Effects of Salicylic Acid and Methyl Jasmonate Treatments on Flavonoid and Carotenoid Accumulation in the Juice Sacs of Satsuma Mandarin In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. In Vitro Culture System and Treatment
2.3. Extraction and Determination of Flavonoids
2.4. Extraction and Determination of Carotenoids
2.5. Analysis of Gene Expression by Using Real-Time Quantitative RT-PCR
2.6. RNA Isolation and Fluorescent Labeling of Probes
2.7. Statistical Analysis
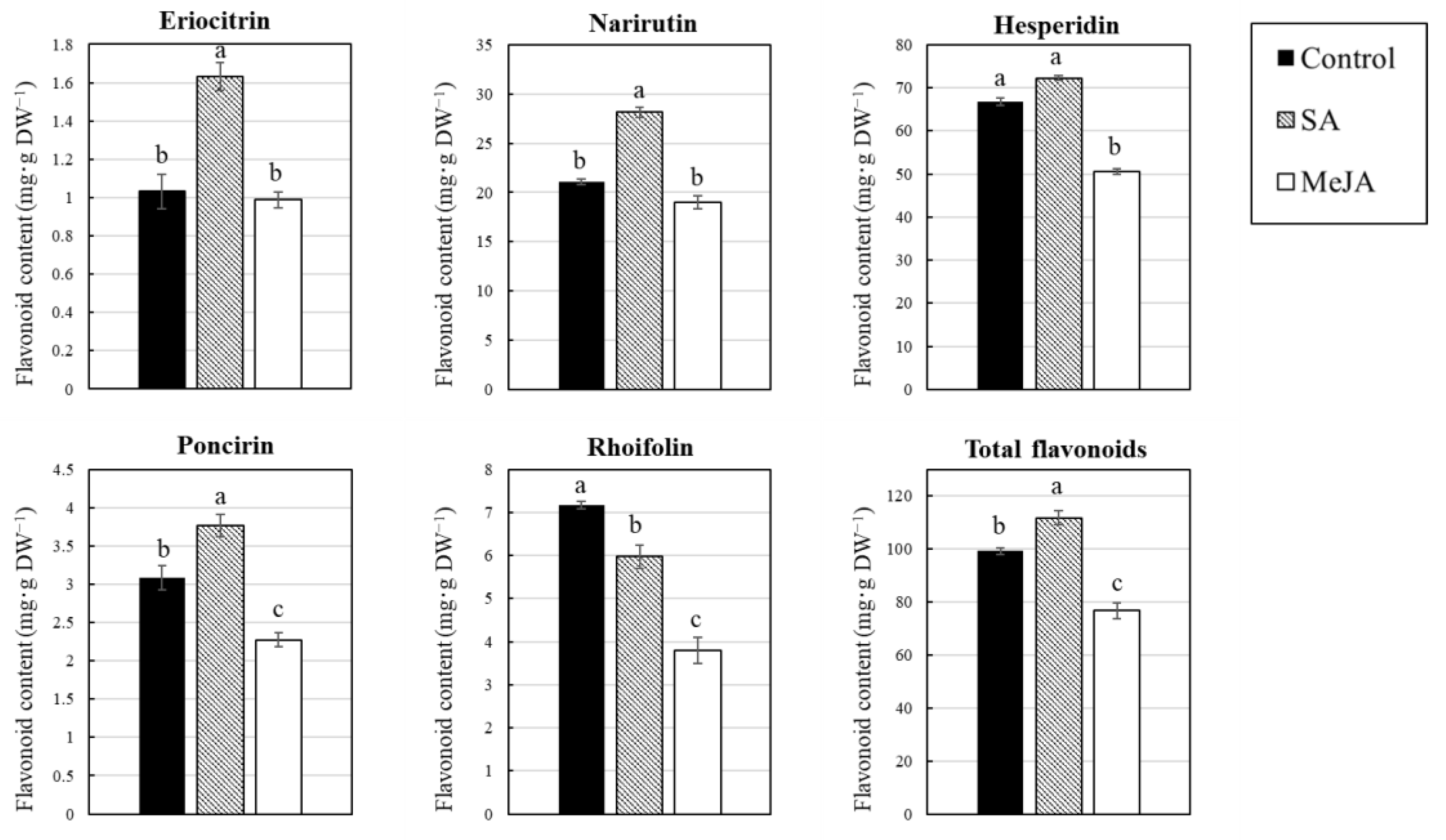
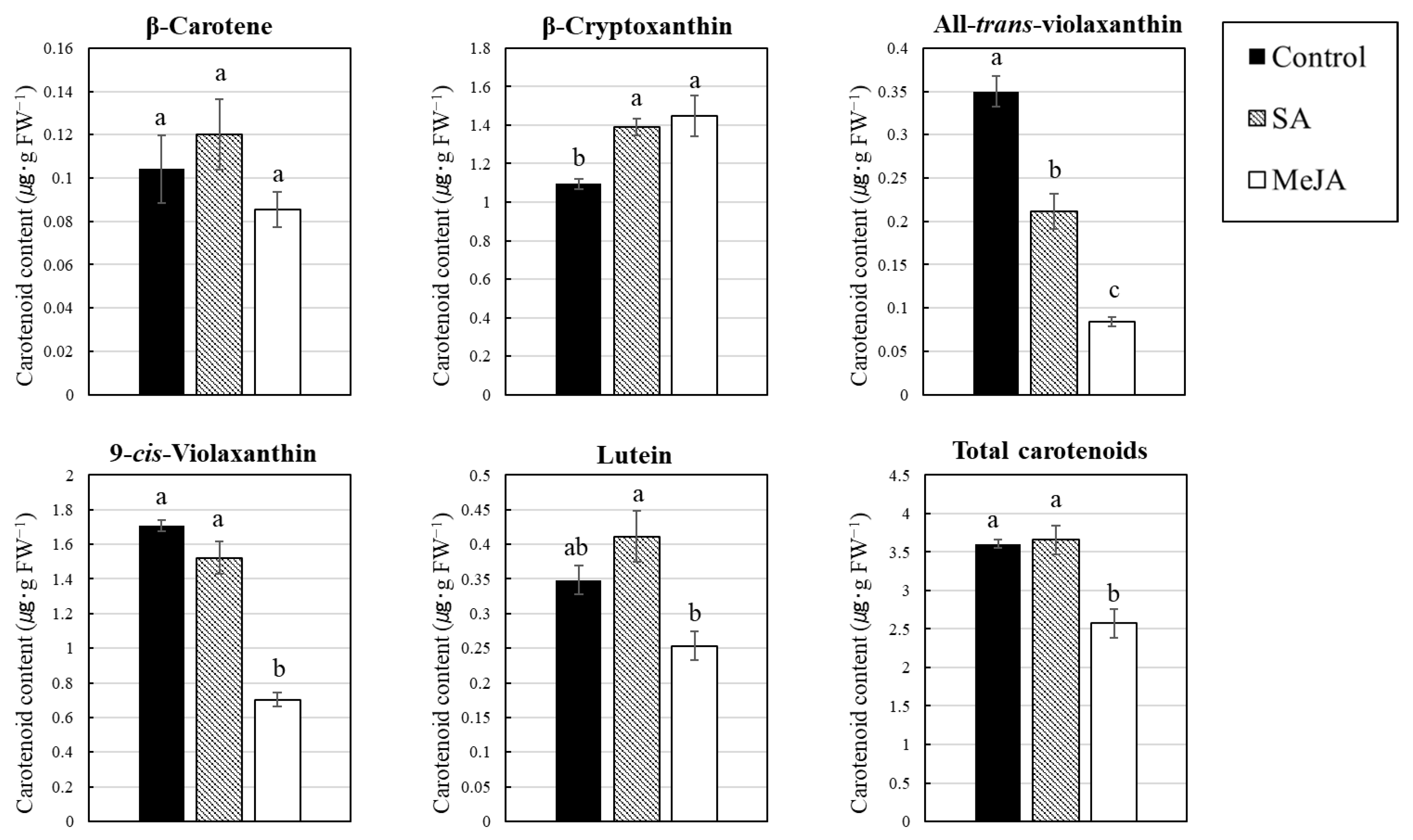
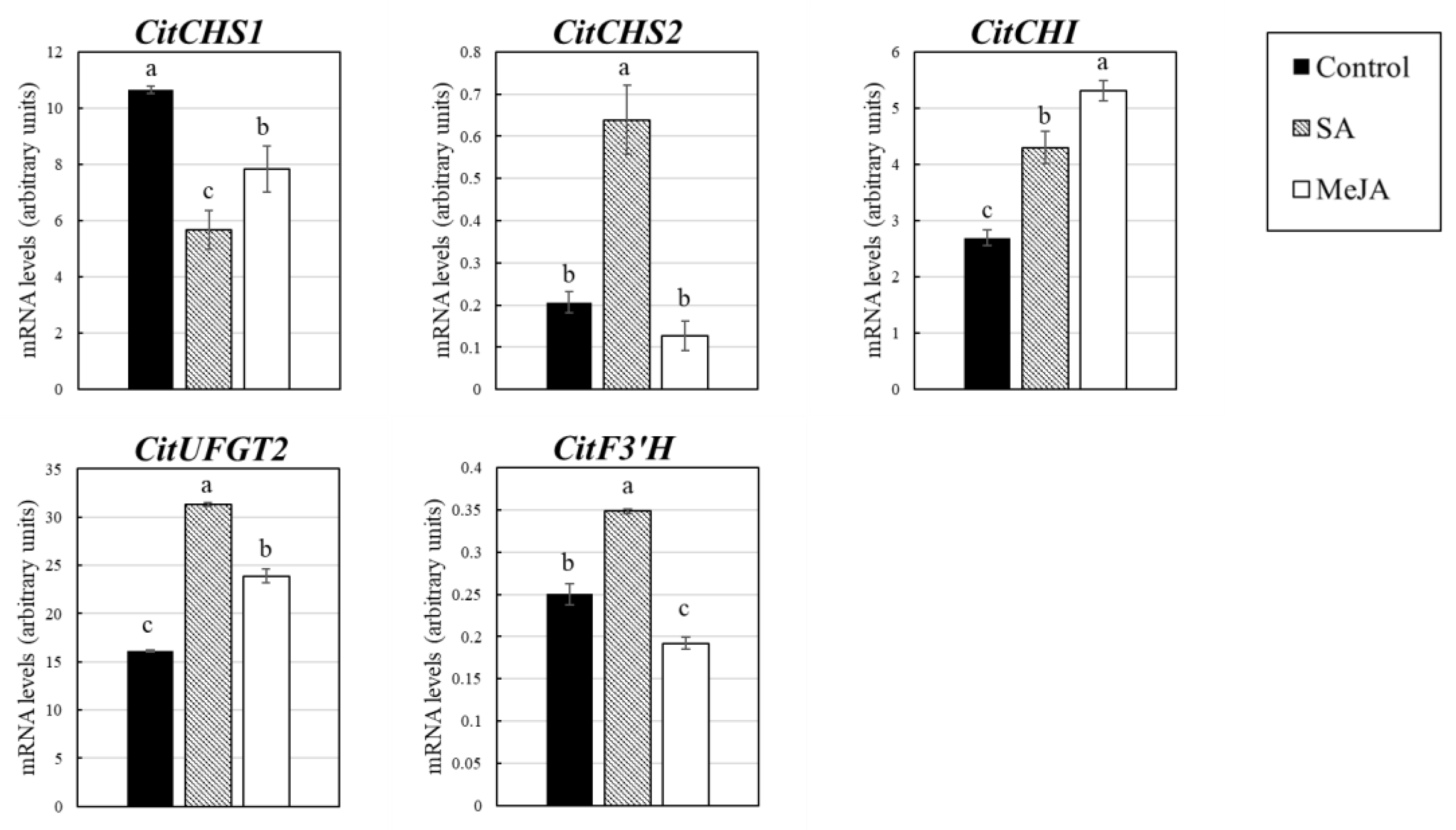
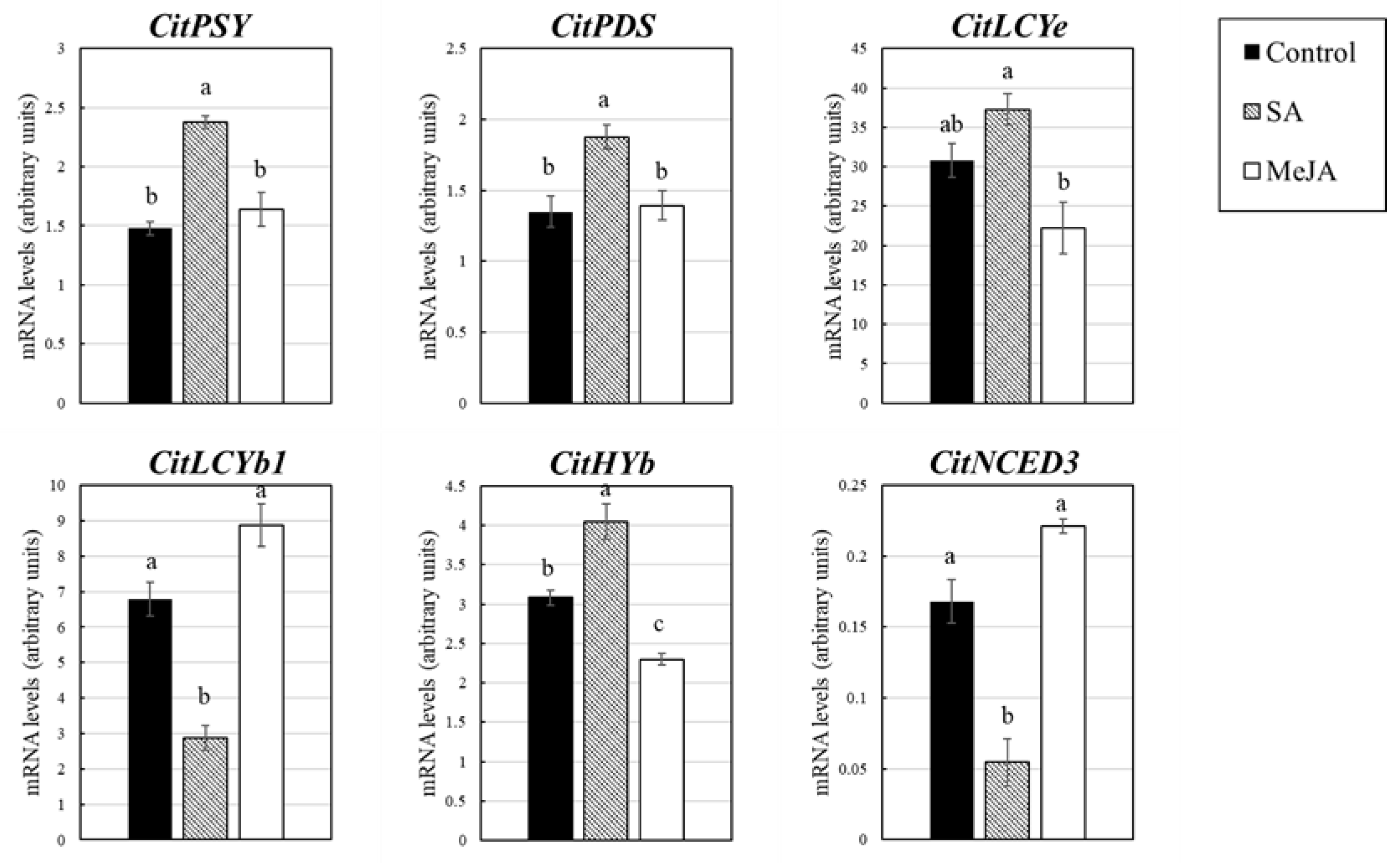
3. Results
3.1. Effect of Salicylic Acid (SA) and Methyl Jasmonate (MeJA) on Flavonoid Content in Citrus Juice Sacs In Vitro
3.2. Effect of SA and MeJA on Carotenoid Content in Citrus Juice Sacs In Vitro
3.3. Effect of SA and MeJA on Expression of the Genes Related to Flavonoid and Carotenoid Accumulation in Citrus Juice Sacs
3.4. Identification of Transcription Factor in Response to Salicylic Acid and Methyl Jasmonate Treatments
4. Discussion
4.1. Effect of SA on the Flavonoid and Carotenoid Accumulation in Citrus Juice Sacs In Vitro
4.2. Effect of MeJA on the Flavonoid and Carotenoid Accumulation in Citrus Juice Sacs In Vitro
4.3. Identification of Transcription Factor in Response to SA and MeJA Treatments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fujii, H.; Shimada, T.; Sugiyama, A.; Nishikawa, F.; Endo, T.; Nakano, M.; Ikoma, Y.; Shimizu, T.; Omura, M. Profiling ethylene-responsive genes in mature mandarin fruit using a citrus 22K oligoarray. Plant Sci. 2007, 173, 340–348. [Google Scholar] [CrossRef]
- Giovannoni, J.J. Genetic Regulation of Fruit Development and Ripening. Plant Cell 2004, 16 (Suppl. 1), S170–S180. [Google Scholar] [CrossRef]
