Energy Recovery via Thermal Gasification from Waste Insulation Electrical Cables (WIEC)
Abstract
1. Introduction
2. Materials and Methods
2.1. Analyses of Fuels
2.1.1. Ultimate Analysis
2.1.2. Thermogravimetric Analysis
2.1.3. Higher Heating Value Analysis
2.1.4. X-Ray Fluorescence (XRF)
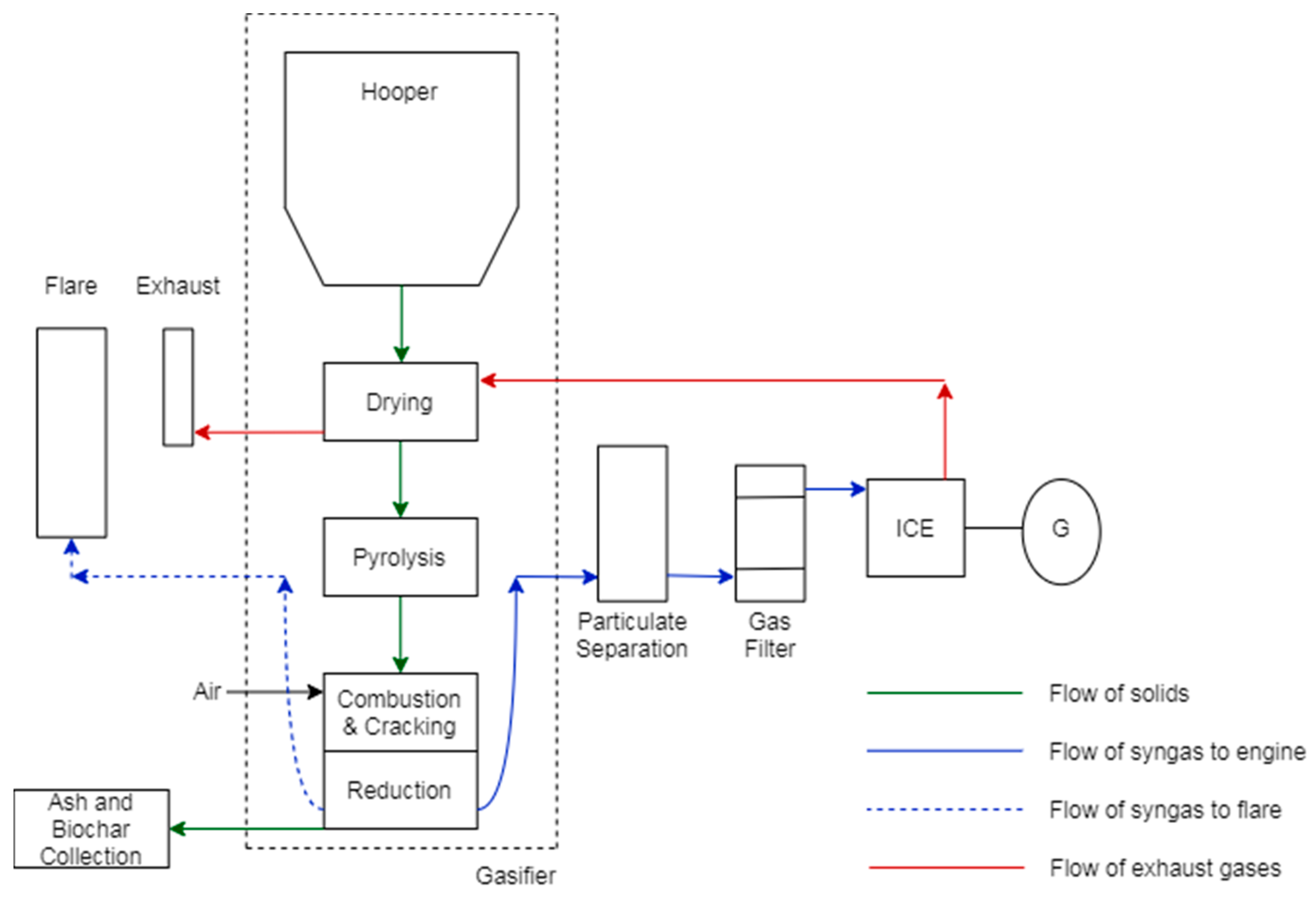
2.2. Gasification Test
2.3. Gasification Products Analysis
2.3.1. Synthesis Gas
2.3.2. Tars
2.3.3. Chars
2.3.4. Theoretical Parameters
3. Results and Discussions
3.1. Biomass Characterization
3.2. Heating Values
3.3. Proximate Analyses
3.4. Ultimate Analysis
4. Results
4.1. Controlled Gasification Results
4.2. Controlled Gasification Syngas Composition
4.3. Controlled Gasification Theoretical Parameters
4.4. Controlled Gasification: Chars and Tars
4.5. Load Gasification Experiments
4.6. Load Gasification Analysis
4.7. System Efficiency for the Blending Ratio 80:20 for PFB/WIEC
5. Conclusions
- A mixtures of PFB and WIEC can be applied in fixed bed gasifiers, since biomass was used as a bed for polymeric residues because they were inserted in the gasifier in a homogeneous way. In downdraft fixed flow beds, it was necessary for the biomass to have a size and weight that descended with gravity, the mixture of forest biomass and polymeric WIEC made the waste enter the system more easily.
