Biomechanical Particularities in the Therapy of the Rheumatic Knee
Abstract
1. Introduction
2. Cellular and Molecular Mechanisms of Joint Manifestation
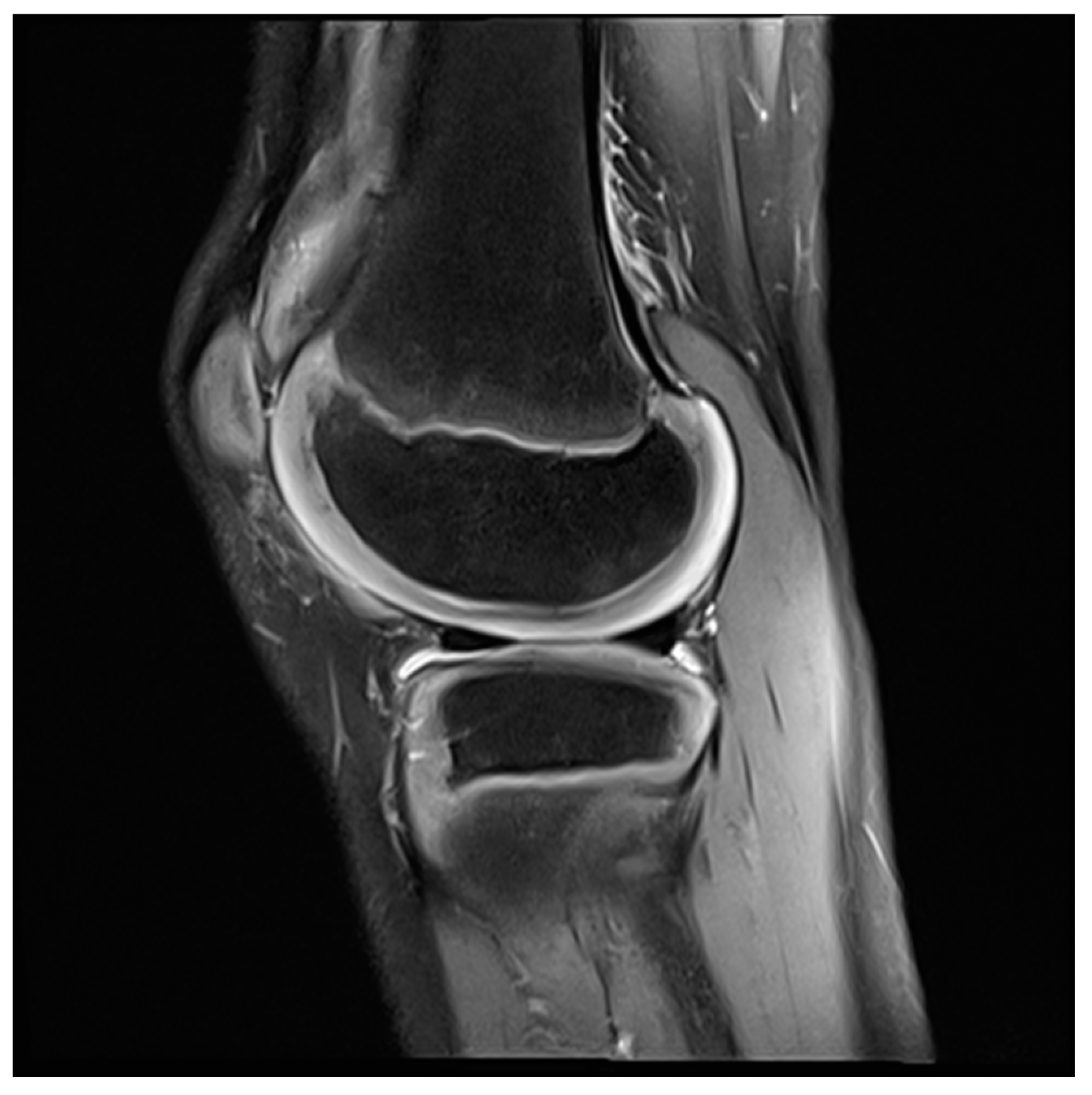
3. Biomechanics and Clinic of the Rheumatic Knee
4. Conservative Therapy
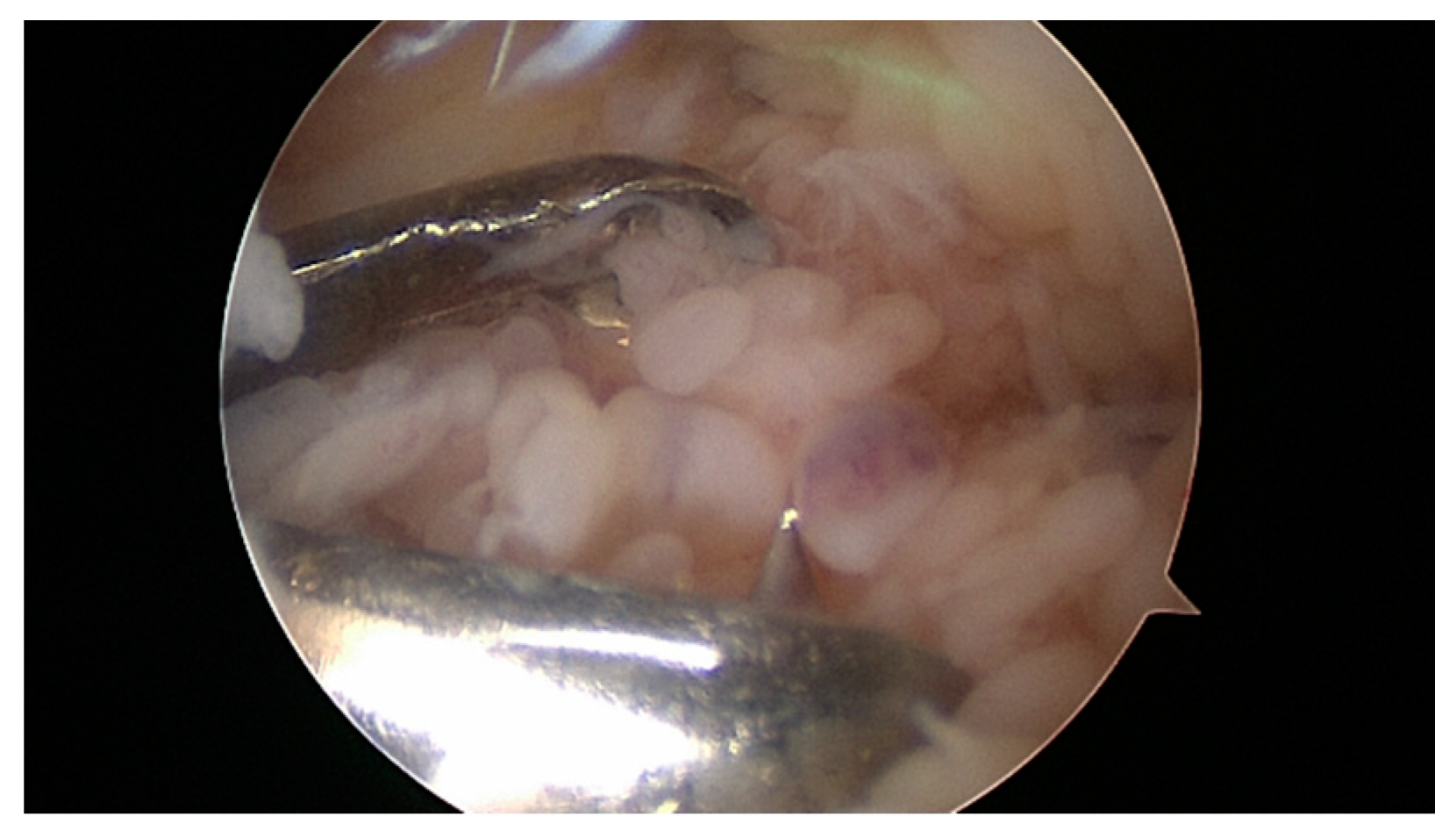
5. Surgical Therapy
6. Joint Replacement Therapy/Endo-Prosthetic Treatment
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rehart, S.; Sell, S.; Arbogast, M.; Aringer, M.; Arnold, I. Expertise Orthopädische Rheumatologie; Georg Thieme Verlag: New York, NY, USA, 2015. [Google Scholar]
- Chen, Z.; Bozec, A.; Ramming, A.; Schett, G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat. Rev. Rheumatol. 2019, 15, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Zafar, A.; Moin, S.; Khan, A.Q.; Zubair, S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin. Chim. Acta 2016, 455, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Baboolal, T.G.; Mastbergen, S.C.; Jones, E.; Calder, S.J.; Lafeber, F.P.; McGonagle, D. Synovial fluid hyaluronan mediates MSC attachment to cartilage, a potential novel mechanism contributing to cartilage repair in osteoarthritis using knee joint distraction. Ann. Rheum. Dis. 2016, 75, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Gaulke, R. Orthopädisch-Rheumatologische Jahresuntersuchung (ORJ). Z. Orthopädie Unf. 2018, 156, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A.; Dale, K.; Eek, M. Radiographic Evaluation of Rheumatoid Arthritis and Related Conditions by Standard Reference Films. Acta Radiol. Diagn. 1977, 18, 481–491. [Google Scholar] [CrossRef]
- Biehl, C.; Thormann, U.; Heiß, C. Complications in Knee Surgery of Rheumatic Patients. Aktuelle Rheumatol. 2016, 43, 73–81. [Google Scholar] [CrossRef]
- Schöniger, A.; Henniger, M.; Rehart, S. Gelenkinfektionen in der orthopädischen Rheumatologie. OUP 2013, 2, 396–399. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- De Lange-Brokaar, B.J.; Kloppenburg, M.; Andersen, S.N.; Dorjée, A.L.; Yusuf, E.; Herb-van Toorn, L.; Kroon, H.M.; Zuurmond, A.M.; Stojanovic-Susulic, V.; Bloem, J.L.; et al. Characterization of synovial mast cells in knee osteoarthritis: Association with clinical parameters. Osteoarthr. Cartil. 2016, 24, 664–671. [Google Scholar] [CrossRef]
- Ouboussad, L.; Burska, A.N.; Melville, A.; Buch, M.H. Synovial Tissue Heterogeneity in Rheumatoid Arthritis and Changes With Biologic and Targeted Synthetic Therapies to Inform Stratified Therapy. Front. Med. 2019, 6, 45. [Google Scholar] [CrossRef]