- Takayanagi, K.; Morimoto, S.; Shirakura, Y.; Mukai, K.; Sugiyama, T.; Tokuji, Y.; Ohnishi, M. Mechanism of Visceral Fat Reduction in Tsumura Suzuki Obese, Diabetes (TSOD) Mice Orally Administered β-Cryptoxanthin from Satsuma Mandarin Oranges (Citrus Unshiu Marc). J. Agric. Food Chem. 2011, 59, 12342–12351. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Iida, K.; Madono, Y.; Yungyuen, W.; Yahata, M.; Yamawaki, K.; Kato, M. Identification and quantitative analysis of β-cryptoxanthin and β-citraurin esters in satsuma mandarin fruit during the ripening process. Food Chem. 2017, 234, 356–364. [Google Scholar] [CrossRef]
- Yamaguchi, M. Role of carotenoid β-cryptoxanthin in bone homeostasis. J. Biomed. Sci. 2012, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, A.R.; Liu, C.; Smith, D.E.; Hu, K.-Q.; Choi, S.-W.; Ausman, L.M.; Wang, X.-D. β-Cryptoxanthin Restores Nicotine-Reduced Lung SIRT1 to Normal Levels and Inhibits Nicotine-Promoted Lung Tumorigenesis and Emphysema in A/J Mice. Cancer Prev. Res. 2013, 6, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, Y.; Ando, F.; Shimokata, H.; Yano, M. Dietary patterns of antioxidant vitamin and carotenoid intake associated with bone mineral density: Findings from Post-Menopausal Japanese Female Subjects. Osteoporos. Int. 2011, 22, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Zhang, L.; Sugiura, M.; Kato, M. Citrus and health. In The Genus Citrus; Elsevier: Amsterdam, The Netherlands, 2020; pp. 495–511. [Google Scholar]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; YANO, M.; Ohta, H. Flavonoid Composition of Fruit Tissues of Citrus Species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-G.; Kim, H.; Hur, H.-G.; Lim, Y.; Ahn, J.-H. Regioselectivity of 7-O-methyltransferase of poplar to flavones. J. Bbiotechnol. 2006, 126, 241–247. [Google Scholar] [CrossRef]
