Study of Eco-Friendly Belite-Calcium Sulfoaluminate Cements Obtained from Special Wastes
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Proportioning of CSA Clinker-Generating Raw Mixtures
2.2. Burning of Raw Mixtures
2.3. Preparation of BCSA Cements
2.4. Hydration Procedure
2.5. Characterization Techniques
2.5.1. X-ray Fluorescence Analysis
2.5.2. DT–TG Analysis
2.5.3. XRD Analysis
2.5.4. Isothermal Calorimetry
2.5.5. Mercury Intrusion Porosimetry (MIP)
3. Results and Discussion
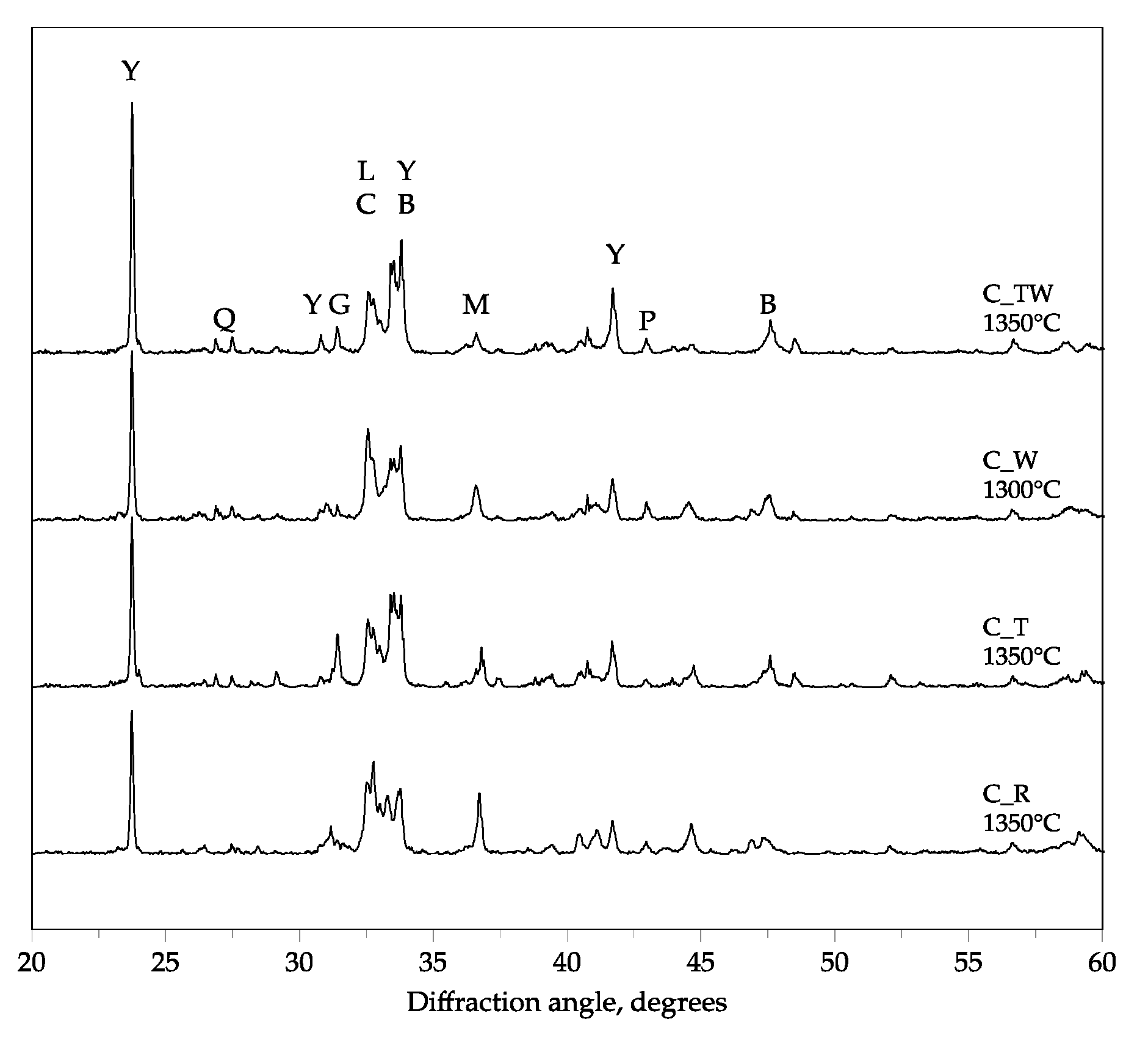
3.1. Synthesis of BCSA Clinkers
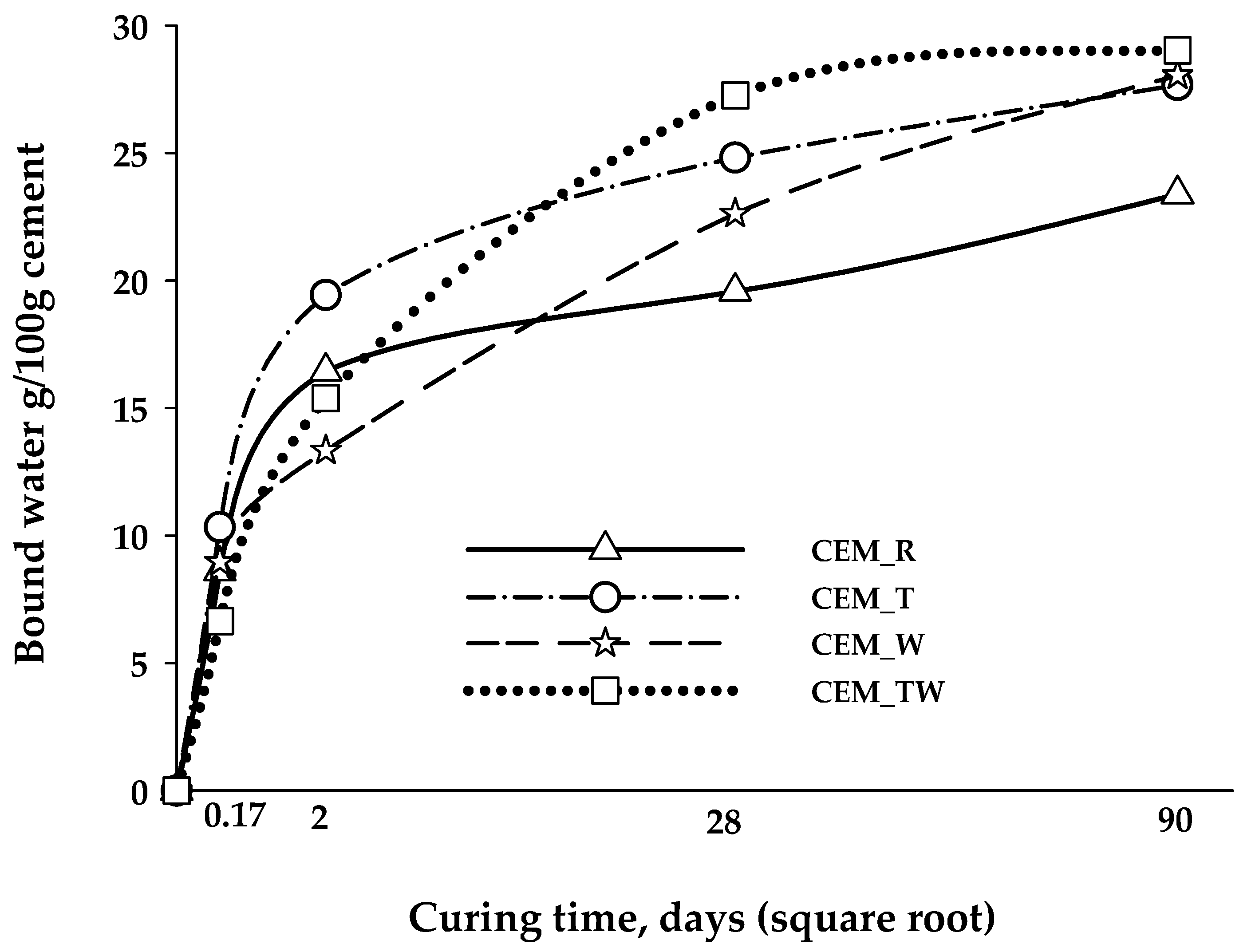
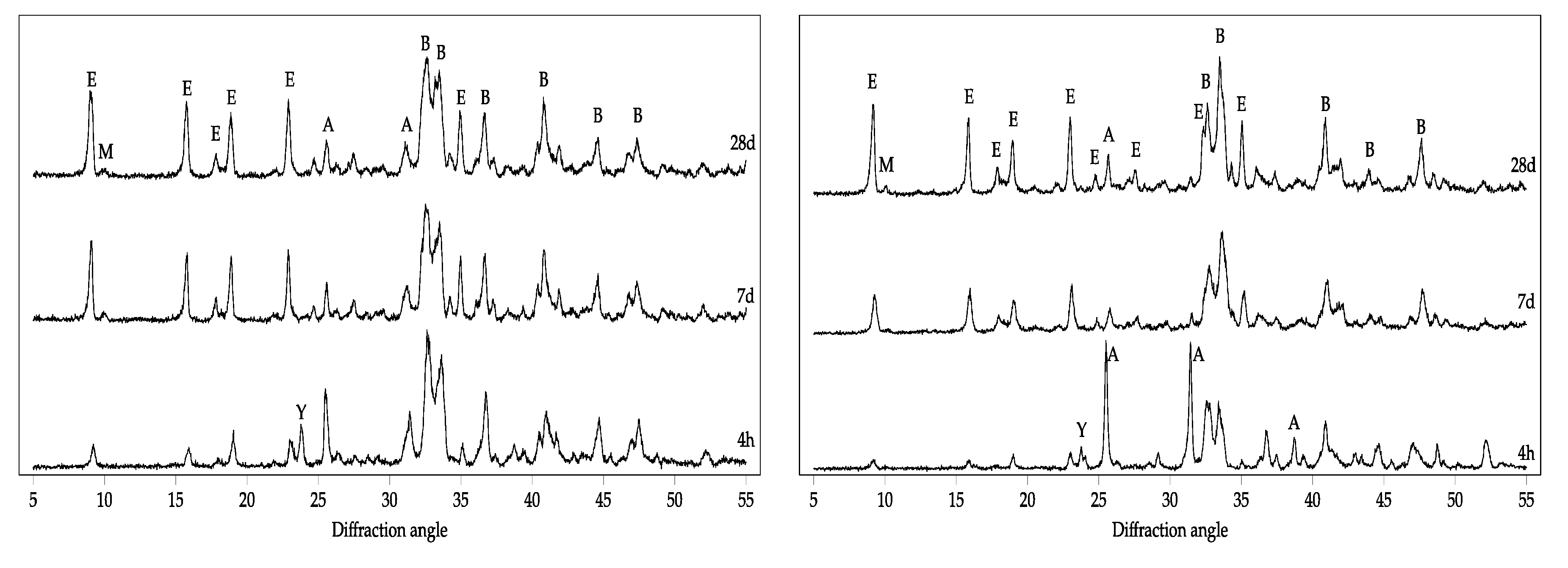
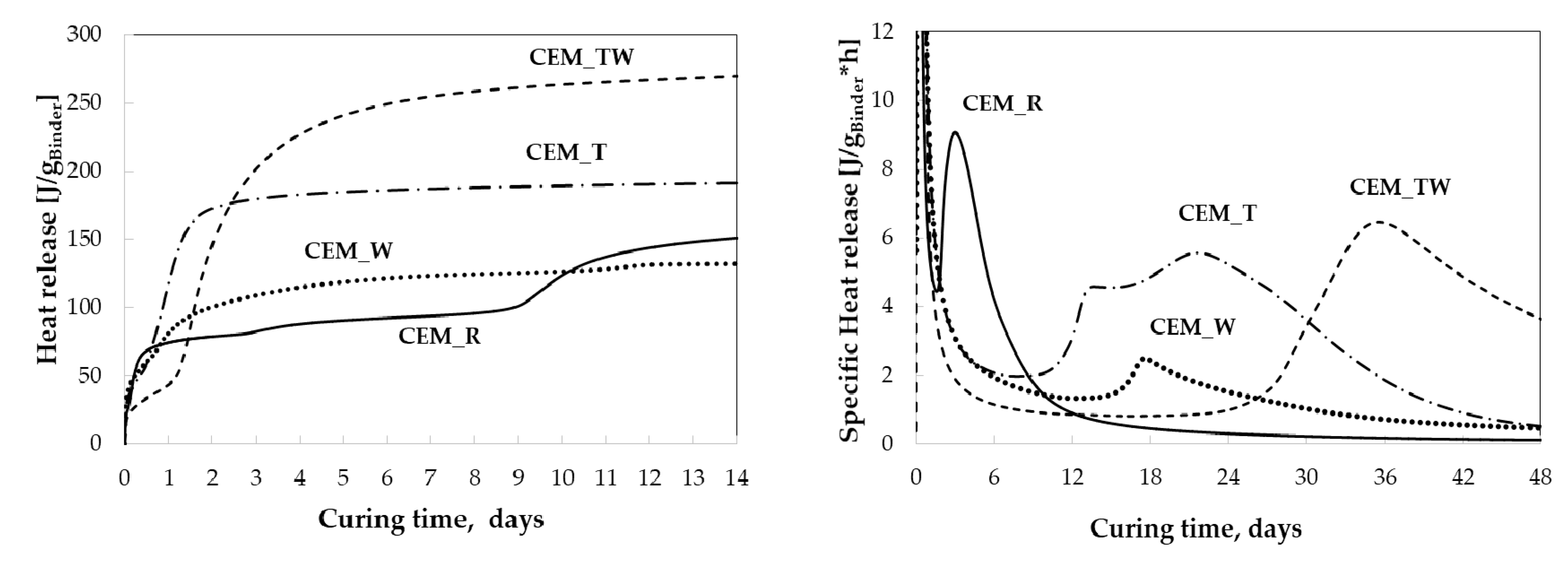
3.2. Hydration of BCSA Cements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Activity Report 2019. The European Cement Association. Available online: https://cembureau.eu/media/clkdda45/activity-report-2019.pdf (accessed on 26 November 2020).
- Tregambi, C.; Solimene, R.; Montagnaro, F.; Salatino, P.; Marroccoli, M.; Ibrid, N.; Telesca, A. Solar-driven production of lime for ordinary Portland cement formulation. Sol. Energy 2018, 173, 759–768. [Google Scholar] [CrossRef]
- Barcelo, L.; Kline, J.; Walenta, G.; Gartner, E. Cement and carbon emissions. Mater. Struct. 2014, 47, 1055–1065. [Google Scholar] [CrossRef]
- Xu, D.; Cui, Y.; Li, H.; Yang, K.; Xu, W.; Chen, Y. On the future of Chinese cement industry. Cem. Concr. Res. 2015, 78, 2–13. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Ibris, N.; Lupiáñez, C.; Díez, L.I.; Romeo, L.M.; Montagnaro, F. Use of oxyfuel combustion ash for the production of blended cements: A synergetic solution toward reduction of CO2 emissions. Fuel Proc. Technol. 2017, 156, 211–220. [Google Scholar] [CrossRef]
- Schneider, M. The cement industry on the way to a low-carbon future. Cem. Concr. Res. 2019, 124, 105792. [Google Scholar] [CrossRef]
