Biorefinery: The Production of Isobutanol from Biomass Feedstocks
Abstract
1. Introduction
2. Research on Isobutanol Production
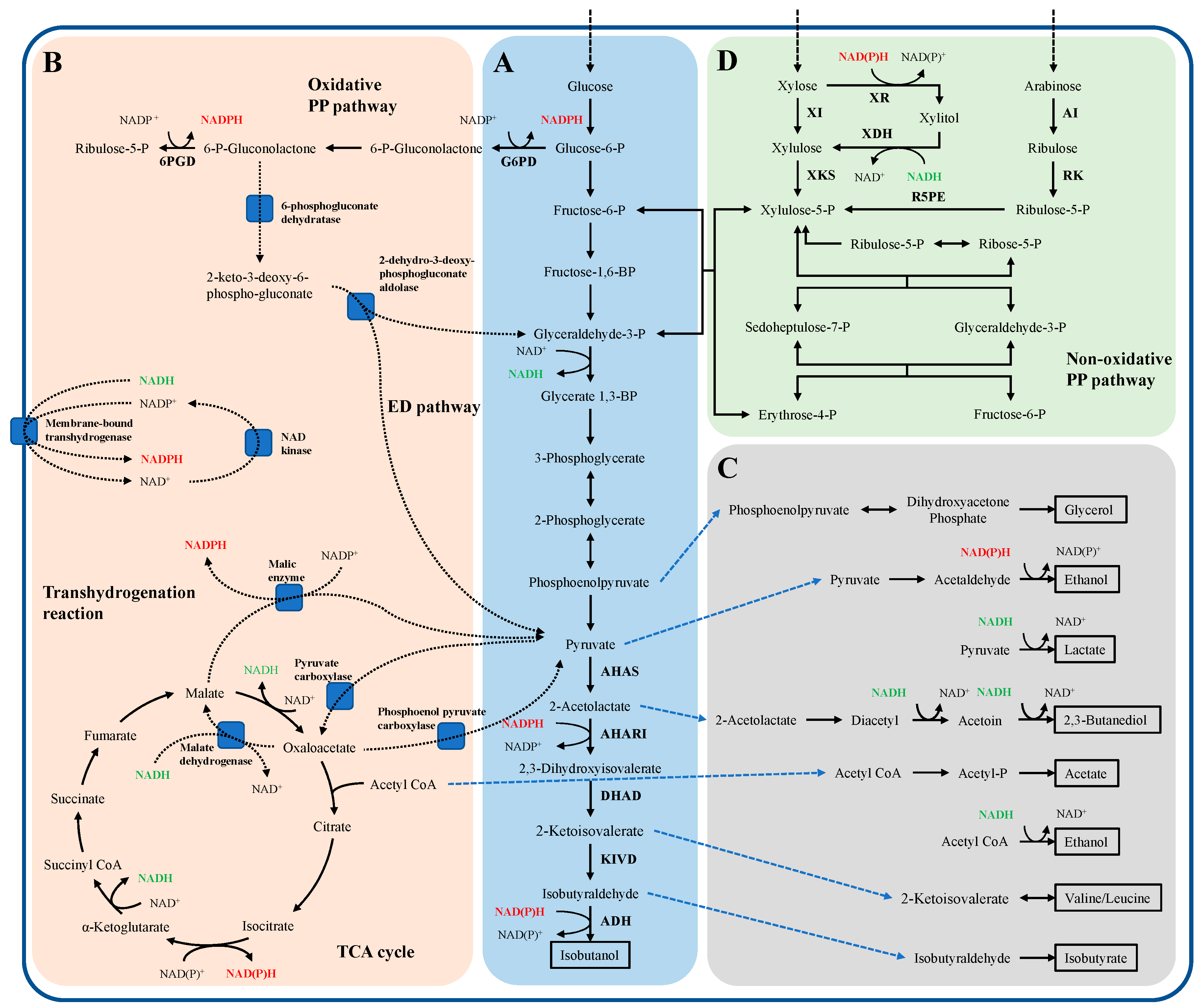
3. Biomass Isobutanol Production
3.1. Isobutanol Production from Lignocellulose
3.1.1. Cellulosic Isobutanol Produced by Natural Cellulose-Degrading Microorganisms
3.1.2. Cellulosic Isobutanol Produced by Non-Native Cellulose-Degrading Microorganisms
3.2. Isobutanol Production from Protein
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cherubini, F.; Strømman, A.H. Life cycle assessment of bioenergy systems: State of the art and future challenges. Bioresour. Technol. 2011, 102, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Chou, H.; Ham, T.S.; Lee, T.S.; Keasling, J.D. Metabolic engineering of microorganisms for biofuels production: From bugs to synthetic biology to fuels. Curr. Opin. Biotechnol. 2008, 19, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Raud, M.; Kikas, T.; Sippula, O.; Shurpali, N. Potentials and challenges in lignocellulosic biofuel production technology. Renew. Sustain. Energy Rev. 2019, 111, 44–56. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, J.; Jang, Y.-S.; Lee, S.Y. Metabolic engineering of microorganisms for the production of higher alcohols. mBio 2014, 5, e01524-14. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.C.; Mi, L.; Pontrelli, S.; Luo, J.C.L.L.M.S.P.S. Fuelling the future: Microbial engineering for the production of sustainable biofuels. Nat. Rev. Genet. 2016, 14, 288–304. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.; Hu, H.; Wang, W.; Zhang, X. Metabolic engineering and enzyme-mediated processing: A biotechnological venture towards biofuel production—A review. Renew. Sustain. Energy Rev. 2018, 82, 436–447. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Valdivia, M.; Galan, J.L.; Laffarga, J.; Ramos, J. Biofuels 2020: Biorefineries based on lignocellulosic materials. Microb. Biotechnol. 2016, 9, 585–594. [Google Scholar] [CrossRef]
- Lara-Flores, A.A.; Araújo, R.G.; Rodríguez-Jasso, R.M.; Aguedo, M.; Aguilar, C.N.; Trajano, H.L.; Ruiz, H.A. Bioeconomy and Biorefinery: Valorization of Hemicellulose from Lignocellulosic Biomass and Potential Use of Avocado Residues as a Promising Resource of Bioproducts; Singhania, R., Agarwal, R., Kumar, R., Sukumaran, R., Eds.; Waste to Wealth; Springer: Singapore, 2018; pp. 141–170. [Google Scholar]
- Weber, C.; Farwick, A.; Benisch, F.; Brat, D.; Dietz, H.; Subtil, T.; Boles, E. Trends and challenges in the microbial production of lignocellulosic bioalcohol fuels. Appl. Microbiol. Biotechnol. 2010, 87, 1303–1315. [Google Scholar] [CrossRef]
- Amoah, J.; Kahar, P.; Ogino, C.; Kondo, A. Bioenergy and biorefinery: Feedstock, biotechnological conversion, and products. Biotechnol. J. 2019, 14, e1800494. [Google Scholar] [CrossRef]
- Toor, M.; Kumar, S.S.; Malyan, S.K.; Bishnoi, N.R.; Mathimani, T.; Rajendran, K.; Pugazhendhi, A. An overview on bioethanol production from lignocellulosic feedstocks. Chemosphere 2020, 242, 125080. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-W.; Shi, A.-Q.; Tu, R.; Zhang, X.-L.; Wang, Q.; Bai, F. Branched-chain higher alcohols. Process Integr. Biochem. Eng. 2011, 128, 101–118. [Google Scholar] [CrossRef]