- Napal, G.N.D.; Carpinella, M.C.; Palacios, S.M. Antifeedant activity of ethanolic extract from Flourensia oolepis and isolation of pinocembrin as its active principle compound. Bioresour. Technol. 2009, 100, 3669–3673. [Google Scholar] [CrossRef]
- Cheng, H.-L.; Hsieh, M.-J.; Yang, J.-S.; Lin, C.-W.; Lue, K.-H.; Lu, K.-H.; Yang, S.-F. Nobiletin inhibits human osteosarcoma cells metastasis by blocking ERK and JNK-mediated MMPs expression. Oncotarget 2016, 7, 35208. [Google Scholar] [CrossRef]
- Goh, J.X.H.; Tan, L.T.-H.; Goh, J.K.; Chan, K.G.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Nobiletin and Derivatives: Functional Compounds from Citrus Fruit Peel for Colon Cancer Chemoprevention. Cancers 2019, 11, 867. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Osakabe, N.; Sanbongi, C.; Yanagisawa, R.; Inoue, K.-I.; Yasuda, A.; Natsume, M.; Baba, S.; Ichiishi, E.-I.; Yoshikawa, T. Extract of Perilla frutescens Enriched for Rosmarinic Acid, a Polyphenolic Phytochemical, Inhibits Seasonal Allergic Rhinoconjunctivitis in Humans. Exp. Biol. Med. 2004, 229, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.-P.; Zhang, L.; Yan, P.; Ahammed, G.J.; Han, W.-Y. Methyl Salicylate Enhances Flavonoid Biosynthesis in Tea Leaves by Stimulating the Phenylpropanoid Pathway. Molecules 2019, 24, 362. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Esplá, A.; Zapata, P.J.; Valero, D.; Martínez-Romero, D.; Díaz-Mula, H.M.; Serrano, M. Preharvest treatments with salicylates enhance nutrient and antioxidant compounds in plum at harvest and after storage. J. Sci. Food Agric. 2018, 98, 2742–2750. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.G.; Sanz, C.; Richardson, D.G.; Olías, J.M. Methyl Jasmonate Vapor Promotes β-Carotene Synthesis and Chlorophyll Degradation in Golden Delicious Apple Peel. J. Plant Growth Regul. 1993, 12, 163. [Google Scholar] [CrossRef]