- Cecchi, I.; De La Rosa, I.A.; Menegatti, E.; Roccatello, D.; Collantes-Estevez, E.; Lopez-Pedrera, C.; Barbarroja, N. Neutrophils: Novel key players in Rheumatoid Arthritis. Current and future therapeutic targets. Autoimmun. Rev. 2018, 17, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, M.J.; Nissim, A.; Knight, A.R.; Whiteman, M.; Haigh, R.; Winyard, P.G. Oxidative stress in autoimmune rheumatic diseases. Free Radic. Biol. Med. 2018, 125, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Veselinovic, M.; Barudzic, N.; Vuletic, M.; Zivkovic, V.; Tomic-Lucic, A.; Djuric, D.; Jakovljević, V. Oxidative stress in rheumatoid arthritis patients: Relationship to diseases activity. Mol. Cell. Biochem. 2014, 391, 225–232. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Anzaghe, M.; Schülke, S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells 2020, 9, 880. [Google Scholar] [CrossRef]
- Najm, A.; Le Goff, B.; Orr, C.; Thurlings, R.; Canete, J.D.; Humby, F.; Alivernini, S.; Manzo, A.; Just, S.A.; Romao, V.C.; et al. Standardisation of synovial biopsy analyses in rheumatic diseases: A consensus of the EULAR Synovitis and OMERACT Synovial Tissue Biopsy Groups. Arthritis Res. Ther. 2018, 20, 265. [Google Scholar] [CrossRef]
- Carmona-Rivera, C.; Carlucci, P.M.; Moore, E.; Lingampalli, N.; Uchtenhagen, H.; James, E.; Liu, Y.; Bicker, K.L.; Wahamaa, H.; Hoffmann, V.; et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci. Immunol. 2017, 2, eaag3358. [Google Scholar] [CrossRef]
- Hirose, J.; Nishioka, H.; Tsukano, M.; Matsubara, S.; Usuku, K.; Mizuta, H. Matrix changes in articular cartilage in the knee of patients with rheumatoid arthritis after biological therapy: 1-year follow-up evaluation by T2 and T1ρ MRI quantification. Clin. Radiol. 2018, 73, 984.e911–984.e918. [Google Scholar] [CrossRef]
- Bottini, N.; Firestein, G.S. Duality of fibroblast-like synoviocytes in RA: Passive responders and imprinted aggressors. Nat. Rev. Rheumatol. 2013, 9, 24–33. [Google Scholar] [CrossRef]
- Harre, U.; Schett, G. Association between bone catabolism and anti-citrullinated protein antibodies in rheumatoid arthritis. Z. Rheumatol. 2016, 75, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.S.; Cush, J.J.; Schulze-Koops, H.; Lipsky, P.E. Rheumatoid synovial CD4+ T cells exhibit a reduced capacity to differentiate into IL-4-producing T-helper-2 effector cells. Arthritis Res. 2001, 3, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Conigliaro, P.; Triggianese, P.; De Martino, E.; Fonti, G.L.; Chimenti, M.S.; Sunzini, F.; Viola, A.; Canofari, C.; Perricone, R. Challenges in the treatment of Rheumatoid Arthritis. Autoimmun. Rev. 2019, 18, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Evangelatos, G.; Fragoulis, G.E.; Koulouri, V.; Lambrou, G.I. MicroRNAs in rheumatoid arthritis: From pathogenesis to clinical impact. Autoimmun. Rev. 2019, 18, 102391. [Google Scholar] [CrossRef]