- Atsumi, S.; Hanai, T.; Liao, J.C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nat. Cell Biol. 2008, 451, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Connor, M.R.; Liao, J.C. Microbial production of advanced transportation fuels in non-natural hosts. Curr. Opin. Biotechnol. 2009, 20, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Shahsavan, M.; Mack, J.H. Numerical study of a boosted HCCI engine fueled with n-butanol and isobutanol. Energy Convers. Manag. 2018, 157, 28–40. [Google Scholar] [CrossRef]
- Savage, N. Fuel options: The ideal biofuel. Nat. Cell Biol. 2011, 474, S9–S11. [Google Scholar] [CrossRef]
- Elfasakhany, A. Investigations on performance and pollutant emissions of spark-ignition engines fueled with n-butanol-, isobutanol-, ethanol-, methanol-, and acetone-gasoline blends: A comparative study. Renew. Sustain. Energy Rev. 2017, 71, 404–413. [Google Scholar] [CrossRef]
- Baez, A.; Cho, K.-M.; Liao, J.C. High-flux isobutanol production using engineered Escherichia coli: A bioreactor study with in situ product removal. Appl. Microbiol. Biotechnol. 2011, 90, 1681–1690. [Google Scholar] [CrossRef]
- Smith, K.M.; Liao, J.C. An evolutionary strategy for isobutanol production strain development in Escherichia coli. Metab. Eng. 2011, 13, 674–681. [Google Scholar] [CrossRef]
- Bastian, S.; Liu, X.; Meyerowitz, J.T.; Snow, C.D.; Chen, M.M.; Arnold, F.H. Engineered ketol-acid reductoisomerase and alcohol dehydrogenase enable anaerobic 2-methylpropan-1-ol production at theoretical yield in Escherichia coli. Metab. Eng. 2011, 13, 345–352. [Google Scholar] [CrossRef]
- Smith, K.M.; Cho, K.-M.; Liao, J.C. Engineering Corynebacterium glutamicum for isobutanol production. Appl. Microbiol. Biotechnol. 2010, 87, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Blombach, B.; Riester, T.; Wieschalka, S.; Ziert, C.; Youn, J.-W.; Wendisch, V.F.; Eikmanns, B.J. Corynebacterium glutamicum tailored for efficient isobutanol production. Appl. Environ. Microbiol. 2011, 77, 3300–3310. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Jojima, T.; Suda, M.; Inui, M. Isobutanol production in Corynebacterium glutamicum: Suppressed succinate by-production by pckA inactivation and enhanced productivity via the Entner-Doudoroff pathway. Metab. Eng. 2020, 59, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wen, J.; Jia, X. Engineering Bacillus subtilis for isobutanol production by heterologous Ehrlich pathway construction and the biosynthetic 2-ketoisovalerate precursor pathway overexpression. Appl. Microbiol. Biotechnol. 2011, 91, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Shouliang, Y.; Huang, D.; Li, Y.; Wen, J.; Jia, X. Rational improvement of the engineered isobutanol-producing Bacillus subtilis by elementary mode analysis. Microb. Cell Factories 2012, 11, 101. [Google Scholar] [CrossRef]
- Qi, H.; Li, S.; Zhao, S.; Huang, D.; Xia, M.; Wen, J. Model-driven redox pathway manipulation for improved isobutanol production in bacillus subtilis complemented with experimental validation and metabolic profiling analysis. PLoS ONE 2014, 9, e93815. [Google Scholar] [CrossRef]
- Liu, S.; Qureshi, N. How microbes tolerate ethanol and butanol. New Biotechnol. 2009, 26, 117–121. [Google Scholar] [CrossRef]
- Chen, X.; Nielsen, K.F.; Borodina, I.; Kielland-Brandt, M.C.; Karhumaa, K. Increased isobutanol production in Saccharomyces cerevisiae by overexpression of genes in valine metabolism. Biotechnol. Biofuels 2011, 4, 21. [Google Scholar] [CrossRef]