- Liu, L.; Wei, J.; Zhang, M.; Zhang, L.; Li, C.; Wang, Q. Ethylene independent induction of lycopene biosynthesis in tomato fruits by jasmonates. J. Exp. Bot. 2012, 63, 5751–5761. [Google Scholar] [CrossRef]
- Huang, R.; Xia, R.; Lu, Y.; Hu, L.; Xu, Y. Effect of pre-harvest salicylic acid spray treatment on post-harvest antioxidant in the pulp and peel of ‘Cara cara’ navel orange (Citrus sinenisis L. Osbeck). J. Sci. Food Agric. 2008, 88, 229–236. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, G.; Kato, M.; Yamawaki, K.; Takagi, T.; Kiriiwa, Y.; Ikoma, Y.; Matsumoto, H.; Yoshioka, T.; Nesumi, H. Regulation of carotenoid accumulation and the expression of carotenoid metabolic genes in citrus juice sacs in vitro. J. Exp. Bot. 2012, 63, 871–886. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Yungyuen, W.; Sato, Y.; Furuya, T.; Yahata, M.; Yamawaki, K.; Kato, M. Accumulation of carotenoids in a novel citrus cultivar ’Seinannohikari’ during the fruit maturation. Plant Physiol. Biochem. 2018, 129, 349–356. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, G.; Yamawaki, K.; Ikoma, Y.; Matsumoto, H.; Yoshioka, T.; Ohta, S.; Kato, M. Effect of Blue LED light intensity on carotenoid accumulation in citrus juice sacs. Physiol. Plant. 2015, 188, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Seoka, M.; Ma, G.; Zhang, L.; Yahata, M.; Yamawaki, K.; Kan, T.; Kato, M. Expression and functional analysis of the nobiletin biosynthesis-related gene CitOMT in citrus fruit. Sci. Rep. 2020, 10, 15288. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Ikoma, Y.; Matsumoto, H.; Sugiura, M.; Hyodo, H.; Yano, M. Accumulation of Carotenoids and Expression of Carotenoid Biosynthetic Genes during Maturation in Citrus Fruit. Physiol. Plant. 2004, 134, 824–837. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, Y.; Yano, M.; Ogawa, K.; Yoshioka, T.; Xu, Z.C.; Hisada, S.; Omura, M.; Moriguchi, T. Isolation and Evaluation of RNA from Polysaccharide-Rich Tissues in Fruit for Quality by CDNA Library Construction and RT-PCR. J. Jpn. Soc. Hort. Sci. 1996, 64, 809–814. [Google Scholar] [CrossRef]
- Preciado-Rangel, P.; Reyes-Pérez, J.J.; Ramírez-Rodríguez, S.C.; Salas-Pérez, L.; Fortis-Hernández, M.; Murillo-Amador, B.; Troyo-Diéguez, E. Foliar Aspersion of Salicylic Acid Improves Phenolic and Flavonoid Compounds, and Also the Fruit Yield in Cucumber (Cucumis Sativus L.). Plants 2019, 8, 44. [Google Scholar] [CrossRef]
- Gondor, O.K.; Janda, T.; Soós, V.; Pál, M.; Majláth, I.; Adak, M.K.; Balázs, E.; Szalai, G. Salicylic Acid Induction of Flavonoid Biosynthesis Pathways in Wheat Varies by Treatment. Front. Plant Sci. 2016, 7, 1447. [Google Scholar] [CrossRef]
- Moriguchi, T.; Kita, M.; Tomono, Y.; Endo-Inagaki, T.; Omura, M. Gene expression in flavonoid biosynthesis: Correlation with flavonoid accumulation in developing citrus fruit. Physiol Plant. 2001, 111, 66–74. [Google Scholar] [CrossRef]
- Baba, S.A.; Ashraf, N. Functional characterization of flavonoid 3′-hydroxylase, CsF3′ H, from Crocus sativus L: Insights into substrate specificity and role in abiotic stress. Arch. Biochem. Biophys. 2019, 667, 70–78. [Google Scholar] [CrossRef]