- Sardar, R.; Satish, D.; Gupta, D. Identification of Novel SARS-CoV-2 Drug Targets by Host MicroRNAs and Transcription Factors Co-regulatory Interaction Network Analysis. Front. Genet. 2020, 11, 571274. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Diller, M.; Hasseli, R.; Hülser, M.-L.; Aykara, I.; Frommer, K.; Rehart, S.; Müller-Ladner, U.; Neumann, E. Targeting Activated Synovial Fibroblasts in Rheumatoid Arthritis by Peficitinib. Front. Immunol. 2019, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Meireles, S.M.; Oliveira, L.M.; Andrade, M.S.; Silva, A.C.; Natour, J. Isokinetic evaluation of the knee in patients with rheumatoid arthritis. Jt. Bone Spine 2002, 69, 566–573. [Google Scholar] [CrossRef]
- Weiss, R.J.; Wretenberg, P.; Stark, A.; Palmblad, K.; Larsson, P.; Grondal, L.; Broström, E. Gait pattern in rheumatoid arthritis. Gait Posture 2008, 28, 229–234. [Google Scholar] [CrossRef]
- Van Der Straaten, R.; Wesseling, M.; Jonkers, I.; Vanwanseele, B.; Bruijnes, A.; Malcorps, J.; Bellemans, J.; Truijen, J.; De Baets, L.; Timmermans, A. Functional movement assessment by means of inertial sensor technology to discriminate between movement behaviour of healthy controls and persons with knee osteoarthritis. J. Neuroeng. Rehabil. 2020, 17, 65. [Google Scholar] [CrossRef]
- Kawamura, K.; Momohara, S.; Tomatsu, T. Alignment of lower extremity in rheumatoid arthritis patients with a history of both total hip replacement and total knee replacement. Ryumachi 2003, 43, 638–643. [Google Scholar]
- Imagama, T.; Tokushige, A.; Seki, K.; Taguchi, T. Weight Bearing Joints Destruction in Rheumatoid Arthritis. Curr. Rheumatol. Rev. 2017, 13, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Tittinger, T.; Słoniak, R.; Szczepański, D.; Gaździk, T.S.; Kulesa-Mrowiecka, M.; Kikowski, Ł. Lateral instability of the knee joint and disorder of the ankle joint extension disorder in men. Wiad. Lek. 2019, 72, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, J.; Kasten, P. Physiologie der Sehnenheilung. Orthopädie Unf. Up2date 2015, 10, 75–87. [Google Scholar] [CrossRef]
- Biehl, C.; Kappl, S.; Rehart, S. Operative Therapie an den Gelenken bei der Spondyloarthritis. Aktuelle Rheumatol. 2013, 38, 104–108. [Google Scholar] [CrossRef]