- Brat, D.; Weber, C.; Lorenzen, W.; Bode, H.B.; Boles, E. Cytosolic re-localization and optimization of valine synthesis and catabolism enables inseased isobutanol production with the yeast Saccharomyces cerevisiae. Biotechnol. Biofuels 2012, 5, 65. [Google Scholar] [CrossRef]
- Wess, J.; Brinek, M.; Boles, E. Improving isobutanol production with the yeast Saccharomyces cerevisiae by successively blocking competing metabolic pathways as well as ethanol and glycerol formation. Biotechnol. Biofuels 2019, 12, 1–15. [Google Scholar] [CrossRef]
- Zhao, E.M.; Zhang, Y.; Mehl, J.; Park, H.; Lalwani, M.A.; Toettcher, J.E.; Avalos, J.L. Optogenetic regulation of engineered cellular metabolism for microbial chemical production. Nat. Cell Biol. 2018, 555, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.; Brancoli, P.; Agnihotri, S.; Bolton, K.; Taherzadeh, M.J. A review of integration strategies of lignocelluloses and other wastes in 1st generation bioethanol processes. Process. Biochem. 2018, 75, 173–186. [Google Scholar] [CrossRef]
- Liu, C.-G.; Xiao, Y.; Xia, X.-X.; Zhao, X.-Q.; Peng, L.; Srinophakun, P.; Bai, F. Cellulosic ethanol production: Progress, challenges and strategies for solutions. Biotechnol. Adv. 2019, 37, 491–504. [Google Scholar] [CrossRef]
- Xin, F.; Dong, W.; Zhang, W.; Ma, J.; Jiang, M. Biobutanol production from crystalline cellulose through consolidated bioprocessing. Trends Biotechnol. 2019, 37, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Li, Q.; Liu, J.; Jin, M.; Yang, S. Consolidated bioprocessing for butanol production of cellulolytic Clostridia: Development and optimization. Microb. Biotechnol. 2019, 13, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Tan, E.C.D.; McCormick, R.L.; Zhang, M.; Aden, A.; He, X.; Zigler, B.T. Techno-economic analysis and life-cycle assessment of cellulosic isobutanol and comparison with cellulosic ethanol and n-butanol. Biofuels Bioprod. Biorefining 2014, 8, 30–48. [Google Scholar] [CrossRef]
- Hal, W.J. Iso-Butanol Platform Rotterdam (IBPR); Policy Studies; ECN Biomass & Energy Efficiency: Schagen, The Netherlands, 2016. [Google Scholar]
- Higashide, W.; Li, Y.; Yang, Y.; Liao, J.C. Metabolic engineering of Clostridium cellulolyticum for production of isobutanol from cellulose. Appl. Environ. Microbiol. 2011, 77, 2727–2733. [Google Scholar] [CrossRef]
- Guedon, E.; Desvaux, M.; Payot, S.; Petitdemange, H. Growth inhibition of Clostridium cellulolyticum by an inefficiently regulated carbon flow. Microbiology 1999, 145, 1831–1838. [Google Scholar] [CrossRef]
- Li, Y.; Xu, T.; Tschaplinski, T.J.; Engle, N.L.; Yang, Y.; Graham, D.E.; He, Z.; Zhou, J. Improvement of cellulose catabolism in Clostridium cellulolyticum by sporulation abolishment and carbon alleviation. Biotechnol. Biofuels 2014, 7, 25. [Google Scholar] [CrossRef][Green Version]
- Lin, P.P.; Rabe, K.S.; Takasumi, J.L.; Kadisch, M.; Arnold, F.H.; Liao, J.C. Isobutanol production at elevated temperatures in thermophilic Geobacillus thermoglucosidasius. Metab. Eng. 2014, 24, 1–8. [Google Scholar] [CrossRef]
- Lin, P.P.; Mi, L.; Morioka, A.H.; Yoshino, K.M.; Konishi, S.; Xu, S.C.; Papanek, B.A.; Riley, L.A.; Guss, A.M.; Liao, J.C. Consolidated bioprocessing of cellulose to isobutanol using Clostridium thermocellum. Metab. Eng. 2015, 31, 44–52. [Google Scholar] [CrossRef]