- Moharekar, S.T.; Lokhande, S.D.; Hara, T.; Tanaka, R.; Tanaka, A.; Chavan, P.D. Effect of salicylic acid on chlorophyll and carotenoid contents of wheat and moong seedlings. Photosynthetica 2003, 41, 315. [Google Scholar] [CrossRef]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Zapata, P.J.; Castillo, S.; Serrano, M. Postharvest methyl salicylate treatments delay ripening and maintain quality attributes and antioxidant compounds of ‘Early Lory’ sweet cherry. Postharvest Biol. Technol. 2016, 117, 102–109. [Google Scholar] [CrossRef]
- Yang, Y.-X.; Ahammed, G.J.; Wu, C.; Fan, S.; Zhou, Y.-H. Crosstalk among Jasmonate, Salicylate and Ethylene Signaling Pathways in Plant Disease and Immune Responses. Curr. Protein Pept. Sci. 2015, 16, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Rudell, D.R.; Mattheis, J.P.; Fan, X.; Fellman, J.K. Methyl Jasmonate Enhances Anthocyanin Accumulation and Modifies Production of Phenolics and Pigments InFuji’Apples. J. Am. Soc. Hort. Sci. 2002, 127, 435–441. [Google Scholar] [CrossRef]
- Ni, J.; Zhao, Y.; Tao, R.; Yin, L.; Gao, L.; Strid, A.; Qian, M.; Li, J.; Li, Y.; Shen, J. Ethylene mediates the branching of the jasmonate-induced flavonoid biosynthesis pathway by suppressing anthocyanin biosynthesis in red Chinese pear fruits. Plant Biotechnol. J. 2020, 18, 1223–1240. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.; del Castillo, M.L.R. Influence of preharvest and postharvest methyl jasmonate treatments on flavonoid content and metabolomic enzymes in red raspberry. Postharvest Biol. Technol. 2014, 97, 77–82. [Google Scholar] [CrossRef]
- Wang, S.Y.; Bowman, L.; Ding, M. Methyl jasmonate enhances antioxidant activity and flavonoid content in blackberries (Rubus sp.) and promotes antiproliferation of human cancer cells. Food Chem. 2008, 107, 1261–1269. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, J.; Nageswaran, D.; Li, L. Carotenoid metabolism and regulation in horticultural crops. Hortic. Res. 2015, 2, 1–11. [Google Scholar] [CrossRef]