- Shi, C.; Qu, B.; Provis, J.L. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Gartner, E.; Sui, T. Alternative cement clinkers. Cem. Concr. Res. 2018, 114, 27–39. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Myers, R.J.; Lothenbach, B.; Bernal, S.A.; Provis, J.L. Thermodynamic modelling of alkali-activated slag cements. Appl. Geochem. 2015, 61, 233–247. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Alkali-Activated Materials: State-of-the-Art Report, RILEM TC 224-AAM.; Springer/RILEM: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Dung, N.T.; Unluer, C. Development of MgO concrete with enhanced hydration and carbonation mechanisms. Cem. Concr. Res. 2018, 103, 160–169. [Google Scholar] [CrossRef]
- Gomez, C.M.; de Oliveira, A.D.S. Chemical phases and microstructural analysis of pastes based on magnesia cement. Constr. Build. Mater. 2018, 188, 615–620. [Google Scholar] [CrossRef]
- Walling, S.A.; Provis, J.L. Magnesia-based cements: A journey of 150 years, and cements for the future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef] [PubMed]
- Mobili, A.; Telesca, A.; Marroccoli, M.; Tittarelli, F. Calcium sulfoaluminate and alkali-activated fly ash cements as alternative to Portland cement: Study on chemical, physical-mechanical, and durability properties of mortars with the same strength class. Constr. Build. Mater. 2020, 246, 118436. [Google Scholar] [CrossRef]
- Ben Haha, M.; Winnefeld, F.; Pisch, A. Advances in understanding ye’elimite-rich cements. Cem. Concr. Res. 2019, 123, 105778. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Tomasulo, M.; Valenti, G.L.; Dieter, H.; Montagnaro, F. Low-CO2 cements from fluidized bed process wastes and other industrial byproducts. Combust. Sci. Technol. 2016, 188, 492–503. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Pace, M.L.; Tomasulo, M.; Valenti, G.L.; Monteiro, P.J.M. A hydration study of various calcium sulfoaluminate cements. Cem. Concr. Comp. 2014, 53, 224–232. [Google Scholar] [CrossRef]