- Regarding the operational problems detected during the tests, it was observed that limiting the incorporation of WIEC by a maximum of 30% was necessary for better gas quality, and thus recommended operational parameters.
- The incorporation of WIEC increased the temperature in the gasifier bed. With an increase in temperature, the biomass conversion rate increased and, consequently, the energy efficiency as a result of favoring reduction reactions. This means that the CO/CO2 ratio would increase as the incorporation of WIEC increases.
- As the amount of WIEC in the blending ratio with PFB increased, we noticed that the temperature also rose. The incorporation of WIEC should not exceed 30% to prevent damage to the equipment.
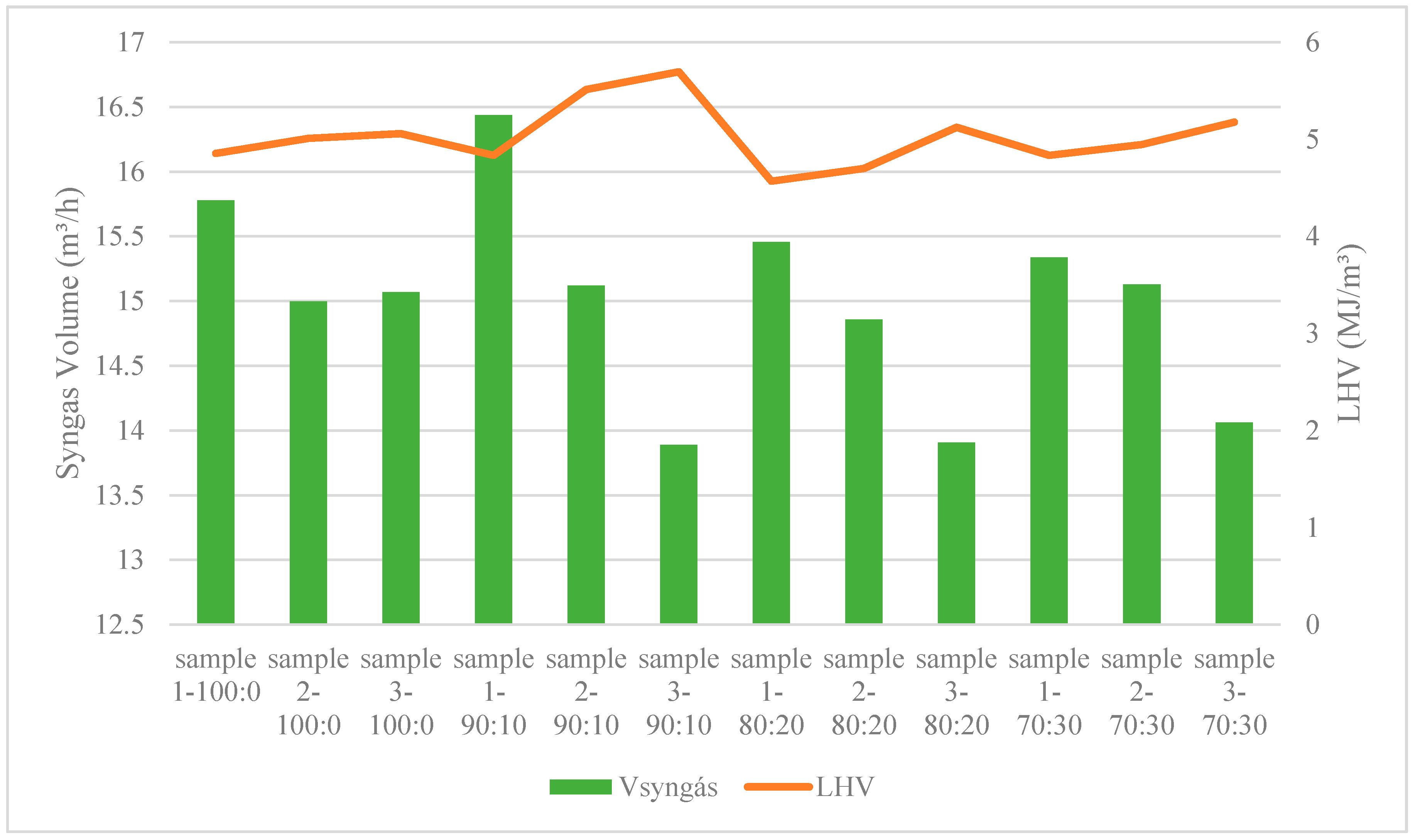
- It was still possible to verify that the gas produced had a relatively stable composition, as well as LHV. It was noted that as the percentage of incorporation increased, the ER tended to suffer an increase. The highest LHV and ER obtained during the tests were 5.2 MJ/Nm3 and 0.35.