- Luo, H.; He, W.; Li, D.; Bao, Y.; Riaz, A.; Xiao, Y.; Song, J.; Liu, C. Effect of methyl jasmonate on carotenoids biosynthesis in germinated maize kernels. Food Chem. 2020, 307, 125525. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Z. Potentiation of Developmentally Regulated Plant Defense Response by AtWRKY18, a Pathogen-Induced Arabidopsis Transcription Factor. Physiol. Plant 2002, 129, 706–716. [Google Scholar] [CrossRef]
- Du, L.; Chen, Z. Identification of genes encoding receptor-like protein kinases as possible targets of pathogen-and salicylic acid-induced WRKY DNA-binding proteins in Arabidopsis. Plant J. 2000, 24, 837–847. [Google Scholar] [CrossRef]
- Robatzek, S.; Somssich, I.E. A new member of the Arabidopsis WRKY transcription factor family, AtWRKY6, is associated with both senescence-and defence-related processes. Plant J. 2001, 28, 123–133. [Google Scholar] [CrossRef]
- Xie, T.; Chen, C.; Li, C.; Liu, J.; Liu, C.; He, Y. Genome-wide investigation of WRKY gene family in pineapple: Evolution and expression profiles during development and stress. BMC Genom. 2018, 19, 490. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Chen, C.; Chen, Z. Evidence for an Important Role of WRKY DNA Binding Proteins in the Regulation of NPR1 Gene Expression. Plant Cell 2001, 13, 1527–1540. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Brader, G.; Palva, E.T. The WRKY70 Transcription Factor: A Node of Convergence for Jasmonate-Mediated and Salicylate-Mediated Signals in Plant Defense. Plant Cell 2004, 16, 319–331. [Google Scholar] [CrossRef] [PubMed]
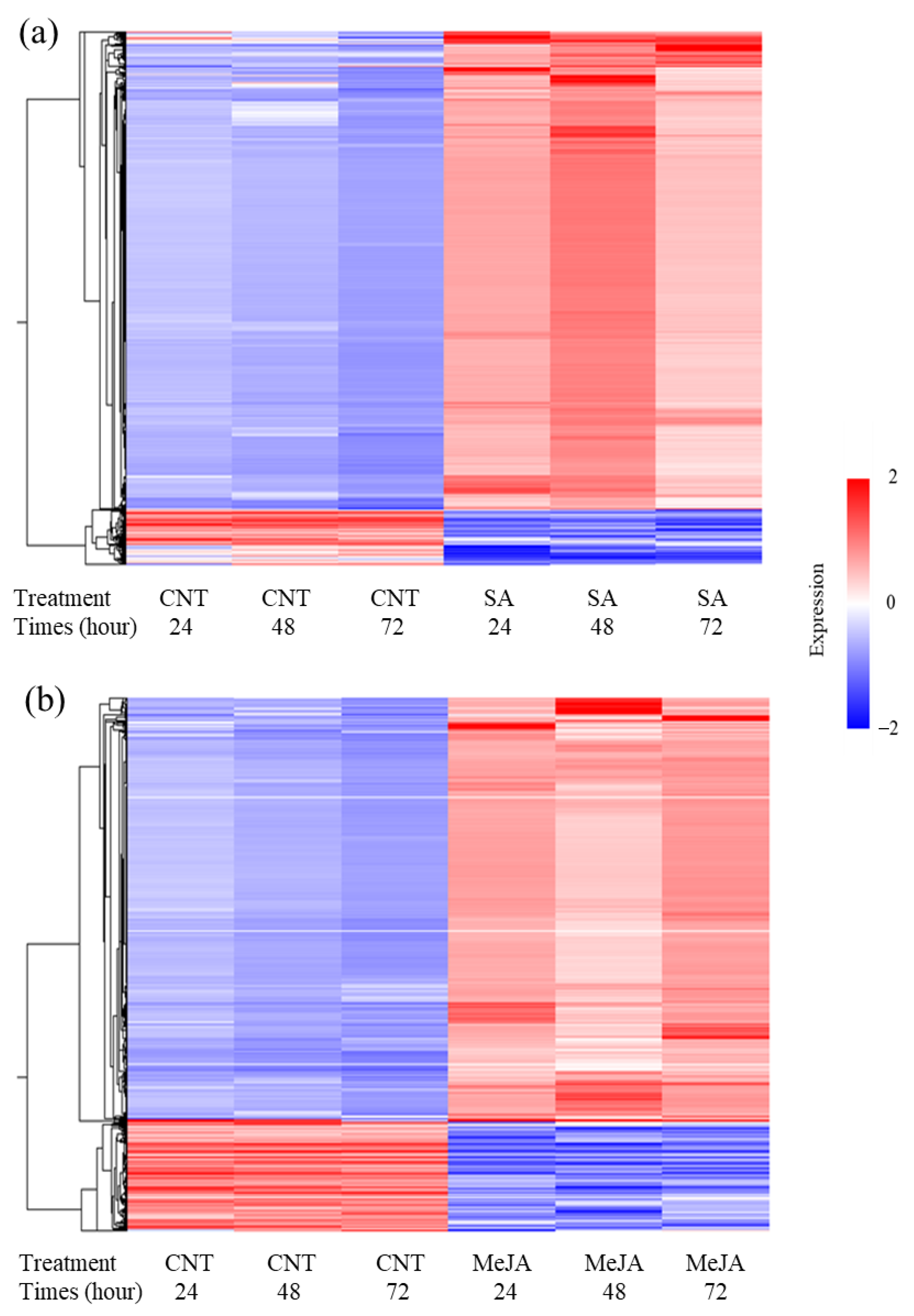
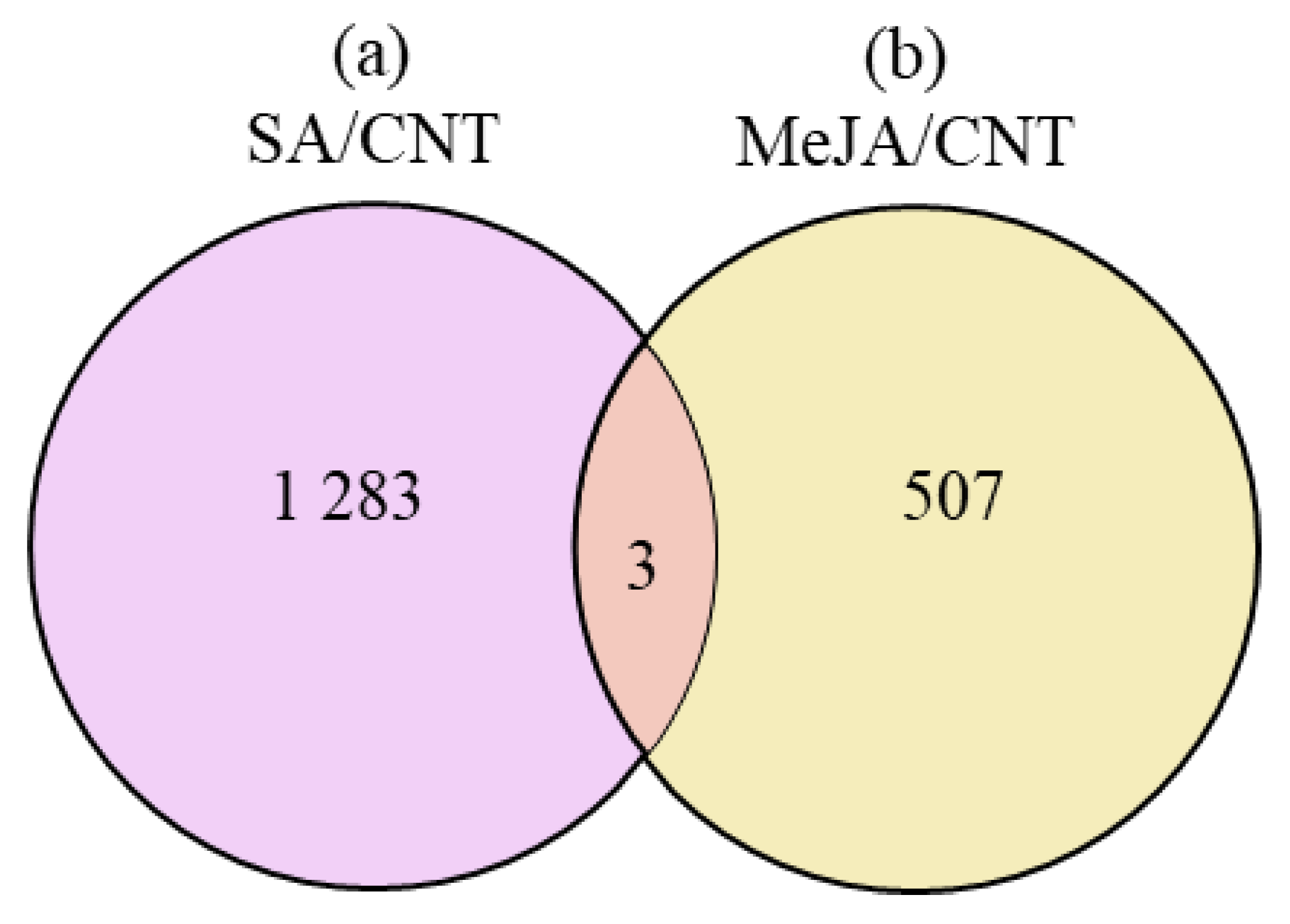
| ID | mRNA | JGI_AGI | Abbreviations | Enzyme | SA/CNT | MeJA/CNT | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |||||