- Irico, S.; Gastaldi, D.; Canonico, F.; Magnacca, G. Investigation of the microstructural evolution of calcium sulfoaluminate cements by thermoporometry. Cem. Concr. Res. 2013, 53, 239–247. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Selçuk, N.; Soner, I.; Selçuk, E. Synthesis of special cement with fluidized bed combustion ashes. Adv. Cem. Res. 2010, 22, 107–113. [Google Scholar] [CrossRef]
- Marroccoli, M.; Pace, M.L.; Telesca, A.; Valenti, G.L. Synthesis of calcium sulfoaluminate cements from Al2O3-rich by-products from aluminium manufacture. In Proceedings of the 2nd International Conference on Sustainable Construction Materials and Technologies, Ancona, Italy, 28–30 June 2010; Zachar, J., Claisse, P., Naik, T.R., Ganjiam, E., Eds.; UWM Center for By-Products Utilization: Milwaukee, WI, USA, 2010. [Google Scholar]
- Marroccoli, M.; Montagnaro, F.; Telesca, A.; Valenti, G.L. Environmental implications of the manufacture of calcium sulfoaluminate-based cements. In Proceedings of the 2nd International Conference on Sustainable Construction Materials and Technologies, Ancona, Italy, 28–30 June 2010; Zachar, J., Claisse, P., Naik, T.R., Ganjiam, E., Eds.; UWM Center for By-Products Utilization: Milwaukee, WI, USA, 2010. [Google Scholar]
- Marroccoli, M.; Montagnaro, F.; Pace, M.L.; Valenti, G.L. Use of fluidized bed combustion ash and other industrial wastes as raw materials for the manufacture of calcium sulphoaluminate cements. In Proceedings of the 20th International Conference on Fluidized Bed Combustion, Xian, China, 18–21 May 2009; Yue, G., Zhang, H., Zhao, C., Luo, Z., Eds.; Springer: Berlin, Heidelberg, Germany, 2009; pp. 389–395. [Google Scholar]
- Chaunsali, P.; Vaishnav, K. Calcium-sulfoaluminate-belite cements: Opportunities and challenges. Indian Concr. J. 2020, 94, 18–25. [Google Scholar]