- The synthesis gas obtained could be considered for use in internal combustion engines, as could be seen in the tests performed.
- In the tests using the engine, it was possible to observe that there was an increase in the gasification temperature. This factor was related to the suction of the generator.
- In tests with a load of 5 kW, the efficiency of the syngas was on average 80%. For loads of 10 and 15 kW, the efficiency was slightly lower, around 74%.
- Loads of 5, 10, and 15 kW were used for the 80:20 mixture. The efficiency of the engine increased as energy increased.
- The efficiency of the engine for the different applied loads averaged approximately 18%.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A/F | Air/fuel |
| C | Carbon |
| C2H2 | Ethyne |
| C2H6 | Ethane |
| CGE | Cold gas efficiency |
| CH4 | Methane |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| EEE | Electrical and electronic equipment |
| Eel | Electrical energy |
| ER | Equivalence ratio |
| H2 | Hydrogen |
| HHV | High heating value |
| ICE | Internal combustion engine |
| LHV | Low heating value |
| mbio | Mass of biomass |
| N | Nitrogen |
| NOx | Nitrogen oxides |
| O | Oxygen |
| Pcomb | Combustion pressure |
| PE | Polyethylene |
| PET | Polyethylene glycol terephthalate |
| PFB | Pine forest biomass |
| Pfilt | Filter pressure |
| PP | Polypropylene |
| PReact | Reaction pressure |
| PS | Polystyrene |
| PVC | Polyvinyl chloride |
| Qbiomass | Biomass flow |
| S | Sulphur |
| Tair | Air temperature |
| Tred | Temperature of reduction |
| Trst | Temperature of rest (oxidation temperature) |
| Vair | Volumetric flow of air |
| Vchars | Chars volume |
| VM | Volatile matter |
| Vsyngas | Volumetric flow of syngas |
| Vtars | Tars volume |
| WEEE | Waste electrical and electronic equipment |
| WIEC | Waste insulation electrical cables |
| ηeng | Engine efficiency |
| ηgas | Gasifier efficiency |
| ηgen | Generator efficiency |
| ηtot | Total efficiency |
References
- Veksha, A.; Giannis, A.; Yuan, G.; Tng, J.; Ping, W.; Chang, V.W.; Lisak, G.; Lim, T. Distribution and modeling of tar compounds produced during downdraft gasification of municipal solid waste. Renew. Energy 2018, 136, 1294–1303. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Mckendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- REN21. Renewables 2019: Global Status Report; REN21: Paris, France, 2019. [Google Scholar]
- Prasertcharoensuk, P.; Hernandez, D.A.; Bull, S.J.; Phan, A.N. Optimisation of a throat downdraft gasifier for hydrogen production. Biomass Bioenergy 2018, 116, 216–226. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification and Pyrolysis: Pratical Design and Theory; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Murzin, D.Y.; Simakova, I.L. 7.21—Catalysis in Biomass Processing. In Comprehensive Inorganic Chemistry II; Elsevier: Amsterdam, The Netherlands, 2013; pp. 559–586. [Google Scholar]
- Lu, Y.J.; Guo, L.J.; Ji, C.M.; Zhang, X.M.; Hao, X.H.; Yan, Q.H. Hydrogen production by biomass gasification in supercritical water: A parametric study. Int. J. Hydrogen Energy 2006, 31, 822–831. [Google Scholar] [CrossRef]
- Kim, J.; Choi, Y. Production of a Clean Hydrogen-Rich Gas by the Staged Gasification of Biomass and Plastic Waste; Scrivener Publishing: New York, NY, USA, 2017; pp. 363–384. [Google Scholar]
- Ponzio, A.; Kalisz, S.; Blasiak, W. Effect of operating conditions on tar and gas composition in high temperature air / steam gasification (HTAG) of plastic containing waste. Fuel Process. Technol. 2006, 87, 223–233. [Google Scholar] [CrossRef]
