Induces G1/S Arrest, Suppresses Proliferation, and Induces Apoptosis in Mantle Cell Lymphoma. Biochem. Pharmacol. 2005, 70, 700–713. [Google Scholar] [CrossRef]
- Choudhuri, T.; Pal, S.; Das, T.; Sa, G. Curcumin Selectively Induces Apoptosis in Deregulated Cyclin D1-expressed Cells at G2Phase of Cell Cycle in a p53-dependent Manner. J. Biol. Chem. 2005, 280, 20059–20068. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Chen, Q.; Siddiqui, I.; Sarva, K.; Shankar, S. Linkage of Curcumin-Induced Cell Cycle Arrest and Apoptosis by Cyclin-Dependent Kinase Inhibitor p21/WAF1/CIP1. Cell Cycle 2007, 6, 2953–2961. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Wu, J.; Cao, W.; Zhang, J.; Liu, W.; Jiang, X.; Zhang, X. Curcumin induces G2/M cell cycle arrest in a p53-dependent manner and upregulates ING4 expression in human glioma. J. Neuro-Oncology 2007, 85, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Srivastava, R.K. Involvement of Bcl-2 family members, phosphatidylinositol 3’-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int. J. Oncol. 2007, 30, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. The paradox of pro-inflammatory cytokines in cancer. Cancer Metastasis Rev. 2006, 25, 307–313. [Google Scholar] [CrossRef]
- Williams, C.S.; Mann, M.; Dubois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999, 18, 7908–7916. [Google Scholar] [CrossRef]
- Kumar, A.; Dhawan, S.; Mukhopadhyay, A.; Aggarwal, B.B. Human Immunodeficiency Virus-1-Tat Induces Matrix Metalloproteinase-9 in Monocytes through Protein Tyrosine Phosphatase-Mediated Activation of Nuclear Transcription Factor NF-kappaB. FEBS Lett. 1999, 462, 140–144. [Google Scholar] [CrossRef]
- John, P.C.L.; Mews, M.; Moore, R. Cyclin/cdk complexes: Their involvement in cell cycle progression and mitotic division. Protoplasma 2001, 216, 119–142. [Google Scholar] [CrossRef]
- Sharma, R.A.; McLelland, H.R.; Hill, K.A.; Ireson, C.R.; Euden, S.A.; Manson, M.M.; Pirmohamed, M.; Marnett, L.J.; Gescher, A.J.; Steward, W.P. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001, 7, 1894–1900. [Google Scholar]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative Preparation of Curcumin for Improved Oral Bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I Clinical Trial of Oral Curcumin: Biomarkers of Systemic Activity and Compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Cheng, A.L.; Hsu, C.-H.; Lin, J.K.; Hsu, M.-M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer. Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.P.; Heath, D.D.; I Murray, S.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and pharmacodynamics of curcumin. Adv. Exp. Med. Biol. 2007, 595, 453–470. [Google Scholar] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S.S.R. Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers. Planta Medica 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Miyazawa, T. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci. 2000, 67, 2785–2793. [Google Scholar] [CrossRef]
- Pan, M.H.; Lin-Shiau, S.Y.; Lin, J.K. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IkappaB kinase and NFkappaB activation in macrophages. Biochem. Pharmacol. 2000, 60, 1665–1676. [Google Scholar] [CrossRef]
- Ireson, C.; Orr, S.; Jones, D.J.; Verschoyle, R.; Lim, C.K.; Luo, J.L.; Howells, L.; Plummer, S.; Jukes, R.; Williams, M.; et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001, 61, 1058–1064. [Google Scholar]
- Oppenheimer, A. Turmeric (Currumin) in Billiary Diseases. Lancet 1937, 229, 619–621. [Google Scholar] [CrossRef]
- Yang, K.-Y.; Lin, L.-C.; Tseng, T.-Y.; Wang, S.-C.; Tsai, T.-H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC–MS/MS. J. Chromatogr. B 2007, 853, 183–189. [Google Scholar] [CrossRef]
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar] [PubMed]
- Antony, B. A Composition to Enhance the Bioavailability of Curcumin. World Patent 2006129323 A1, 7 December 2006. [Google Scholar]
- Giori, A.; Franceschi, F. Phospholipid Complexes of Curcumin Having Improved Bioavailability. World Patent 2007101551, 25 October 2007. [Google Scholar]
- Badmaev, V.; Majeed, M.; Rajendran, R. Bioprotectant Composition, Method of Use and Extraction Process of Curcuminoids. World Patent 1997003674 A1, 6 February 1997. [Google Scholar]
- Madhavi, D.; Kagan, D. Bioavailability of a Sustained Release Formulation of Curcumin. Integr. Med. (Encinitas, Calif.) 2014, 13, 24–30. [Google Scholar]
- Siddiqui, R.A.; Hassan, S.; Harvey, K.A.; Rasool, T.; Das, T.; Mukerji, P.; DeMichele, S. Attenuation of proteolysis and muscle wasting by curcumin c3 complex in MAC16 colon tumour-bearing mice. Br. J. Nutr. 2009, 102, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Anthony, B. Composition to Enhance the Bioavailability of Curcumin. U.S. Patent 7879373 B2, 30 May 2005. [Google Scholar]
- Bombardelli, E. Phytosome: New cosmetic delivery system. Boll. Chim. Farm. 1991, 130, 431–438. [Google Scholar]
- Phytosome Technical Paper. Available online: https://www.indena.com/indena_files/2020/01/wp_phytosome_int.pdf (accessed on 17 November 2020).