- Telesca, A.; Marroccoli, M.; Winnefeld, F. Synthesis and characterisation of calcium sulfoaluminate cements produced by different chemical gypsums. Adv. Cem. Res. 2019, 31, 113–123. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Stabler, C.; Bullerjahn, F.; Ben Haha, M.B. Hydration and performance evolution of belite–ye’elimite–ferrite cement. Adv. Cem. Res. 2019, 31, 124–137. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Ibris, N.; Naik, T.R.; Lupiáñez, C.; Díez, L.I.; Romeo, L.M.; Montagnaro, F. Synthesis and characterization of belite calcium sulfoaluminate cements produced by oxyfuel combustion residues. In Proceedings of the 5th Sustainable Construction Materials and Technologies, Kingston upon Thames, UK, 14–17 July 2019; Ganjian, E., Claisse, P., Ghafoori, N., Limbchiya, M., Bagheri, M., Eds.; Volume 2. Code 149940. [Google Scholar]
- Mobili, A.; Belli, A.; Giosué, C.; Telesca, A.; Marroccoli, M.; Tittarelli, F. Calcium sulfoaluminate, geopolimeric, and cementitious mortars for structural applications. Environments 2017, 4, 64. [Google Scholar] [CrossRef]
- Álvarez-Pinazo, G.; Santacruz, I.; Aranda, M.A.G.; De La Torre, Á.G. Hydration of belite–ye’elimite–ferrite cements with different calcium sulfate sources. Adv. Cem. Res. 2016, 28, 529–543. [Google Scholar] [CrossRef]
- Borštnara, M.; Daneuc, N.; Doleneca, S. Phase development and hydration kinetics of belite-calcium sulfoaluminate cements at different curing temperatures. Cer. Int. 2020, 46, 29421–29428. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, J. Utilization of phosphogypsum for sulfate-rich belite sulfoaluminate cement production. Adv. Cem. Res. 2015, 27, 515–525. [Google Scholar] [CrossRef]
- Ma, B.; Li, X.; Shen, X.; Mao, Y.; Huang, H. Enhancing the addition of fly ash from thermal power plants in activated high belite sulfoaluminate cement. Constr. Build. Mater. 2014, 52, 261–266. [Google Scholar] [CrossRef]