- Ilankoon, I.M.S.K.; Ghorbani, Y.; Nan, M.; Herath, G.; Moyo, T. E-waste in the international context—A review of trade flows, regulations, hazards, waste management strategies and technologies for value recovery. Waste Manag. 2018, 82, 258–275. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Yeh, C.H.; Liu, Y. Evaluating WEEE recycling innovation strategies with interacting sustainability-related criteria. J. Clean. Prod. 2018, 190, 618–629. [Google Scholar] [CrossRef]
- Wang, R.; Xu, Z. Recycling of non-metallic fractions from waste electrical and electronic equipment (WEEE): A review. Waste Manag. 2014, 34, 1455–1469. [Google Scholar] [CrossRef]
- Işıldar, A.; van Hullebusch, E.D.; Lenz, M.; Du Laing, G.; Marra, A.; Cesaro, A.; Panda, S.; Akcil, A.; Kucuker, M.A.; Kucht, K. Biotechnological strategies for the recovery of valuable and critical raw materials from waste electrical and electronic equipment (WEEE)—A review. J. Hazard. Mater. 2019, 362, 467–481. [Google Scholar] [CrossRef]
- De Meester, S.; Nachtergaele, P.; Debaveye, S.; Dewulf, J.; Vos, P. Using material flow analysis and life cycle assessment in decision support: A case study on WEEE valorization in Belgium. Resour. Conserv. Recycl. 2019, 142, 1–9. [Google Scholar] [CrossRef]
- Queralt, I.; Chimenos, J.M.; Formosa, J.; Maldonado-Alameda, A.; Pérez-Martínez, S.; Giro-Paloma, J. Characterisation and partition of valuable metals from WEEE in weathered municipal solid waste incineration bottom ash, with a view to recovering. J. Clean. Prod. 2019, 218, 61–68. [Google Scholar]
- Luda, M.P. Pyrolysis of WEEE plastics. Waste Electr. Electron. Equip. Handb. 2012, 239–263. [Google Scholar]
- Yang, X.; Sun, L.; Xiang, J.; Hu, S.; Su, S. Pyrolysis and dehalogenation of plastics from waste electrical and electronic equipment (WEEE): A review. Waste Manag. 2013, 33, 462–473. [Google Scholar] [CrossRef]
- Ebin, B.; Isik, M.I. Chapter 5—Pyrometallurgical Processes for the Recovery of Metals from WEEE. In WEEE Recycling; Elsevier: Amsterdam, The Netherlands, 2016; pp. 107–137. [Google Scholar]
- Panizio, R.M.; Calado, L.F.C.; Alves, O.; Nobre, C.; Silveira, J.L.; Brito, P.; Gonçalves, M.M. Effect of the incorporation of biomass in the carbonization of waste electrical and electronic equipment. In Proceedings of the Bioenergy Conference, Portalegre, Portugal, 11–13 September 2019. [Google Scholar]
- Gurgul, A.; Szczepaniak, W.; Zabłocka-Malicka, M. Incineration and pyrolysis vs. steam gasification of electronic waste. Psychol. Bull. 2018, 624, 1119–1124. [Google Scholar] [CrossRef]
- Kasper, A.C.; Berselli, G.B.T.; Freitas, B.D.; Tenório, J.A.S.; Bernardes, A.M.; Veit, H.M. Printed wiring boards for mobile phones: Characterization and recycling of copper. Waste Manag. 2011, 31, 2536–2545. [Google Scholar] [CrossRef]
- Gramatyka, P.; Nowosielski, R.; Sakiewicz, P. Recycling of waste electrical and electronic Recycling of waste electrical and electronic equipment. J. Achiev. Mater. Manuf. Eng. 2015, 20, 535–538. [Google Scholar]
- Zhu, H.L.; Zhang, Y.S.; Materazzi, M.; Aranda, G.; Brett, D.J.L.; Shearing, P.R.; Manos, G. Co-gasification of beech-wood and polyethylene in a fluidized-bed reactor. Fuel Process. Technol. 2019, 190, 29–37. [Google Scholar] [CrossRef]
- Carmo-Calado, L.; Hermoso-Orzáez, M.; Mota-Panizio, R.; Guilherme-Garcia, B.; Brito, P. Co-Combustion of Waste Tires and Plastic-Rubber Wastes with Biomass Technical and Environmental Analysis. Sustainability 2020, 12, 1036. [Google Scholar] [CrossRef]
- Pinto, F.; Franco, C.; André, R.N.; Tavares, C.; Dias, M.; Gulyurtlu, I.; Cabrita, I. Effect of experimental conditions on co-gasification of coal, biomass and plastics wastes with air/steam mixtures in a fluidized bed system. Fuel 2003, 82, 1967–1976. [Google Scholar] [CrossRef]
- Diretiva 2012/19/UE do Parlamento Europeu e do Conselho e do conselho de 4 de julho de 2012. Relativa aos resíduos de equipamentos elétricos e eletrónicos (REEE). J. Of. da União Eur. 2012, L 197/38-71.