- Holwerda, E.K.; Olson, D.G.; Ruppertsberger, N.M.; Stevenson, D.M.; Murphy, S.J.L.; Maloney, M.I.; Lanahan, A.A.; Amador-Noguez, D.; Lynd, L.R. Metabolic and evolutionary responses of Clostridium thermocellum to genetic interventions aimed at improving ethanol production. Biotechnol. Biofuels 2020, 13, 1–20. [Google Scholar] [CrossRef]
- Minty, J.J.; Singer, M.E.; Scholz, S.A.; Bae, C.-H.; Ahn, J.-H.; Foster, C.E.; Liao, J.C.; Lin, X.N. Design and characterization of synthetic fungal-bacterial consortia for direct production of isobutanol from cellulosic biomass. Proc. Nat. Acad. Sci. USA 2013, 110, 14592–14597. [Google Scholar] [CrossRef]
- Rubinstein, G.M.; Lipscomb, G.L.; Williams-Rhaesa, A.M.; Schut, G.J.; Kelly, R.M.; Adams, M.W.W. Engineering the cellulolytic extreme thermophile Caldicellulosiruptor bescii to reduce carboxylic acids to alcohols using plant biomass as the energy source. J. Ind. Microbiol. Biotechnol. 2020, 47, 1–13. [Google Scholar] [CrossRef]
- Nakashima, N.; Tamura, T. A new carbon catabolite repression mutation of Escherichia coli, mlc, and its use for producing isobutanol. J. Biosci. Bioeng. 2012, 114, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Akita, H.; Nakashima, N.; Hoshino, T. Bacterial production of isobutanol without expensive reagents. Appl. Microbiol. Biotechnol. 2014, 99, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Brat, D.; Boles, E. Isobutanol production fromd-xylose by recombinant Saccharomyces cerevisiae. FEMS Yeast Res. 2013, 13, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Cheng, J.-S.; Wang, B.L.; Fink, G.R.; Stephanopoulos, G. Xylose isomerase overexpression along with engineering of the pentose phosphate pathway and evolutionary engineering enable rapid xylose utilization and ethanol production by Saccharomyces cerevisiae. Metab. Eng. 2012, 14, 611–622. [Google Scholar] [CrossRef]
- Zhang, Y.; Lane, S.; Chen, J.-M.; Hammer, S.K.; Luttinger, J.; Yang, L.; Jin, Y.-S.; Avalos, J.L. Xylose utilization stimulates mitochondrial production of isobutanol and 2-methyl-1-butanol in Saccharomyces cerevisiae. Biotechnol. Biofuels 2019, 12, 1–15. [Google Scholar] [CrossRef]
- Promdonkoy, P.; Mhuantong, W.; Champreda, V.; Tanapongpipat, S.; Runguphan, W. Improvement in d-xylose utilization and isobutanol production in S. cerevisiae by adaptive laboratory evolution and rational engineering. J. Ind. Microbiol. Biotechnol. 2020, 47, 497–510. [Google Scholar] [CrossRef]
- Kim, S.R.; Skerker, J.M.; Kang, W.; Lesmana, A.; Wei, N.; Arkin, A.P.; Jin, Y.-S. Rational and evolutionary engineering approaches uncover a small set of genetic changes efficient for rapid xylose fermentation in Saccharomyces cerevisiae. PLoS ONE 2013, 8, e57048. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.; Zhang, Y.; Yun, E.J.; Ziolkowski, L.; Zhang, G.; Jin, Y.-S.; Avalos, J.L. Xylose assimilation enhances the production of isobutanol in engineered Saccharomyces cerevisiae. Biotechnol. Bioeng. 2019, 117, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.; Müller, F.; Takors, R.; Blombach, B. Harnessing novel chromosomal integration loci to utilize an organosolv-derived hemicellulose fraction for isobutanol production with engineered Corynebacterium glutamicum. Microb. Biotechnol. 2017, 11, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Krause, F.S.; Blombach, B.; Eikmanns, B.J. Metabolic Engineering of Corynebacterium glutamicum for 2-Ketoisovalerate Production. Appl. Environ. Microbiol. 2010, 76, 8053–8061. [Google Scholar] [CrossRef]