- Valenti, G.L.; Marroccoli, M.; Pace, M.L.; Telesca, A. Discussion of the paper ‘Understanding expansion in calcium sulfoaluminate–belite cements’ by Chen, I.A. et al., Cem. Concr. Res. 2012, 42, 51–60. Cem. Concr. Res. 2012, 42, 1555–1559. [Google Scholar] [CrossRef]
- Chen, I.A.; Juenger, M.C. Incorporation of coal combustion residuals into calcium sulfoaluminate-belite cement clinkers. Cem. Concr. Comp. 2012, 34, 893–902. [Google Scholar] [CrossRef]
- Irvin, A.C.; Juenger, M.C.G. Synthesis and hydration of calcium sulfoaluminate-belite cements with varied phase compositions. J. Mat. Sci. 2011, 46, 2568–2577. [Google Scholar] [CrossRef]
- Cuberos, A.J.M.; De La Torre, A.G.; Martin-Sedeno, M.C.; Schollbach, K.; Pollmann, H.; Aranda, M.A.G. Active iron-rich belite sulfoaluminate cements: Clinkering and hydration. Environ. Sci. Technol. 2010, 44, 6855–6862. [Google Scholar] [CrossRef] [PubMed]
- Pimraksa, K.; Hanjitsuwan, S.; Chindaprasirt, P. Synthesis of belite cement from lignite fly ash. Ceram. Int. 2009, 35, 2415–2425. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate-belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Quillin, K. Performance of belite-sulfoaluminate cements. Cem. Concr. Res. 2001, 31, 1341–1349. [Google Scholar] [CrossRef]
- Arjunan, P.; Silsbee, M.R.; Roy, D.M. Sulfoaluminate-belite cement from low-calcium fly ash and sulfur-rich and other industrial by-products. Cem. Concr. Res. 1999, 29, 1305–1311. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Zhang, J.; Zhang, Q.; Wang, Y. Synergistic use of industrial solid wastes to prepare belite-rich sulphoaluminate cement and its feasibility use in repairing materials. Constr. Build. Mater. 2020, 264, 120201. [Google Scholar] [CrossRef]
- Su, D.; Li, Q.; Guo, Y.; Yue, G.; Wang, L. Effect of residual CaSO4 in clinker on properties of high belite sulfoaluminate cement based on solid wastes. Materials 2020, 13, 429. [Google Scholar] [CrossRef]