- Marczylo, T.; Verschoyle, R.D.; Cooke, D.N.; Morazzoni, P.; Steward, W.P.; Gescher, A.J. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother. Pharmacol. 2007, 60, 171–177. [Google Scholar] [CrossRef]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.G.; Togni, S.; Appendino, G. Product-evaluation registry of Meriva®, a curcumin-phosphatidylcholine complex, for the complementary management of osteoarthritis. Panminerva Med. 2010, 52, 55–62. [Google Scholar]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.G.; Togni, S.; Appendino, G. Efficacy and safety of Meriva®, a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern. Med. Rev. A J. Clin. Ther. 2010, 15, 337–344. [Google Scholar]
- Liposomal Curcumin—16 Fluid Ounces. Available online: http://www.healthyitems.com/curcumin-p/607.htm (accessed on 17 November 2020).
- Amy Myers MD—Liposomal Curcumin. Available online: https://store.amymyersmd.com/products/liposomal-curcumin (accessed on 17 November 2020).
- Actinovo Liposomal Curcumin (Turmeric, Curcuminoids). Available online: https://www.actinovo.com/en/liposomal-curcumin-turmeric (accessed on 17 November 2020).
- Valimenta Liposomal Curcumin. Available online: https://www.valimenta.com/product/liposomal-curcumin/ (accessed on 17 November 2020).
- Cavacurmin®: Highly Bioavailable Curcumin. Available online: https://www.wacker.com/cms/en/industries/food/curcumin.jsp (accessed on 5 December 2020).
- Atal, C.K.; Dubey, R.K.; Singh, J. Biochemical basis of enhanced drug bioavailability by piperine: Evidence that piperine is a potent inhibitor of drug metabolism. J. Pharmacol. Exp. Ther. 1985, 232, 258–262. [Google Scholar] [PubMed]
- Han, H.-K. The effects of black pepper on the intestinal absorption and hepatic metabolism of drugs. Expert Opin. Drug Metab. Toxicol. 2011, 7, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Zhai, G.; Zhang, L. Self-Micro-Emulsion Colon Site-Specific Delivery Capsule Comprises Curcumin, Oil Phase, Surfactant, Cosurfactant, and Folic Acid Grease Material. Chinese Patent CN102266287, 7 December 2011. [Google Scholar]
- Catalan-Latorre, A.; Ravaghi, M.; Manca, M.L.; Caddeo, C.; Marongiu, F.; Ennas, G.; Escribano-Ferrer, E.; Peris, J.E.; Diez-Sales, O.; Fadda, A.M.; et al. Freeze-dried eudragit-hyaluronan multicompartment liposomes to improve the intestinal bioavailability of curcumin. Eur. J. Pharm. Biopharm. 2016, 107, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.B.; Rios, C.N.; Gupta, V.; Aseh, A. Preparation and Methodology of Silk Fibroin Nanoparticles. World Patent WO2010059963 A2, 27 May 2010. [Google Scholar]
- Kim, S.G.; Veena, M.S.; Basak, S.K.; Han, E.; Tajima, T.; Gjertson, D.W.; Starr, J.; Eidelman, O.; Pollard, H.B.; Srivastava, M.; et al. Curcumin Treatment Suppresses IKK Kinase Activity of Salivary Cells of Patients with Head and Neck Cancer: A Pilot Study. Clin. Cancer Res. 2011, 17, 5953–5961. [Google Scholar] [CrossRef] [PubMed]
- Kurzrock, R.; Li, L.; Mehta, K.; Aggarawal, B.B. Liposomal Curcumin for the Treatment of Cancer. U.S. Patent 20060067998 A1, 30 March 2006. [Google Scholar]
- Kurzrock, R.; Li, L. Liposome-encapsulated curcumin: In vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. J. Clin. Oncol. 2005, 23. [Google Scholar] [CrossRef]
- Clinicaltrials.Gov: A Phase IB Dose Escalation Study of Lipocurc in Patients With Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02138955 (accessed on 13 November 2019).