- Goodman, S.M.; Springer, B.; Guyatt, G.; Abdel, M.P.; Dasa, V.; George, M.D.; Gewurz-Singer, O.; Giles, J.T.; Johnson, B.; Lee, S.; et al. 2017 American College of Rheumatology/American Association of Hip and Knee Surgeons Guideline for the Perioperative Management of Antirheumatic Medication in Patients With Rheumatic Diseases Undergoing Elective Total Hip or Total Knee Arthroplasty. Arthritis Rheumatol. 2017, 69, 1538–1551. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Prim. 2018, 4, 18001. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; Van Vollenhoven, R.F.; De Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 Update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef]
- Goodman, S.M.; George, M.D. Should we stop or continue conventional synthetic (including glucocorticoids) and targeted DMARDs before surgery in patients with inflammatory rheumatic diseases? RMD Open 2020, 6, e001214. [Google Scholar] [CrossRef]
- George, M.D.; Baker, J.F. Perioperative management of immunosuppression in patients with rheumatoid arthritis. Curr. Opin. Rheumatol. 2019, 31, 300–306. [Google Scholar] [CrossRef]
- Yeganeh, M.H.; Kheir, M.M.; Shahi, A.; Parvizi, J. Rheumatoid Arthritis, Disease Modifying Agents, and Periprosthetic Joint Infection: What Does a Joint Surgeon Need to Know? J. Arthroplast. 2018, 33, 1258–1264. [Google Scholar] [CrossRef]
- Krüger, K.; Der Dgrh, K.P.; Albrecht, K.; Rehart, S.; Scholz, R. Empfehlungen der Deutschen Gesellschaft für Rheumatologie zur perioperativen Vorgehensweise unter Therapie mit DMARD und Biologicals bei entzündlich-rheumatischen Erkrankungen. Z. Rheumatol. 2014, 73, 77–84. [Google Scholar] [CrossRef]
- Kostuj, T.; Rehart, S.; Matta-Hurtado, R.; Biehl, C.; Willburger, R.E.; Schmidt, K. Pilot study for the registry of complications in rheumatic diseases from the German Society of Surgery (DGORh): Evaluation of methods and data from the first 1000 patients. BMJ Open 2017, 7, e015987. [Google Scholar] [CrossRef]
- Kobayashi, S.; Niki, Y.; Harato, K.; Nagura, T.; Nakamura, M.; Matsumoto, M. Rheumatoid Arthritis Patients Achieve Better Satisfaction but Lower Functional Activities as Compared to Osteoarthritis Patients After Total Knee Arthroplasty. J. Arthroplast. 2019, 34, 478–482.e1. [Google Scholar] [CrossRef] [PubMed]
- Rahimnia, A.; Alishiri, G.; Bayatpoor, M.E.; Hosseini, M.A.; Najafizadeh-Sari, S.; Yaribeygi, H.; Sahebkar, A. Evaluation of Disease Severity and Health-Related Quality of Life in Patients with Rheumatoid Arthritis Undergoing Total Knee Arthroplasty. Curr. Rheumatol. Rev. 2020, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Jaiswal, K.S.; Gupta, B. Managing Rheumatoid Arthritis with Dietary Interventions. Front. Nutr. 2017, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Siracusa, R.; Impellizzeri, D.; D’Amico, R.; Peritore, A.F.; Crupi, R.; Gugliandolo, E.; Fusco, R.; Di Paola, R.; Schievano, C.; et al. Safety and efficacy of a new micronized formulation of the ALIAmide palmitoylglucosamine in preclinical models of inflammation and osteoarthritis pain. Arthritis Res. 2019, 21, 254. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, V.; Yakoub, K.M.; Caruso, G.; Lazzarino, G.; Signoretti, S.; Barbey, A.K.; Tavazzi, B.; Lazzarino, G.; Belli, A.; Amorini, A.M. Antioxidant Therapies in Traumatic Brain Injury. Antioxidants 2020, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Dudics, S.; Langan, D.; Meka, R.R.; Venkatesha, S.H.; Berman, B.M.; Che, C.-T.; Moudgil, K.D. Natural Products for the Treatment of Autoimmune Arthritis: Their Mechanisms of Action, Targeted Delivery, and Interplay with the Host Microbiome. Int. J. Mol. Sci. 2018, 19, 2508. [Google Scholar] [CrossRef]
- Izzi, V.; Masuelli, L.; Tresoldi, I.; Sacchetti, P.; Modesti, A.; Galvano, F.; Bei, R. The effects of dietary flavonoids on the regulation of redox inflammatory networks. Front. Biosci. Landmark Ed. 2012, 17, 2396–2418. [Google Scholar] [CrossRef]
- Bang, J.S.; Oh, D.H.; Choi, H.M.; Sur, B.J.; Lim, S.J.; Kim, J.Y.; Yang, H.I.; Yoo, M.C.; Hahm, D.H.; Kim, K.S. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1β-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res. Ther. 2009, 11, R49. [Google Scholar] [CrossRef]
- Richardson, D.; Pearson, R.G.; Kurian, N.; Latif, M.L.; Garle, M.J.; Barrett, D.A.; Kendall, D.A.; Scammell, B.E.; Reeve, A.J.; Chapman, V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, R43. [Google Scholar] [CrossRef] [PubMed]
- Osthoff, A.K.R.; Niedermann, K.; Braun, J.; Adams, J.; Brodin, N.; Dagfinrud, H.; Duruoz, T.; Esbensen, B.A.; Günther, K.-P.; Hurkmans, E.; et al. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann. Rheum. Dis. 2018, 77, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Viggiani, M.T.; Anelli, M.G.; Fanizzi, R.; Lorusso, O.; Lopalco, G.; Cantarini, L.; Di Leo, A.; Lapadula, G.; Iannone, F. Sarcopenia in Patients with Rheumatic Diseases: Prevalence and Associated Risk Factors. J. Clin. Med. 2018, 7, 504. [Google Scholar] [CrossRef]
- Ngeuleu, A.; Allali, F.; Medrare, L.; Madhi, A.; Rkain, H.; Hajjaj-Hassouni, N. Sarcopenia in rheumatoid arthritis: Prevalence, influence of disease activity and associated factors. Rheumatol. Int. 2017, 37, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.; Kosz, M.; Schwarting, A. Physical activity, exercise and nutrition in rheumatism: Adjuvant treatment options for inflammatory-rheumatic diseases. Orthopade 2019, 48, 917–926. [Google Scholar] [CrossRef]