| C22820 | Ciclev10022863m | PROTEIN NIM1-INTERACTING 1 | 2.53 | 2.26 | 4.18 | 0.31 | 0.42 | 0.35 | ||
| C25898 | Ciclev10025958m | AT4G10490.1 | DMR-6 | 2-oxoglutarate (2OG) and Fe (Ⅱ)-dependent | 17.93 | 12.21 | 67.54 | 0.23 | 0.12 | 0.10 |
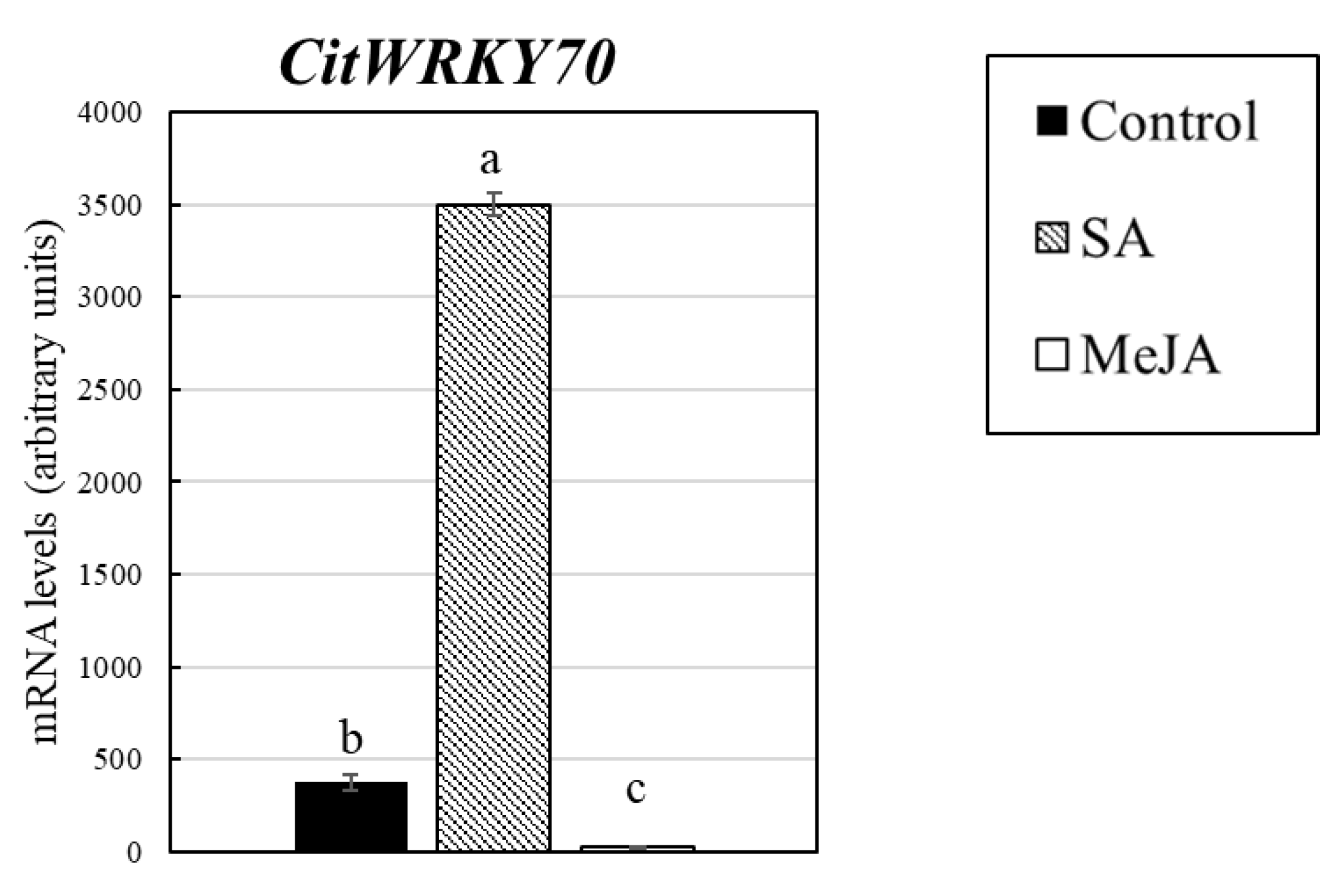
| C12031 | Ciclev10012055m | AT3G56400.1 | WRKY70 | WRKY DNA-binding protein 70 | 6.52 | 5.51 | 10.30 | 0.30 | 0.16 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, R.; Ma, G.; Zhang, L.; Hirai, M.; Yahata, M.; Yamawaki, K.; Shimada, T.; Fujii, H.; Endo, T.; Kato, M. Effects of Salicylic Acid and Methyl Jasmonate Treatments on Flavonoid and Carotenoid Accumulation in the Juice Sacs of Satsuma Mandarin In Vitro. Appl. Sci. 2020, 10, 8916. https://doi.org/10.3390/app10248916
Yamamoto R, Ma G, Zhang L, Hirai M, Yahata M, Yamawaki K, Shimada T, Fujii H, Endo T, Kato M. Effects of Salicylic Acid and Methyl Jasmonate Treatments on Flavonoid and Carotenoid Accumulation in the Juice Sacs of Satsuma Mandarin In Vitro. Applied Sciences. 2020; 10(24):8916. https://doi.org/10.3390/app10248916
Chicago/Turabian StyleYamamoto, Risa, Gang Ma, Lancui Zhang, Miki Hirai, Masaki Yahata, Kazuki Yamawaki, Takehiko Shimada, Hiroshi Fujii, Tomoko Endo, and Masaya Kato. 2020. "Effects of Salicylic Acid and Methyl Jasmonate Treatments on Flavonoid and Carotenoid Accumulation in the Juice Sacs of Satsuma Mandarin In Vitro" Applied Sciences 10, no. 24: 8916. https://doi.org/10.3390/app10248916
APA StyleYamamoto, R., Ma, G., Zhang, L., Hirai, M., Yahata, M., Yamawaki, K., Shimada, T., Fujii, H., Endo, T., & Kato, M. (2020). Effects of Salicylic Acid and Methyl Jasmonate Treatments on Flavonoid and Carotenoid Accumulation in the Juice Sacs of Satsuma Mandarin In Vitro. Applied Sciences, 10(24), 8916. https://doi.org/10.3390/app10248916