- Desai, S.H.; Rabinovitch-Deere, C.A.; Tashiro, Y.; Atsumi, S. Isobutanol production from cellobiose in Escherichia coli. Appl. Microbiol. Biotechnol. 2014, 98, 3727–3736. [Google Scholar] [CrossRef]
- Desai, S.H.; A Rabinovitch-Deere, C.; Fan, Z.; Atsumi, S. Isobutanol production from cellobionic acid in Escherichia coli. Microb. Cell Factories 2015, 14, 1–10. [Google Scholar] [CrossRef]
- Su, H.; Lin, J.; Wang, G. Metabolic engineering of Corynebacterium crenatium for enhancing production of higher alcohols. Sci. Rep. 2016, 6, 39543. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kim, J.K.; Ahn, J.-O.; Song, Y.-H.; Shin, C.-S.; Park, Y.-C.; Kim, K.H. Isobutanol production from empty fruit bunches. Renew. Energy 2020, 157, 1124–1130. [Google Scholar] [CrossRef]
- Jung, H.-M.; Lee, J.Y.; Lee, J.-H.; Oh, M.-K. Improved production of isobutanol in pervaporation-coupled bioreactor using sugarcane bagasse hydrolysate in engineered Enterobacter aerogenes. Bioresour. Technol. 2018, 259, 373–380. [Google Scholar] [CrossRef]
- Jung, H.M.; Kim, Y.H.; Oh, M.K. Formate and nitrate utilization in Enterobacter aerogenes for semi-anaerobic production of isobutanol. Biotechnol. J. 2017, 12, 1700121. [Google Scholar] [CrossRef]
- Huo, Y.-X.; Cho, K.M.; Rivera, J.G.L.; Monte, E.; Shen, C.R.; Yan, Y.; Liao, J.C. Conversion of proteins into biofuels by engineering nitrogen flux. Nat. Biotechnol. 2011, 29, 346–351. [Google Scholar] [CrossRef]
- Wu, W.; Tran-Gyamfi, M.B.; Jaryenneh, J.D.; Davis, R.W. Cofactor engineering of ketol-acid reductoisomerase (IlvC) and alcohol dehydrogenase (YqhD) improves the fusel alcohol yield in algal protein anaerobic fermentation. Algal Res. 2016, 19, 162–167. [Google Scholar] [CrossRef]
- Liu, F.; Wu, W.; Tran-Gymfi, M.B.; Jaryenneh, J.D.; Zhuang, X.; Davis, R.W. Bioconversion of distillers’ grains hydrolysates to advanced biofuels by an Escherichia coli co-culture. Microb. Cell Factor. 2017, 16, 192. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-J.; Seo, D.; Choi, K.-Y. Bioalcohol production from spent coffee grounds and okara waste biomass by engineered Bacillus subtilis. Biomass. Convers. Biorefinery 2019, 10, 167–173. [Google Scholar] [CrossRef]
- Tian, L.; Papanek, B.; Olson, D.G.; Rydzak, T.; Holwerda, E.K.; Zheng, T.; Zhou, J.; Maloney, M.; Jiang, N.; Giannone, R.J.; et al. Simultaneous achievement of high ethanol yield and titer in Clostridium thermocellum. Biotechnol. Biofuels 2016, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.A.; Olson, D.G.; Argyros, D.A.; Miller, B.B.; Barrett, T.F.; Murphy, D.M.; McCool, J.D.; Warner, A.K.; Rajgarhia, V.B.; Lynd, L.R.; et al. Development of pyrF-based genetic system for targeted gene deletion in Clostridium thermocellum and creation of a pta mutant. Appl. Environ. Microbiol. 2010, 76, 6591–6599. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.G.; Lynd, L.R. Transformation of Clostridium thermocellum by electroporation. Cellulases 2012, 510, 317–330. [Google Scholar] [CrossRef]
- Mearls, E.B.; Olson, D.G.; Herring, C.D.; Lynd, L.R. Development of a regulatable plasmid-based gene expression system for Clostridium thermocellum. Appl. Microbiol. Biotechnol. 2015, 99, 7589–7599. [Google Scholar] [CrossRef]