- McMeeken, J.; Stillman, B.; Story, I.; Kent, P.; Smith, J. The effects of knee extensor and flexor muscle training on the timed-up-and-go test in individuals with rheumatoid arthritis. Physiother. Res. Int. 1999, 4, 55–67. [Google Scholar] [CrossRef]
- Oda, R.; Fujiwara, H.; Tokunaga, D.; Nakamura, S.; Taniguchi, D.; Kawahito, Y.; Seno, T.; Matsui, T.; Kubo, T. How do anti-TNF therapies affect gait function in patients with rheumatoid arthritis? Int. J. Rheum. Dis. 2013, 17, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Pu, D.; Luo, J.; Wang, Y.; Ju, B.; Lv, X.; Fan, P.; He, L. Prevalence of depression and anxiety in rheumatoid arthritis patients and their associations with serum vitamin D level. Clin. Rheumatol. 2018, 37, 179–184. [Google Scholar] [CrossRef]
- Bauer, N.B.; Khassawna, T.E.; Goldmann, F.; Stirn, M.; Ledieu, D.; Schlewitz, G.; Govindarajan, P.; Zahner, D.; Weisweiler, D.; Schliefke, N.; et al. Characterization of bone turnover and energy metabolism in a rat model of primary and secondary osteoporosis. Exp. Toxicol. Pathol. 2015, 67, 287–296. [Google Scholar] [CrossRef]
- Dusad, A.; Pedro, S.; Mikuls, T.R.; Hartman, C.W.; Garvin, K.L.; O’Dell, J.R.; Michaud, K. Impact of Total Knee Arthroplasty as Assessed Using Patient-Reported Pain and Health-Related Quality of Life Indices: Rheumatoid Arthritis Versus Osteoarthritis. Arthritis Rheumatol. 2015, 67, 2503–2511. [Google Scholar] [CrossRef]
- Lipina, M.; Makarov, M.; Mukhanov, V.; Karpashevich, A.; Maglevaniy, S.; Amirdjaпova, V.; Archipov, S. Arthroscopic synovectomy of the knee joint for rheumatoid arthritis. Int. Orthop. 2019, 43, 1859–1863. [Google Scholar] [CrossRef] [PubMed]
- Kerschbaumer, F.; Kandziora, F.; Herresthal, J.; Hertel, A.; Hör, G. Combined arthroscopic and radiation synovectomy in rheumatoid arthritis. Orthopade 1998, 27, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Maderbacher, G.; Greimel, F.; Schaumburger, J.; Grifka, J.; Baier, C. The knee joint in rheumatoid arthritis-current orthopaedic surgical treatment options. Z. Rheumatol. 2018, 77, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, P.N.; Sherman, S.L.; Raphael, B.S.; Su, E.P. Rheumatoid Synovectomy: Does the Surgical Approach Matter? Clin. Orthop. Relat. Res. 2011, 469, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Beil, F.T.; Ruther, W. Indications and contraindications for radiosynoviorthesis. Z. Rheumatol. 2015, 74, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Carl, H.D.; Swoboda, B. Effectiveness of arthroscopic synovectomy in rheumatoid arthritis. Z. Rheumatol. 2008, 67, 485–490. [Google Scholar] [CrossRef]