- Ayol, A.; Tezer Yurdakos, O.; Gurgen, A. Investigation of municipal sludge gasification potential: Gasification characteristics of dried sludge in a pilot-scale downdraft fixed bed gasifier. Int. J. Hydrogen Energy 2019, 44, 17397–17410. [Google Scholar] [CrossRef]
- Martínez-lera, S.; Ranz, J.P. On the development of a wood gasi fi cation modelling approach with special emphasis on primary devolatilization and tar formation and destruction phenomena. Energy 2016, 113. [Google Scholar] [CrossRef]
- Allesina, G.; Pedrazzi, S.; Allegretti, F.; Morselli, N.; Puglia, M.; Santunione, G.; Tartarini, P. Gasification of cotton crop residues for combined power and biochar production in Mozambique. Appl. Therm. Eng. 2018, 139, 387–394. [Google Scholar] [CrossRef]
- Salaudeen, S.A.; Arku, P.; Dutta, A. Gasification of Plastic Solid Waste and Competitive Technologies; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128131404. [Google Scholar]
- Jiang, K.; Cheng, C.; Ran, M.; Lu, Y.; Wu, Q. Preparation of a biochar with a high calorific value from chestnut shells. New Carbon Mater. 2018, 33, 183–187. [Google Scholar] [CrossRef]
- Dabai, F.; Paterson, N.; Millan, M.; Fennell, P.; Kandiyoti, R. Tar formation and destruction in a fixed-bed reactor simulating downdraft gasification: Equipment development and characterization of tar-cracking products. Energy Fuels 2010, 24, 4560–4570. [Google Scholar] [CrossRef]
- Viana, H.; Rodrigues, A.; Lopes, D.M.M.; Godina, R.; Nunes, L.J.R.; Matias, J.C.O. Pinus Pinaster and Eucalyptus Globulus Energetic Properties and Ash Characterization. In Proceedings of the 2018 IEEE International Conference on Environment and Electrical Engineering and 2018 IEEE Industrial and Commercial Power Systems Europe (EEEIC/I&CPS Europe), Palermo, Italy, 12–15 June 2018; pp. 1–4. [Google Scholar]
- Pio, D.T.; Tarelho, L.A.C.; Tavares, A.M.A.; Matos, M.A.A.; Silva, V. Co-gasification of refused derived fuel and biomass in a pilot-scale bubbling fl uidized bed reactor. Energy Convers. Manag. 2020, 206. [Google Scholar] [CrossRef]
- Miranda, M.; Gulyurtlu, I.; Cabrita, I.; Pinto, F.; Franco, C.; Andre, R.N. Co-gasication study of biomass mixed with plastic wastes. Fuel 2002, 81, 291–297. [Google Scholar]
- Ahmed, I.I.; Gupta, A.K. Kinetics of woodchips char gasification with steam and carbon dioxide. Appl. Energy 2011, 88, 1613–1619. [Google Scholar] [CrossRef]
- van den Bergh, A. The Co-Gasification of Wood and Polyethylene the Influence of Temperature, Equivalence Ratio, Steam and the Feedstock Composition on the Gas Yield and Composition. Master’s Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2005. [Google Scholar]
- Kannan, P.; Al Shoaibi, A.; Srinivasakannan, C. Optimization of Waste Plastics Gasification Process Using Aspen-Plus. In Gasification for Practical Applications; Scitus Academics: Wilmington DE, USA, 2012. [Google Scholar]
- Zeng, J.; Xiao, R.; Yuan, J. High-quality syngas production from biomass driven by chemical looping on a PY-GA coupled reactor. Energy 2021, 214, 118846. [Google Scholar] [CrossRef]
- Innovative, O.; Recycling, P. Co-gasification of wood and polyethylene with the aim of CO and H2 production. J. Mater. Cycles Waste Manag. 2006, 95–98. [Google Scholar]