- Helson, L.; Chiu, S. Intravenous Curcumin and Derivatives for Treatment of Neurodegenerative and Stress Disorders. U.S. Patent 20110229555 A1, 22 September 2011. [Google Scholar]
- Bisht, S.; Feldmann, G.; Soni, S.; Ravi, R.; Karikar, C.; Maitra, A.; Maitra, A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): A novel strategy for human cancer therapy. J. Nanobiotechnol. 2007, 5. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Mizuma, M.; Feldmann, G.; Ottenhof, N.A.; Hong, S.-M.; Pramanik, D.; Chenna, V.; Karikari, C.; Sharma, R.; Goggins, M.G.; et al. Systemic Administration of Polymeric Nanoparticle-Encapsulated Curcumin (NanoCurc) Blocks Tumor Growth and Metastases in Preclinical Models of Pancreatic Cancer. Mol. Cancer Ther. 2010, 9, 2255–2264. [Google Scholar] [CrossRef]
- Chiu, S.S.; Lui, E.; Majeed, M.; Vishwanatha, J.K.; Ranjan, A.P.; Maitra, A.; Pramanik, D.; Smith, J.A.; Helson, L. Differential distribution of intravenous curcumin formulations in the rat brain. Anticancer. Res. 2011, 31, 907–911. [Google Scholar]
- Gou, M.; Men, K.; Shi, H.; Xiang, M.; Zhang, J.; Song, J.; Long, J.; Wan, Y.; Luo, F.; Zhao, X.; et al. Curcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo. Nanoscale 2011, 3, 1558–1567. [Google Scholar] [CrossRef]
- Yang, X.; Li, Z.; Wang, N.; Li, L.; Song, L.; He, T.; Sun, L.; Wang, Z.; Wu, Q.; Luo, N.; et al. Curcumin-Encapsulated Polymeric Micelles Suppress the Development of Colon Cancer In Vitro and In Vivo. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Shahani, K.; Swaminathan, S.K.; Freeman, D.; Blum, A.; Ma, L.; Panyam, J. Injectable Sustained Release Microparticles of Curcumin: A New Concept for Cancer Chemoprevention. Cancer Res. 2010, 70, 4443–4452. [Google Scholar] [CrossRef]
- Ranjan, A.P.; Mukerjee, A.; Vishwanatha, J.K. Solid in Oil/Water Emulsion-Diffusion Evaporation Formulation for Preparing Curcumin-Loaded PLGA Nanoparticles. U.S. Patent 20100290982 A1, 18 November 2010. [Google Scholar]
- Keck, C.M.; Santini, A. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur. J. Pharm. Biopharm. 2006, 62, 3–16. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Sun, M.; Guo, C.; Yu, A.; Xi, Y.; Cui, J.; Lou, H.; Zhai, G. Preparation and characterization of intravenously injectable curcumin nanosuspension. Drug Deliv. 2010, 18, 131–142. [Google Scholar] [CrossRef]
- Kurniawansyah, F.; Duong, H.T.T.; Luu, T.D.; Mammucari, R.; Vittorio, O.; Boyer, C.; Foster, N.R. Inhalable curcumin formulations: Micronization and bioassay. Chem. Eng. J. 2015, 279, 799–808. [Google Scholar] [CrossRef]
- McClure, R.; Yanagisawa, D.; Stec, D.; Abdollahian, D.; Koktysh, D.; Xhillari, D.; Jaeger, R.; Stanwood, G.; Chekmenev, E.; Tooyama, I.; et al. Inhalable Curcumin: Offering the Potential for Translation to Imaging and Treatment of Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 44, 283–295. [Google Scholar] [CrossRef]