- Galluccio, S.; Beirau, T.; Pollmann, H. Maximization of the reuse of industrial residues for the production of eco-friendly CSA-belite clinker. Constr. Build. Mater. 2019, 208, 250–257. [Google Scholar] [CrossRef]
- Kiventerä, J.; Piekkari, K.; Isteri, V.; Ohenoja, K.; Tanskanen, P.; Illikainen, M. Solidification/stabilization of gold mine tailings using calcium sulfoaluminate-belite cement. J. Clean. Prod. 2019, 239, 118008. [Google Scholar] [CrossRef]
- Julphunthong, P. Synthesizing of calcium sulfoaluminate-belite (CSAB) cements from industrial waste materials. Mater. Today Proc. 2018, 5, 14933–14938. [Google Scholar] [CrossRef]
- Rungchet, A.; Poon, C.S.; Chindaprasirt, P.; Pimraksa, K. Synthesis of low-temperature calcium sulfoaluminate-belite cements from industrial wastes and their hydration: Comparative studies between lignite fly ash and bottom ash. Cem. Concr. Comp. 2017, 83, 10–19. [Google Scholar] [CrossRef]
- El-Alfi, E.A.; Gado, R.A. Preparation of calcium sulfoaluminate-belite cement from marble sludge waste. Constr. Build. Mater. 2016, 113, 764–772. [Google Scholar] [CrossRef]
- Xue, P.; Xu, A.; He, D.; Yang, Q.; Liu, G.; Engstrom, F.; Bjorkman, B. Research on the sintering process and characteristics of belite sulphoaluminate cement produced by BOF slag. Constr. Build. Mater. 2016, 122, 567–576. [Google Scholar] [CrossRef]
- Rungchet, A.; Chindaprasirt, P.; Wansom, S.; Pimraksa, K. Hydrothermal synthesis of calcium sulfoaluminate-belite cement from industrial waste materials. J. Clean. Prod. 2016, 115, 273–283. [Google Scholar] [CrossRef]
- Senff, L.; Castela, A.; Hajjaji, W.; Hotza, D.; Labrincha, J.A. Formulations of sulfobelite cement through design of experiments. Constr. Build. Mater. 2011, 25, 3410–3416. [Google Scholar] [CrossRef]
- Ferone, C.; Capasso, I.; Bonati, A.; Roviello, G.; Montagnaro, F.; Santoro, L.; Turco, R.; Cioffi, R. Sustainable management of water potabilization sludge by means of geopolymers production. J. Clean. Prod. 2019, 229, 1–9. [Google Scholar] [CrossRef]