- Wirth, C.J.; Kerschbaumer, F.; Weise, K.U. Operative Zugangswege in Orthopädie und Traumatologie: Begründet von Rudolf Bauer, Fridun Kerschbaumer und Sepp Poisel; Thieme: New York, NY, USA, 2013. [Google Scholar]
- Krenn, V.; Morawietz, L.; Burmester, G.R.; Haupl, T. Synovialitis score: Histopathological grading system for chronic rheumatic and non-rheumatic synovialitis. Z. Rheumatol. 2005, 64, 334–342. [Google Scholar] [CrossRef]
- Carl, H.D.; Gelse, K.; Swoboda, B. Total knee arthroplasty for rheumatoid arthritis. Z. Rheumatol. 2011, 70, 411–416. [Google Scholar] [CrossRef]
- Mirza, R.; Ishaq, S.; Khan, M.O.; Memon, A. Rituximab therapy for flare-up of rheumatoid arthritis after total knee replacement surgery. J. Pak. Med. Assoc. 2012, 62, 1120–1123. [Google Scholar]
- Dierkes, B.; Oda, A.; Thabe, H. [Long- and medium-term results after unicondylar prosthesis at the knee joint in patients with RA.]. In Proceedings of the 37th Congress of the German Society of Rheumatology, Cologne, Germany, 23–26 September 2009. [Google Scholar]
- Thabe, H.; Brackertz, D. Praktische Rheumaorthopädie; Chapman and Hall: London, UK, 1997. [Google Scholar]
- Wang, W.; Niu, D. Balancing of soft tissues in total knee arthroplasty for patients with rheumatoid arthritis with knee flexion contracture. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2008, 22, 1173–1176. [Google Scholar]
- Hou, H.B.; Cao, B.; Shi, S.M.; Huo, A.X.; Liu, Y.H. Total knee arthroplasty for treatment of rheumatoid arthritis: A protocol for a systematic review of randomized controlled trial. Medicine 2019, 98, e16558. [Google Scholar] [CrossRef] [PubMed]
- Fink, B.; Berger, I.; Siegmüller, C.; Fassbender, H.-G.; Meyer-Scholten, C.; Tillmann, K.; Rüther, W. Recurring synovitis as a possible reason for aseptic loosening of knee endoprostheses in patients with rheumatoid arthritis. J. Bone Jt. Surg. 2001, 83, 604–608. [Google Scholar] [CrossRef]
- Li, Z.; Feng, B.; Du, Y.; Wang, Y.; Bian, Y.; Weng, X. Complications of total knee arthroplasty in patients with haemophilia compared with osteoarthritis and rheumatoid arthritis: A 20-year single-surgeon cohort. Haemophilia 2020, 26, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, Y.; Teramoto, A.; Jimbo, S.; Okada, Y.; Kamiya, T.; Imamura, R.; Takashima, H.; Watanabe, K.; Nagoya, S. Denosumab prevents periprosthetic bone mineral density loss in the tibial metaphysis in total knee arthroplasty. Knee 2020, 27, 580–586. [Google Scholar] [CrossRef]