- Saraf, G.J.S. Topical Delivery of Curcuma longa Extract Loaded Nanosized Ethosomes to Combat Facial Wrinkles Research Article. J. Pharm. Drug Deliv. Res. 2014, 3, 1. [Google Scholar] [CrossRef]
- Azuine, M.A.; Bhide, S.V. Chemopreventive effect of turmeric against stomach and skin tumors induced by chemical carcinogens in Swiss mice. Nutr. Cancer 1992, 17, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Lü, Y.; Chang, R.L.; Lou, Y.-R.; Huang, M.-T.; Newmark, H.L.; Reuhl, K.R.; Conney, A.H. Effect of curcumin on 12-O-tetradecanoylphorbol-13-acetate- and ultraviolet B light-induced expression of c-Jun and c-Fos in JB6 cells and in mouse epidermis. Carcinogenesis 1994, 15, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, C.; Oguro, T.; Yoshida, T.; Wen, C.; Sueki, H.; Iijima, M. Enhancing Effect of Ultraviolet A on Ornithine Decarboxylase Induction and Dermatitis Evoked by 12-o-Tetradecanoylphorbol-13-Acetate and Its Inhibition by Curcumin in Mouse Skin. Dermatology 1996, 193, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Villase~Or, I.M.; Simon, M.K.B.; Villanueva, A.M.A. Comparative Potencies of Nutraceuticals in Chemically Induced Skin Tumor Prevention. Nutr. Cancer 2002, 44, 66–70. [Google Scholar] [CrossRef]
- Phillips, J.M.; Clark, C.; Herman-Ferdinandez, L.; Moore-Medlin, T.; Rong, X.; Gill, J.R.; Clifford, J.L.; Abreo, F.; Nathan, C.-A.O. Curcumin Inhibits Skin Squamous Cell Carcinoma Tumor Growth In Vivo. Otolaryngol. Neck Surg. 2011, 145, 58–63. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Crowther, O.; Chua, D.; Eppley, W.; Meyer, B.; Salomon, M.; Driedger, A.; Morgan, M. Lithium-Air Cell Protective Membranes Comprising Polytetrafluroethylene Coated Fiberglass Cloth. U.S. Patent 2011/0177401 A1, 21 July 2011. [Google Scholar]
- Zhao, Y.-Z.; Lu, C.-T.; Zhang, Y.; Xiao, J.; Zhao, Y.-P.; Tian, J.-L.; Xu, Y.-Y.; Feng, Z.-G.; Xu, C.-Y. Selection of high efficient transdermal lipid vesicle for curcumin skin delivery. Int. J. Pharm. 2013, 454, 302–309. [Google Scholar] [CrossRef]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123‒126, 369–385. [Google Scholar] [CrossRef]
- Lakshmi, P.K.; Mounica, V.; Manoj Kumar, Y.; Prasanthi, D. Preparation and Evaluation of Curcumin Invasomes. Int. J. Drug Deliv. 2014, 6, 113–120. [Google Scholar]
- Liu, C.-H.; Chang, F.-Y.; Hung, D.-K. Terpene microemulsions for transdermal curcumin delivery: Effects of terpenes and cosurfactants. Colloids Surfaces B Biointerfaces 2011, 82, 63–70. [Google Scholar] [CrossRef]
- Patra, S.; Roy, E.; Madhuri, R.; Sharma, P.K. Retracted Article: The next generation cell-penetrating peptide and carbon dot conjugated nano-liposome for transdermal delivery of curcumin. Biomater. Sci. 2016, 4, 418–429. [Google Scholar] [CrossRef]
- Mangalathillam, S.; Rejinold, N.S.; Nair, A.; Lakshmanan, V.-K.; Nair, S.V.; Jayakumar, R. Curcumin loaded chitin nanogels for skin cancer treatment via the transdermal route. Nanoscale 2012, 4, 239–250. [Google Scholar] [CrossRef]