- El-Hendawy, A.-N.A. Surface and adsorptive properties of carbons prepared from biomass. Appl. Surf. Sci. 2005, 252, 287–295. [Google Scholar] [CrossRef]
- El-Hendawy, A.N.A. Variation in the FTIR spectra of a biomass under impregnation, carbonization and oxidation conditions. J. Anal. Appl. Pyrolysis 2006, 75, 159–166. [Google Scholar] [CrossRef]
- Kiln, R.; Biomass, T.A. The concept, design and performance of a novel rotary kiln type air-staged biomass gasifier. Energies 2016, 9, 67. [Google Scholar]
- Rahman, A.; Sudarmanta, B.; Fansuri, H.; Muraza, O. Syngas production from municipal solid waste with a reduced tar yield by three-stages of air inlet to a downdraft gasifier. Fuel 2020, 263, 116509. [Google Scholar]
- Bhoi, P.R.; Huhnke, R.L.; Kumar, A.; Indrawan, N. Co-gasification of municipal solid waste and biomass in a commercial scale downdraft gasifier. Energy 2018, 163, 513–518. [Google Scholar] [CrossRef]
- Patel, V.R.; Upadhyay, D.S.; Patel, R.N. Gasification of lignite in a fixed bed reactor: Influence of particle size on performance of downdraft gasifier. Energy 2014, 78, 323–332. [Google Scholar] [CrossRef]
- Aznar, P.; Caballero, M.A.; Sancho, J.A.; Francés, E. Plastic waste elimination by co-gasification with coal and biomass in fluidized bed with air in pilot plant. Fuel Process. Technol. 2006, 87, 409–420. [Google Scholar] [CrossRef]
- Kim, J.; Mun, T.; Kim, J.; Kim, J. Air gasification of mixed plastic wastes using a two-stage gasifier for the production of producer gas with low tar and a high caloric value. Fuel 2011, 90, 2266–2272. [Google Scholar] [CrossRef]
- Toledo, J.M.; Aznar, M.P.; Sancho, J.A. Catalytic Air Gasification of Plastic Waste (Polypropylene) in a Fluidized Bed. Part II: Effects of Some Operating Variables on the Quality of the Raw Gas Produced Using Olivine as the In-Bed Material. Ind. Eng. Chem. Res. 2011, 50, 11815–11821. [Google Scholar] [CrossRef]
- Cho, M.; Mun, T.; Kim, J. Air gasification of mixed plastic wastes using calcined dolomite and activated carbon in a two-stage gasifier to reduce tar. Energy 2013, 53, 299–305. [Google Scholar] [CrossRef]
- Xiao, R.; Jin, B.; Zhou, H.; Zhong, Z.; Zhang, M. Air gasification of polypropylene plastic waste in fluidized bed gasifier. Energy Convers. Manag. 2007, 48, 778–786. [Google Scholar] [CrossRef]
- Raman, P.; Ram, N.K. Performance analysis of an internal combustion engine operated on producer gas, in comparison with the performance of the natural gas and diesel engines. Energy 2013, 63, 317–333. [Google Scholar] [CrossRef]


| Analysis | Parameters | Units | Fuels | |
|---|---|---|---|---|
| Pine | WIEC | |||
| Proximate | Moisture | % | 7.43 | 0.79 |
| Volatile matter | % | 53.61 | 68.4 | |
| Fixed carbon | % | 36.36 | 30.51 | |
| Ashes | % | 2.6 | 2.3 | |
| Ultimate | Nitrogen | % | 0.6 | 0.2 |
| Carbon | % | 49.7 | 52.3 | |
| Hydrogen | % | 7.5 | 2.5 | |
| Sulphur | % | 0 | 0 | |
| Oxygen | % | 39.8 | 42.7 | |
| HHV | MJ/kg | 18.4 | 22.7 | |
| XRF | % | 0.04 | 0.55 | |
| 100:0 | 90:10 | 80:20 | 70:30 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Units | Sample 1 | Sample 2 | Sample 3 | Sample 1 | Sample 2 | Sample 3 | Sample 1 | Sample 2 | Sample 3 | Sample 1 | Sample 2 | Sample 3 |