- Ahmad, T.; Ahmad, K.; Alam, M. Sustainable management of water treatment sludge through 3′R’ concept (Review). J. Clean. Prod. 2019, 124, 1–13. [Google Scholar] [CrossRef]
- CEN. EN 196-2. Method of Testing Cement-Part 2: Chemical Analysis of Cement; CEN: Brussels, Belgium, 2013. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford: London, UK, 1997. [Google Scholar]
- Bernardo, G.; Telesca, A.; Valenti, G.L. A porosimetric study of calcium sulfoaluminate cement pastes cured at early ages. Cem. Concr. Res. 2006, 36, 1042–1047. [Google Scholar] [CrossRef]
- Winslow, D.N.; Diamond, S. A mercury porosimetry study of the evolution of porosity in Portland cement. ASTM J. Mater. 1970, 3, 564–585. [Google Scholar]
- Garboczi, E.J. Permeability, diffusivity, and microstructural parameters: A critical review. Cem. Concr. Res. 1990, 20, 591–601. [Google Scholar] [CrossRef]




| L | B | G | C | T | W | |
|---|---|---|---|---|---|---|
| CaO | 55.20 | - | 34.08 | 12.20 | 30.89 | 4.95 |
| SiO2 | - | 7.20 | 0.51 | 54.60 | 1.00 | 37.71 |
| Al2O3 | - | 56.30 | 0.71 | 10.00 | 0.52 | 27.52 |
| Fe2O3 | - | 6.30 | 0.30 | 3.70 | 2.24 | 2.37 |
| SO3 | - | - | 44.37 | - | 40.13 | 0.53 |
| Others | 1.40 | 2.70 | 0.81 | 5.20 | 3.54 | 4.70 |
| l.o.i. * | 43.40 | 27.50 | 19.02 | 14.30 | 21.82 | 22.12 |
| L | B | G | C | T | W | |
|---|---|---|---|---|---|---|
| RM_R | 55.0 | 13.0 | 9.0 | 23.0 | - | - |
| RM_T | 55.0 | 12.0 | - | 23.0 | 10.0 | - |
| RM_W | 55.0 | - | 9.0 | - | - | 36.0 |
| RM_TW | 55.0 | - | - | - | 10.0 | 35.0 |
| C2S | C4A3$ | C4AF | C$ | Cf | |
|---|---|---|---|---|---|
| C_R | 57.5 | 25.4 | 7.6 | 4.4 | 1.6 |
| C_T | 57.6 | 23.5 | 8.3 | 4.9 | 1.9 |
| C_W | 58.8 | 28.2 | 4.0 | 4.4 | 0.7 |
| C_WT | 56.4 | 27.1 | 3.9 | 5.6 | - |
| CEM_R | CEM_T | CEM_W | CEM_TW | |