- Nedelcu, I.-A.; Ficai, A.; Sonmez, M.; Ficai, D.; Oprea, O.; Andronescu, E. Silver Based Materials for Biomedical Applications. Curr. Org. Chem. 2014, 18, 173–184. [Google Scholar] [CrossRef]
- Franco-Molina, M.A.; Mendoza-Gamboa, E.; Sierra-Rivera, C.A.; Gomez-Flores, R.; Zapata-Benavides, P.; Castillo-Tello, P.; Alcocer-González, J.M.; Miranda-Hernández, D.F.; Tamez-Guerra, R.S.; Rodríguez-Padilla, C. Antitumor activity of colloidal silver on MCF-7 human breast cancer cells. J. Exp. Clin. Cancer Res. 2010, 29. [Google Scholar] [CrossRef] [PubMed]
- Sangiliyandi, G.; Sriram, M.I.; Kanth, S.B.M.; Kalishwaralal, K.; Gurunathan, S. Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. Int. J. Nanomed. 2010, 5, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Rutberg, F.G.; Dubina, M.V.; Kolikov, V.A.; Moiseenko, F.V.; Ignat’Eva, E.V.; Volkov, N.M.; Snetov, V.N.; Stogov, A.Y. Effect of silver oxide nanoparticles on tumor growth in vivo. Dokl. Biochem. Biophys. 2008, 421, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Lilienfeld, S.; Dimauro, T.M. Iontophoretic Delivery of Curcumin and Curcumin Analogs for the Treatment of Alzheimer’s Disease. World Patent 2009158407 A1, 30 December 2009. [Google Scholar]
- Chang, C.-C.; Yang, W.-T.; Ko, S.-Y.; Hsu, Y.-C. Liposomal Curcuminoids for Transdermal Delivery: Iontophoresis Potential for Breast Cancer Chemotherapeutics. Dig. J. Nanomater. Biostruct. 2012, 7, 59–71. [Google Scholar]
- Pröhl, M.; Schubert, U.S.; Weigand, W.; Gottschaldt, M. Metal complexes of curcumin and curcumin derivatives for molecular imaging and anticancer therapy. Co-Ord. Chem. Rev. 2016, 307, 32–41. [Google Scholar] [CrossRef]
- Dahl, T.A.; Bilski, P.; Reszka, K.J.; Chignell, C.F.; Dahll, T.A. photocytotoxicity of curcumin. Photochem. Photobiol. 1994, 59, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Dahl, T.A.; McGowan, W.M.; Shand, M.A.; Srinivasan, V.S. Photokilling of bacteria by the natural dye curcumin. Arch. Microbiol. 1989, 151, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Ellerkamp, V.; Bortel, N.; Schmid, E.; Kirchner, B.; Armeanu-Ebinger, S.; Fuchs, J. Photodynamic Therapy Potentiates the Effects of Curcumin on Pediatric Epithelial Liver Tumor Cells. Anticancer. Res. 2016, 36, 3363–3372. [Google Scholar] [PubMed]
- Banerjee, S.; Prasad, P.; Hussain, A.; Khan, I.; Kondaiah, P.; Chakravarty, A.R. Remarkable photocytotoxicity of curcumin in HeLa cells in visible light and arresting its degradation on oxovanadium(iv) complex formation. Chem. Commun. 2012, 48, 7702. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Lin, J.-N.; Ma, J.-W.; Yang, N.-S.; Ho, C.-T.; Kuo, S.-C.; Way, T.-D. Demethoxycurcumin induces autophagic and apoptotic responses on breast cancer cells in photodynamic therapy. J. Funct. Foods 2015, 12, 439–449. [Google Scholar] [CrossRef]