- Walker, J.E.; Lanahan, A.A.; Zheng, T.; Toruno, C.; Lynd, L.R.; Cameron, J.C.; Olson, D.G.; Eckert, C.A. Development of both type I-B and type II CRISPR/Cas genome editing systems in the cellulolytic bacterium Clostridium thermocellum. Metab. Eng. Commun. 2020, 10, e00116. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Y.; Li, Y. Production of fuels and chemicals from renewable resources using engineered Escherichia coli. Biotechnol. Adv. 2019, 37, 107402. [Google Scholar] [CrossRef]
- Hasunuma, T.; Ishii, J.; Kondo, A. Rational design and evolutional fine tuning of Saccharomyces cerevisiae for biomass breakdown. Curr. Opin. Chem. Biol. 2015, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Arora, A. Tracking strategic developments for conferring xylose utilization/fermentation by Saccharomyces cerevisiae. Ann. Microbiol. 2020, 70, 1–17. [Google Scholar] [CrossRef]
- Promdonkoy, P.; Siripong, W.; Downes, J.J.; Tanapongpipat, S.; Runguphan, W. Systematic improvement of isobutanol production from d-xylose in engineered Saccharomyces cerevisiae. AMB Express 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Bengtsson, O.; Hahn-Hägerdal, B.; Gorwa-Grauslund, M.F. Xylose reductase from Pichia stipitis with altered coenzyme preference improves ethanolic xylose fermentation by recombinant Saccharomyces cerevisiae. Biotechnol. Biofuels 2009, 2, 9. [Google Scholar] [CrossRef]
- Petschacher, B.; Nidetzky, B. Altering the coenzyme preference of xylose reductase to favor utilization of NADH enhances ethanol yield from xylose in a metabolically engineered strain of Saccharomyces cerevisiae. Microb. Cell Factories 2008, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Abu Saleh, A.; Pack, S.P.; Annaluru, N.; Kodaki, T.; Makino, K. Ethanol production from xylose by recombinant Saccharomyces cerevisiae expressing protein engineered NADP+ -dependent xylitol dehydrogenase. J. Biotechnol. 2007, 130, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Zhu, B.; Zhang, G.; Wei, N. Co-fermentation of cellobiose and xylose by mixed culture of recombinant Saccharomyces cerevisiae and kinetic modeling. PLoS ONE 2018, 13, e0199104. [Google Scholar] [CrossRef]
- Vinuselvi, P.; Lee, S.K. Engineered Escherichia coli capable of co-utilization of cellobiose and xylose. Enzym. Microb. Technol. 2012, 50, 1–4. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Jiang, J.; Lu, Q.; Zhao, Z.; Xie, T.; Zhao, H.; Wang, M. Engineering Corynebacterium crenatum to produce higher alcohols for biofuel using hydrolysates of duckweed (Landoltia punctata) as feedstock. Microb. Cell Factories 2015, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-Y.; Wernick, D.G.; Tat, C.A.; Liao, J.C. Consolidated conversion of protein waste into biofuels and ammonia using Bacillus subtilis. Metab. Eng. 2014, 23, 53–61. [Google Scholar] [CrossRef]
- Tojo, S.; Satomura, T.; Kumamoto, K.; Hirooka, K.; Fujita, Y. Molecular mechanisms underlying the positive stringent response of the Bacillus subtilis ilv-leu operon, involved in the biosynthesis of branched-chain amino acids. J. Bacteriol. 2008, 190, 6134–6147. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Yang, Y.-H.; Choi, K.-Y. Bioconversion of plant hydrolysate biomass into biofuels using an engineered Bacillus subtilis and Escherichia coli mixed-whole cell biotransformation. Biotechnol. Bioprocess. Eng. 2020, 25, 477–484. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Current state-of-the-art in ethanol production from lignocellulosic feedstocks. Microbiol. Res. 2020, 240, 126534. [Google Scholar] [CrossRef]