- Kumagai, K.; Harigane, K.; Kusayama, Y.; Tezuka, T.; Inaba, Y.; Saito, T. Total knee arthroplasty improves both knee function and disease activity in patients with rheumatoid arthritis. Mod. Rheumatol. 2017, 27, 806–810. [Google Scholar] [CrossRef]
- Thiele, K.; Fussi, J.; Perka, C.; Pfitzner, T. The Berlin diagnostic algorithm for painful knee TKA. Orthopade 2016, 45, 38–46. [Google Scholar] [CrossRef]
- Thabe, H.; Dafferner-Franzmann, M.; Stening, J. Long-Term Results with Different Designs in Knee Replacements. Aktuelle Rheumatol. 2014, 39, 130–134. [Google Scholar] [CrossRef]
- Van Hamersveld, K.T.; Marang-Van De Mheen, P.J.; Van Der Heide, H.J.L.; Van Der Linden-Van Der Zwaag, H.M.J.; Valstar, E.R.; Nelissen, R. Migration and clinical outcome of mobile-bearing versus fixed-bearing single-radius total knee arthroplasty. Acta Orthop. 2018, 89, 190–196. [Google Scholar] [CrossRef]
- Wolterbeek, N.; Garling, E.H.; Mertens, B.; Valstar, E.R.; Nelissen, R.G. Mobile bearing knee kinematics change over time. A fluoroscopic study in rheumatoid arthritis patients. Clin. Biomech. 2009, 24, 441–445. [Google Scholar] [CrossRef]
- Wolterbeek, N.; Nelissen, R.G.; Valstar, E.R. No differences in in vivo kinematics between six different types of knee prostheses. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2012, 20, 559–564. [Google Scholar] [CrossRef]
- Hofstede, S.N.; Nouta, K.A.; Jacobs, W.; van Hooff, M.L.; Wymenga, A.B.; Pijls, B.G.; Nelissen, R.G.; Marang-van de Mheen, P.J. Mobile bearing vs fixed bearing prostheses for posterior cruciate retaining total knee arthroplasty for postoperative functional status in patients with osteoarthritis and rheumatoid arthritis. Cochrane Database Syst. Rev. 2015, 2, CD003130. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biehl, C.; Heinrich, M.; Biehl, L.; Knapp, G.; Heiss, C.; Thormann, U. Biomechanical Particularities in the Therapy of the Rheumatic Knee. Appl. Sci. 2020, 10, 8600. https://doi.org/10.3390/app10238600
Biehl C, Heinrich M, Biehl L, Knapp G, Heiss C, Thormann U. Biomechanical Particularities in the Therapy of the Rheumatic Knee. Applied Sciences. 2020; 10(23):8600. https://doi.org/10.3390/app10238600
Chicago/Turabian StyleBiehl, Christoph, Martin Heinrich, Lotta Biehl, Gero Knapp, Christian Heiss, and Ulrich Thormann. 2020. "Biomechanical Particularities in the Therapy of the Rheumatic Knee" Applied Sciences 10, no. 23: 8600. https://doi.org/10.3390/app10238600
APA StyleBiehl, C., Heinrich, M., Biehl, L., Knapp, G., Heiss, C., & Thormann, U. (2020). Biomechanical Particularities in the Therapy of the Rheumatic Knee. Applied Sciences, 10(23), 8600. https://doi.org/10.3390/app10238600