- Ahn, J.-C.; Kang, J.-W.; Shin, J.-I.; Chung, P. Combination treatment with photodynamic therapy and curcumin induces mitochondria-dependent apoptosis in AMC-HN3 cells. Int. J. Oncol. 2012, 41, 2184–2190. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, A.K.; Agrahari, A.K.; Sharma, K.; Singh, A.S.; Gupta, M.K.; Tiwari, V.K.; Prakash, P. Making of water soluble curcumin to potentiate conventional antimicrobials by inducing apoptosis-like phenomena among drug-resistant bacteria. Sci. Rep. 2020, 10, 1–22. [Google Scholar] [CrossRef]
- Ding, L.; Ma, S.; Lou, H.; Sun, L.-R.; Ji, M. Synthesis and Biological Evaluation of Curcumin Derivatives with Water-Soluble Groups as Potential Antitumor Agents: An in Vitro Investigation Using Tumor Cell Lines. Molecules 2015, 20, 21501–21514. [Google Scholar] [CrossRef]
- Mishra, S.; Narain, U.; Mishra, R.; Misra, K. Design, Development and Synthesis of Mixed Bioconjugates of Piperic Acid-Glycine, Curcumin-Glycine/alanine and Curcumin-Glycine-Piperic Acid and Their Antibacterial and Antifungal Properties. Bioorganic Med. Chem. 2005, 13, 1477–1486. [Google Scholar] [CrossRef]
- Lu, P.; Tong, Q.; Jiang, F.; Zheng, L.; Chen, F.; Zeng, F.; Dong, J.; Du, Y. Preparation of curcumin prodrugs and their in vitro anti-tumor activities. J. Huazhong Univ. Sci. Technol. Med. Sci. 2005, 25, 668–678. [Google Scholar]
- Wichitnithad, W.; Nimmannit, U.; Wacharasindhu, S.; Rojsitthisak, P. Synthesis, Characterization and Biological Evaluation of Succinate Prodrugs of Curcuminoids for Colon Cancer Treatment. Molecules 2011, 16, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Narain, U.; Tripathi, S.; Misra, K. Syntheses of Curcumin Bioconjugates and Study of Their Antibacterial Activities against beta-Lactamase-Producing Microorganisms. Bioconjugate Chem. 2001, 12, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Tripathi, S.; Mishra, R.; Misra, K. Design, Synthesis and Characterisation of a Novel Anticancer Prodrug Having Antiproliferative Activity against Prostrate Tumour. Indian J. Chem. Sect. B Org. Med. Chem. 2005, 44, 2582–2588. [Google Scholar]
- Tang, H.; Murphy, C.J.; Zhang, B.; Shen, Y.; Sui, M.; Van Kirk, E.A.; Feng, X.; Murdoch, W.J. Amphiphilic Curcumin Conjugate-Forming Nanoparticles as Anticancer Prodrug and Drug Carriers: In Vitro and in Vivo Effects. Nanomedicine 2010, 5, 855–865. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, C.; Zhang, Q.; Gao, M.; Zhang, J.; Kong, D.; Zhao, Y. Tuning the architecture of polymeric conjugate to mediate intracellular delivery of pleiotropic curcumin. Eur. J. Pharm. Biopharm. 2015, 90, 53–62. [Google Scholar] [CrossRef]
- Li, M.; Gao, M.; Fu, Y.; Chen, C.; Meng, X.; Fan, A.; Kong, D.; Wang, Z.; Deng, J. Acetal-linked polymeric prodrug micelles for enhanced curcumin delivery. Colloids Surfaces B Biointerfaces 2016, 140, 11–18. [Google Scholar] [CrossRef]
- Massaro, M.; Amorati, R.; Cavallaro, G.; Guernelli, S.; Lazzara, G.; Milioto, S.; Noto, R.; Poma, P.; Riela, S. Direct chemical grafted curcumin on halloysite nanotubes as dual-responsive prodrug for pharmacological applications. Colloids Surfaces B Biointerfaces 2016, 140, 505–513. [Google Scholar] [CrossRef]