|---|---|---|---|---|
| BCSA clinker | 93.1 | 92.5 | 90.8 | 88.6 |
| Anhydrite | 6.9 | 7.5 | 9.2 | 11.4 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 |
| β-C2S | C4A3$ * | C3A | C4AF | Others | |
|---|---|---|---|---|---|
| C_R | |||||
| 1200 °C | 47.2 | 19.7 | 9.1 | 0.9 | 23.1 |
| 1250 °C | 42.0 | 16.7 | 10.4 | 3.4 | 27.5 |
| 1300 °C | 54.3 | 16.7 | 9.4 | 3.9 | 15.7 |
| 1350 °C | 60.0 | 16.7 | 10.9 | 4.4 | 8.0 |
| C_T | |||||
| 1200 °C | 42.3 | 16.7 | 10.2 | 3.2 | 27.6 |
| 1250 °C | 38.1 | 18.8 | 11.0 | 5.7 | 26.4 |
| 1300 °C | 40.4 | 20.7 | 8.6 | 6.7 | 23.6 |
| 1350 °C | 53.3 | 19.7 | 5.3 | 8.1 | 13.6 |
| C_W | |||||
| 1200 °C | 42.8 | 22.0 | 11.0 | 2.0 | 22.2 |
| 1250 °C | 45.6 | 22.6 | 10.9 | 3.1 | 17.8 |
| 1300 °C | 54.3 | 22.8 | 10.6 | 6.6 | 5.7 |
| 1350 °C | 46.9 | 24.5 | 9.6 | 4.8 | 14.2 |
| C_TW | |||||
| 1200 °C | 44.5 | 19.7 | 8.6 | 0.8 | 26.4 |
| 1250 °C | 34.6 | 30.5 | 15.2 | 4.7 | 15.0 |
| 1300 °C | 36.8 | 29.9 | 14.1 | 5.1 | 14.1 |
| 1350 °C | 45.7 | 28.9 | 13.8 | 9.0 | 12.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Telesca, A.; Matschei, T.; Marroccoli, M. Study of Eco-Friendly Belite-Calcium Sulfoaluminate Cements Obtained from Special Wastes. Appl. Sci. 2020, 10, 8650. https://doi.org/10.3390/app10238650
Telesca A, Matschei T, Marroccoli M. Study of Eco-Friendly Belite-Calcium Sulfoaluminate Cements Obtained from Special Wastes. Applied Sciences. 2020; 10(23):8650. https://doi.org/10.3390/app10238650
Chicago/Turabian StyleTelesca, Antonio, Thomas Matschei, and Milena Marroccoli. 2020. "Study of Eco-Friendly Belite-Calcium Sulfoaluminate Cements Obtained from Special Wastes" Applied Sciences 10, no. 23: 8650. https://doi.org/10.3390/app10238650
APA StyleTelesca, A., Matschei, T., & Marroccoli, M. (2020). Study of Eco-Friendly Belite-Calcium Sulfoaluminate Cements Obtained from Special Wastes. Applied Sciences, 10(23), 8650. https://doi.org/10.3390/app10238650