- Kuroda, K.; Hammer, S.K.; Watanabe, Y.; López, J.M.; Fink, G.R.; Stephanopoulos, G.; Ueda, M.; Avalos, J.L. Critical roles of the pentose phosphate pathway and GLN3 in isobutanol-specific tolerance in yeast. Cell Syst. 2019, 9, 534–547.e5. [Google Scholar] [CrossRef]
- Seo, H.-M.; Jeon, J.-M.; Lee, J.H.; Song, H.-S.; Joo, H.-B.; Park, S.-H.; Choi, K.-Y.; Kim, Y.H.; Park, K.; Ahn, J.; et al. Combinatorial application of two aldehyde oxidoreductases on isobutanol production in the presence of furfural. J. Ind. Microbiol. Biotechnol. 2015, 43, 37–44. [Google Scholar] [CrossRef]
- Song, H.-S.; Jeon, J.-M.; Choi, Y.-K.; Kim, J.-Y.; Kim, W.; Rene, E.R.; Park, K.; Ahn, J.-O.; Lee, H.; Yang, Y.-H. L-Glycine alleviates furfural-induced growth inhibition during isobutanol production in Escherichia coli. J. Microbiol. Biotechnol. 2017, 27, 2165–2172. [Google Scholar] [CrossRef]
- Song, H.-S.; Jeon, J.-M.; Kim, H.-J.; Bhatia, S.; Sathiyanarayanan, G.; Kim, J.; Hong, J.W.; Hong, Y.G.; Choi, K.-Y.; Kim, Y.-G.; et al. Increase in furfural tolerance by combinatorial overexpression of NAD salvage pathway enzymes in engineered isobutanol-producing E. coli. Bioresour. Technol. 2017, 245, 1430–1435. [Google Scholar] [CrossRef]
- Andre, A.; Nagy, T.; Toth, A.J.; Haaz, E.; Fozer, D.; Tarjani, J.A.; Mizsey, P. Distillation contra pervaporation: Comprehensive investigation of isobutanol-water separation. J. Clean. Prod. 2018, 187, 804–818. [Google Scholar] [CrossRef]
- Farhadi, M.; Pazuki, G.; Raisi, A. Modeling of the pervaporation process for isobutanol purification from aqueous solution using intelligent systems. Sep. Sci. Technol. 2017, 53, 1383–1396. [Google Scholar] [CrossRef]
| Ethanol | 1-butanol | Isobutanol | Gasoline | |
|---|---|---|---|---|
| Lower Heating Value (MJ/kg) | 27.0 | 33.1 | 33.3 | 43.5 |
| Flash point (°C) | 13 | 37 | 28 | −43 |
| Solubility (20 °C in water, wt %) | Miscible | 7.7 | 8.7 | negligible |
| Boiling temperature (°C) | 78.4 | 117.7 | 108 | 25–215 |
| Vapor toxicity | Toxic | Moderate | Moderate | Moderate |
| Microorganism | Carbon Source | Strategy | Genes Involved | Titer | Time | Reactor | Reference |
| Clostridium cellulolyticum | Cellulose | Engineered isobutanol pathway | ilvDEC, ilvCEC, yqhDEC, alsSBS, kivdLL | 0.66 g/L | 216 h | Tube | [41] |
| Cellulose | Keto acid pathway Promoter engineering | Δspo0A, alsSBS, kivdLL | 0.35 g/L | ~250 h | Unknown | [43] | |
| Geobacillus thermoglucosidasius | Cellobiose | Keto acid pathway Promoter engineering | ilvCGT alsSBS, kivdLL (LLKF_1386) | 0.6 g/L | 48 h | Tube | [44] |
| Clostridium thermocellum | Cellulose | Keto acid pathway Promoter engineering Optimize fermentation conditions | ilvBCT, ilvNCT, ilvCCT, ilvDCT, kivdLL | 5.4 g/L | 75 h | Tube | [45] |
| Cellulose | Inhibition competition pathway Adaptive laboratory evolution | Δhpt, Δldh, Δpta, adhED494G | 5.1 g/L | 220 h | Bioreactor | [46] | |
| Trichoderma reesei and Escherichia coli | Pretreated corn stover | Random mutagenesis Engineered isobutanol pathway Microbial consortium | T. reesei RUTC30: - E. coli NV3: ilvCEC, ilvDEC, alsSBS, kivdLL, Adh2SC | 1.88 g/L | 380 h | Bioreactor | [47] |