- Shakeri, A.; Panahi, Y.; Johnston, T.P.; Sahebkar, A. Biological properties of metal complexes of curcumin. BioFactors 2019, 45, 304–317. [Google Scholar] [CrossRef]
- Wanninger, S.; Lorenz, V.; Subhan, A.; Edelmann, F.T. Metal complexes of curcumin—synthetic strategies, structures and medicinal applications. Chem. Soc. Rev. 2015, 44, 4986–5002. [Google Scholar] [CrossRef]
- Sareen, R.; Jain, N.; Dhar, K.L. Curcumin–Zn(II) complex for enhanced solubility and stability: An approach for improved delivery and pharmacodynamic effects. Pharm. Dev. Technol. 2015, 21, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Pucci, D.; Bellini, T.; Crispini, A.; D’Agnano, I.; Liguori, P.F.; García-Orduña, P.; Pirillo, S.; Valentini, A.; Zanchetta, G. DNA binding and cytotoxicity of fluorescent curcumin-based Zn(ii) complexes. MedChemComm 2012, 3, 462. [Google Scholar] [CrossRef]
- Pucci, D.; Crispini, A.; Mendiguchía, B.S.; Pirillo, S.; Ghedini, M.; Morelli, S.; De Bartolo, L. Improving the bioactivity of Zn(ii)-curcumin based complexes. Dalton Trans. 2013, 42, 9679–9687. [Google Scholar] [CrossRef] [PubMed]
- Hieu, T.Q.; Doan, T.T. A novel study on curcumin metal complexes: Solubility improvement, bioactivity, and trial burn wound treatment in rats. New J. Chem. 2020, 44, 13036–13045. [Google Scholar] [CrossRef]
- Kühlwein, F.; Polborn, K.; Beck, W. Metallkomplexe von Farbstoffen. VIII Übergangsmetallkomplexe des Curcumins und Seiner Derivate. Zeitschrift für Anorg. und Allg. Chemie 1997, 623, 1211–1219. [Google Scholar] [CrossRef]
- Pi, Z.; Wang, J.; Jiang, B.; Cheng, G.; Zhou, S. A curcumin-based TPA four-branched copper(II) complex probe for in vivo early tumor detection. Mater. Sci. Eng. C 2015, 46, 565–571. [Google Scholar] [CrossRef]
- Zhao, X.-Z.; Jiang, T.; Wang, L.; Yang, H.; Zhang, S.; Zhou, P. Interaction of curcumin with Zn(II) and Cu(II) ions based on experiment and theoretical calculation. J. Mol. Struct. 2010, 984, 316–325. [Google Scholar] [CrossRef]
- Valentini, A.; Conforti, F.; Crispini, A.; De Martino, A.; Condello, R.; Stellitano, C.; Rotilio, G.; Ghedini, M.; Federici, G.; Bernardini, S.; et al. Synthesis, Oxidant Properties, and Antitumoral Effects of a Heteroleptic Palladium(II) Complex of Curcumin on Human Prostate Cancer Cells. J. Med. Chem. 2009, 52, 484–491. [Google Scholar] [CrossRef]
- Thompson, K.H.; Böhmerle, K.; Polishchuk, E.; Martins, C.; Toleikis, P.; Tse, J.; Yuen, V.; McNeill, J.H.; Orvig, C. Complementary inhibition of synoviocyte, smooth muscle cell or mouse lymphoma cell proliferation by a vanadyl curcumin complex compared to curcumin alone. J. Inorg. Biochem. 2004, 98, 2063–2070. [Google Scholar] [CrossRef]
- Miklášová, N.; Fischer-Fodor, E.; Mikláš, R.; Kucková, L.; Kožíšek, J.; Liptaj, T.; Soritau, O.; Valentová, J.; Tomuleasa, C. Synthesis and characterization of new biologically active palladium(II) complexes with (1E,6E)-1,7-bis(3,4-diethoxyphenyl)-1,6-heptadiene-3,5-dione. Inorg. Chem. Commun. 2014, 46, 229–233. [Google Scholar] [CrossRef]