| CO2 | % | 12.78 | 9.16 | 8.52 | 11.52 | 10.51 | 10.82 | 9.95 | 9.80 | 9.1 | 10.01 | 10.71 | 9.84 |
| C2H4 | % | 0.82 | 0.57 | 0.17 | 0.84 | 0.68 | 0.63 | 0.74 | 0.21 | 0.22 | 0.59 | 0.35 | 0.26 |
| C2H6 | % | 0.21 | 0.15 | 0.04 | 0.18 | 0.15 | 0.14 | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 |
| C2H2 | % | 0.04 | 0.02 | 0.04 | 0.03 | 0.08 | 0.25 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 |
| H2S | % | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| N2 | % | 57.09 | 56.58 | 52.27 | 58.84 | 53.60 | 50.33 | 57.73 | 55.13 | 51.60 | 56.31 | 51.09 | 49.66 |
| CH4 | % | 3.21 | 2.24 | 2.04 | 3.09 | 2.70 | 2.62 | 1.47 | 1.71 | 1.58 | 2.05 | 1.46 | 1.42 |
| CO | % | 15.11 | 18.77 | 19.91 | 14.90 | 19.24 | 19.44 | 18.04 | 20.11 | 20.50 | 19.02 | 20.85 | 20.47 |
| H2 | % | 10.73 | 12.91 | 15.42 | 11.30 | 14.58 | 15.75 | 12.02 | 12.95 | 16.79 | 12.26 | 14.52 | 17.68 |
| LHV | MJ/m3 | 4.85 | 5.01 | 5.06 | 4.83 | 5.51 | 5.70 | 4.57 | 4.70 | 5.12 | 4.84 | 4.95 | 5.18 |
| Trst | °C | 507.00 | 603.00 | 720.00 | 594.00 | 615.00 | 708.00 | 614.00 | 699.00 | 758.00 | 627.00 | 718.00 | 789.00 |
| Tred | °C | 289.00 | 484.00 | 515.00 | 371.00 | 499.00 | 547.00 | 274.00 | 476.00 | 526.00 | 395.00 | 485.00 | 532.00 |
| Pcomb | KPa | −19.00 | −16.00 | −12.00 | −19.00 | −17.00 | −10.00 | −19.00 | −12.00 | −12.00 | −17.00 | −13.00 | −12.00 |
| PReact | KPa | −46.00 | −23.00 | −22.00 | −22.00 | −22.00 | −25.00 | −26.00 | −27.00 | −24.00 | −25.00 | −23.00 | −28.00 |
| Pfilt | KPa | −60.00 | −46.00 | −40.00 | −45.00 | −39.00 | −32.00 | −49.00 | −45.00 | −45.00 | −48.00 | −45.00 | −52.00 |
| Vair | m3/h | 11.51 | 10.94 | 10.03 | 12.42 | 10.45 | 8.89 | 11.48 | 10.46 | 9.26 | 11.00 | 9.88 | 9.00 |
| Tair | °C | 12.70 | 14.90 | 15.80 | 15.70 | 19.30 | 19.60 | 11.30 | 14.40 | 15.60 | 12.40 | 15.80 | 18.20 |
| Vtars | g/Nm3syngas | 8.54 | 9.88 | 10.97 | 12.31 | ||||||||
| Vchars | kg/h | 0.16 | 0.17 | 0.16 | 0.19 | ||||||||
| ER | - | 0.28 | 0.27 | 0.24 | 0.30 | 0.25 | 0.16 | 0.26 | 0.24 | 0.20 | 0.25 | 0.22 | 0.19 |
| Cold gas efficiency | % | 80.05 | 78.54 | 79.67 | 65.94 | 69.17 | 65.66 | 54.35 | 53.74 | 54.85 | 55.47 | 55.98 | 54.44 |
| Vsyngás | m3/h | 15.78 | 15.00 | 15.07 | 16.44 | 15.12 | 13.89 | 15.46 | 14.86 | 13.91 | 15.34 | 15.13 | 14.06 |
| Sample collection | min | 60 | 120 | 160 | 60 | 120 | 160 | 60 | 120 | 160 | 60 | 120 | 160 |
| Fuel flow | kg/h | 5.20 | 6.40 | 6.90 | 7.10 | ||||||||
| Experiment time | min | 180.00 | 180.00 | 180.00 | 180.00 | ||||||||
| 5 kW | 10 kW | 15 kW | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Units | Sample 1 | Sample 2 | Sample 3 | Sample 1 | Sample 2 | Sample 3 | Sample 1 | Sample 2 | Sample 3 |
| CO2 | % | 12.40 | 12.50 | 11.80 | 13.21 | 12.75 | 12.81 | 13.03 | 13.89 | 12.05 |
| C2H4 | % | 0.20 | 0.08 | 0.09 | 0.10 | 0.07 | 0.03 | 0.05 | 0.05 | 0.06 |
| C2H6 | % | 0.02 | 0.01 | 0.01 | 0.05 | 0.07 | 0.05 | 0.02 | 0.01 | 0.02 |
| C2H2 | % | 0.02 | 0.02 | 0.01 | 0.01 | 0.08 | 0.04 | 0.01 | 0.02 | 0.01 |
| H2S | % | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| N2 | % | 52.12 | 51.78 | 51.03 | 49.69 | 52.07 | 50.10 | 53.98 | 52.82 | 52.02 |
| CH4 | % | 2.58 | 2.35 | 2.41 | 2.37 | 2.04 | 2.38 | 1.87 | 1.04 | 1.01 |