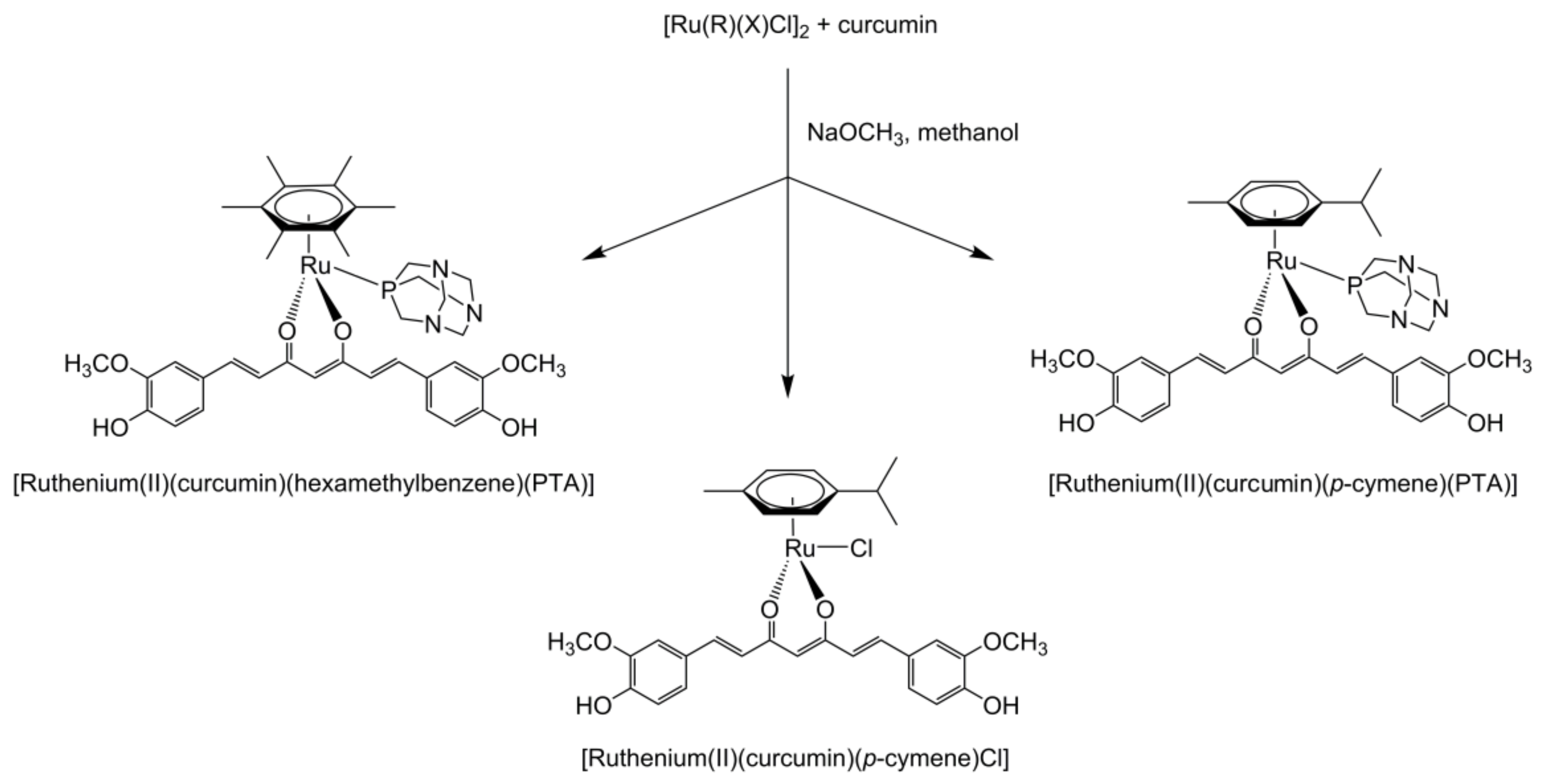
- Pettinari, R.; Marchetti, F.; Condello, F.; Pettinari, C.; Lupidi, G.; Scopelliti, R.; Mukhopadhyay, S.; Riedel, T.; Dyson, P.J. Ruthenium(II)–Arene RAPTA Type Complexes Containing Curcumin and Bisdemethoxycurcumin Display Potent and Selective Anticancer Activity. Organometallics 2014, 33, 3709–3715. [Google Scholar] [CrossRef]
- Caruso, F.; Rossi, M.; Benson, A.; Opazo, C.; Freedman, D.; Monti, E.; Gariboldi, M.B.; Shaulky, J.; Marchetti, F.; Pettinari, R.; et al. Ruthenium-arene Complexes of Curcumin: X-Ray and Density Functional Theory Structure, Synthesis, and Spectroscopic Characterization, in Vitro Antitumor Activity, and DNA Docking Studies of (p-Cymene)Ru(curcuminato)chloro. J. Med. Chem. 2012, 55, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Pettinari, R.; Rossi, M.; Monti, E.; Gariboldi, M.B.; Marchetti, F.; Pettinari, C.; Caruso, A.; Ramani, M.V.; Subbaraju, G.V. The in vitro antitumor activity of arene-ruthenium(II) curcuminoid complexes improves when decreasing curcumin polarity. J. Inorg. Biochem. 2016, 162, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Henriques, M.C.; Faustino, M.A.F.; Silva, A.M.S.; Felgueiras, J.; Fardilha, M.; Braga, S.S. A ruthenium(II)-trithiacyclononane curcuminate complex: Synthesis, characterization, DNA-interaction, and cytotoxic activity. J. Co-Ord. Chem. 2017, 70, 2393–2408. [Google Scholar] [CrossRef]
- Li, S.; Xu, G.; Zhu, Y.; Zhao, J.; Gou, S. Bifunctional ruthenium(ii) polypyridyl complexes of curcumin as potential anticancer agents. Dalton Trans. 2020, 49, 9454–9463. [Google Scholar] [CrossRef]
- Ficai, A.; Marques, C.F.; Ferreira, J.M.; Andronescu, E.; Ficai, D.; Sonmez, M. Multifunctional materials for bone cancer treatment. Int. J. Nanomed. 2014, 9, 2713–2725. [Google Scholar] [CrossRef]
- Chin, D.; Huebbe, P.; Frank, J.; Rimbach, G.; Pallauf, K. Curcumin may impair iron status when fed to mice for six months. Redox Biol. 2014, 2, 563–569. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho Henriques, M.; Faustino, M.A.F.; Santos Braga, S. Curcumin Innovative Delivery Forms: Paving the ‘Yellow Brick Road’ of Antitumoral Phytotherapy. Appl. Sci. 2020, 10, 8990. https://doi.org/10.3390/app10248990
Carvalho Henriques M, Faustino MAF, Santos Braga S. Curcumin Innovative Delivery Forms: Paving the ‘Yellow Brick Road’ of Antitumoral Phytotherapy. Applied Sciences. 2020; 10(24):8990. https://doi.org/10.3390/app10248990
Chicago/Turabian StyleCarvalho Henriques, Magda, Maria Amparo F. Faustino, and Susana Santos Braga. 2020. "Curcumin Innovative Delivery Forms: Paving the ‘Yellow Brick Road’ of Antitumoral Phytotherapy" Applied Sciences 10, no. 24: 8990. https://doi.org/10.3390/app10248990
APA StyleCarvalho Henriques, M., Faustino, M. A. F., & Santos Braga, S. (2020). Curcumin Innovative Delivery Forms: Paving the ‘Yellow Brick Road’ of Antitumoral Phytotherapy. Applied Sciences, 10(24), 8990. https://doi.org/10.3390/app10248990