| CO | % | 17.35 | 18.96 | 18.89 | 18.35 | 18.57 | 19.02 | 16.84 | 17.86 | 19.07 |
| H2 | % | 16.42 | 16.04 | 17.62 | 16.21 | 16.08 | 16.84 | 16.58 | 16.32 | 18.48 |
| LHV | MJ/m3 | 5.03 | 5.03 | 5.22 | 5.01 | 4.94 | 5.15 | 4.64 | 4.44 | 4.82 |
| Trst | °C | 883.00 | 889.00 | 895.00 | 879.00 | 885.00 | 888.00 | 913.00 | 947.00 | 958.00 |
| Tred | °C | 613.00 | 625.00 | 657.00 | 621.00 | 658.00 | 671.00 | 607.00 | 643.00 | 687.00 |
| Pcomb | KPa | −25.00 | −22.00 | −23.00 | −18.00 | −25.00 | −26.00 | −27.00 | −28.00 | −26.00 |
| PReact | KPa | −51.00 | −46.00 | −45.00 | −39.00 | −48.00 | −53.00 | −59.00 | −64.00 | −62.00 |
| Pfilt | KPa | −67.00 | −64.00 | −68.00 | −64.00 | −72.00 | −78.00 | −81.00 | −86.00 | −84.00 |
| Vair | m3/h | 11.76 | 11.21 | 11.07 | 18.88 | 20.63 | 19.35 | 34.97 | 33.86 | 33.56 |
| Tair | ° C | 13.10 | 14.80 | 16.20 | 13.20 | 15.30 | 18.90 | 12.70 | 15.70 | 19.20 |
| Vtars | g/Nm3syngas | 4.20 | 7.54 | 8.79 | ||||||
| Vchars | kg/h | 0.28 | 0.34 | 0.47 | ||||||
| ER | - | 0.27 | 0.25 | 0.25 | 0.23 | 0.25 | 0.23 | 0.28 | 0.27 | 0.27 |
| Cold gas efficiency | % | 80.91 | 77.15 | 80.59 | 71.03 | 73.57 | 74.82 | 74.76 | 70.56 | 77.38 |
| Vsyngas | m3/h | 17.66 | 16.84 | 16.95 | 29.49 | 30.98 | 30.22 | 50,58 | 49.89 | 50.40 |
| Sample collection | min | 60.00 | 120.00 | 160.00 | 60.00 | 120.00 | 160.00 | 60.00 | 120.00 | 160.00 |
| Qbiomass | kg/h | 5.70 | 10.8 | 16.3 | ||||||
| Experiment time | min | 180.00 | 180.00 | 180.00 | ||||||
| 5 kW | 10 kW | 15 kW | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Sample 1 | Sample 2 | Sample 3 | Sample 1 | Sample 2 | Sample 3 | Sample 1 | Sample 2 | Sample 3 |
| ηgas | 80.91 | 77.15 | 80.59 | 71.03 | 73.57 | 74.82 | 74.76 | 70.56 | 77.38 |
| Eel (kW) | 5 | 5 | 5 | 10 | 10 | 10 | 15 | 15 | 15 |
| ηtot | 0.16 | 0.16 | 0.16 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 |
| ηgen | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| ηeng | 0.16 | 0.17 | 0.16 | 0.19 | 0.18 | 0.18 | 0.18 | 0.19 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota-Panizio, R.; Hermoso-Orzáez, M.J.; Carmo-Calado, L.; Campos, V.A.F.d.; Silveira, J.L.; Gonçalves, M.M.; Brito, P. Energy Recovery via Thermal Gasification from Waste Insulation Electrical Cables (WIEC). Appl. Sci. 2020, 10, 8253. https://doi.org/10.3390/app10228253
Mota-Panizio R, Hermoso-Orzáez MJ, Carmo-Calado L, Campos VAFd, Silveira JL, Gonçalves MM, Brito P. Energy Recovery via Thermal Gasification from Waste Insulation Electrical Cables (WIEC). Applied Sciences. 2020; 10(22):8253. https://doi.org/10.3390/app10228253
Chicago/Turabian StyleMota-Panizio, Roberta, Manuel Jesús Hermoso-Orzáez, Luís Carmo-Calado, Victor Arruda Ferraz de Campos, José Luz Silveira, Maria Margarida Gonçalves, and Paulo Brito. 2020. "Energy Recovery via Thermal Gasification from Waste Insulation Electrical Cables (WIEC)" Applied Sciences 10, no. 22: 8253. https://doi.org/10.3390/app10228253
APA StyleMota-Panizio, R., Hermoso-Orzáez, M. J., Carmo-Calado, L., Campos, V. A. F. d., Silveira, J. L., Gonçalves, M. M., & Brito, P. (2020). Energy Recovery via Thermal Gasification from Waste Insulation Electrical Cables (WIEC). Applied Sciences, 10(22), 8253. https://doi.org/10.3390/app10228253