| Caldicellulosiruptor bescii | Switchgrass | Inhibition competition pathway AOR-ADH pathway | Δldh PF0346PF (AOR), Teth514_0564PF (ADHA) | 0.17 g/L | 40 h | Fermentor | [48] |
| Glucose-xylose mixture | Dismantle carbon catabolite repression Inhibition competition pathway Engineered isobutanol pathway | ΔldhA, ΔadhE, ΔpflB, Δpta-ackA, mlc*, ilvCEC, ilvDEC, alsSBS, kivdLL, Adh2SC, | 11 g/L | 182 h | Flask | [49] | |
| Cedar | Dismantle carbon catabolite repression Inhibition competition pathway Promoter engineering Chromosome integration Optimize fermentation conditions | ΔldhA, ΔadhE, ΔpflB, Δpta-ackA, mlc*, ilvCEC, ilvDEC, alsSBS, kivdLL, adhALL | 3.7 g/L | 96 h | Flask | [49,50] | |
| Saccharomyces cerevisiae | Xylose | Xylose XI pathway Cytosolic isobutanol pathway | ΔIlv2, ΔIlv5, ΔIlv3, xylACP, Tal1SC, Xks1SC, Ilv2ΔN54SC, Ilv5ΔN48SC, Ilv3ΔN19SC, Aro10SC, Adh2SC | 1.36 mg/L | 150 h | Flask | [51] |
| Xylose | Xylose XI pathway Chromosome integration Adaptive laboratory evolution Mitochondrial isobutanol pathway Fed-batch fermentation | ΔBAT1, ΔALD6, ΔPHO13, ΔURA3 RKI1SC, RPE1SC, TKL1SC, TAL1SS, XYLAPE, XYL3SS, ILV2SC, ILV5SC, ILV3SC, kivdLL, | 3.1 g/L | 192 h | Tube | [52,53] | |
| Xylose | Xylose XR-XDH pathway Chromosome integration Mitochondrial isobutanol pathway Copy number optimization Adaptive laboratory evolution | ΔPHO13, ΔGRE3, hxt7F79S, XYL1SS, XYL2SS, XYL3SS ILV2SC, ILV5SC, ILV3SC, ADH7SC, kivdLL | 92.9 mg/ L | 144 h | Tube | [53,54] | |
| Xylose | Xylose XR-XDH pathway Chromosome integration Copy number optimization Mitochondrial isobutanol pathway Optimize fermentation conditions Fed-batch fermentation | ΔALD6, ΔPHO13 XYL1SS, XYL2SS, XYL3SS ILV2SC, ILV5SC, ILV3SC, kivdLL, | 2.6 g/L | Unknown | Bioreactor | [55,56] | |
| Corynebacterium glutamicum | Hemicellulose fraction | Inhibition competition pathway Xylose XI pathway Arabinose metabolism pathway Engineered isobutanol pathway | Δpqo, ΔilvE, ΔldhA, Δmdh, xylAXC, xylBCG, araBEC, araAEC, araDEC, ilvBEC, ilvNEC, ilvCEC, ilvDEC, pntABEC, kivdLL, Adh2CG | 0.53 g/L | ~28 h | Flask | [57,58] |
| Cellobiose | Inhibition competition pathway Copy number optimization Engineered isobutanol pathway Cellobiose metabolism pathway | ΔadhE, ΔfrdBC, Δfnr, ΔldhA, Δpta, ΔpflB, ilvCEC, ilvDEC, alsSBS, kivdLL, adhALL, bglCTF | 7.64 g/L | 72 h | Unknown | [59] | |
| Cellobionic | Inhibition competition pathway Engineered isobutanol pathway | ΔadhE, ΔfrdBC, Δfnr, ΔldhA, Δpta, ΔpflB, ilvCEC, ilvDEC, alsSBS, kivdLL, adhALL | 1.4 g/L | 48 h | Unknown | [60] | |
| Corynebacterium crenatum | Duckweed | Engineered isobutanol pathway | ILV2SC, ILV5SC, ILV3SC, kivdLL, Adh2SC | 1.15 g/L | 96 h | Flask | [61] |
| Duckweed | Whole-cell mutagenesis Engineered isobutanol pathway Simultaneous saccharification and fermentation | , , ILV3SC, kivdLL, | 5.6 g/L | 96 h | Flask | [62] | |
| Emptyfruit bunches | Engineered isobutanol pathway Optimize fermentation conditions Separate hydrolysis and fermentation | ilvCEC, ilvDEC, adhPEC, alsSBS, kivDLL | 5.4 g/L | 156 h | Unknown | [62] | |
| Enterobacter aerogenes | Sugarcane bagasse | Inhibition competition pathway Engineered isobutanol pathway Pervaporation-coupled fermentation | ΔldhA, ΔbudA, ΔpflB, ΔptsG, ilvDKP, ilvCKP, budBKP, kivDLL, adhALL | 23 g/L | 72 h | Fermenter | [63,64] |
| Escherichia coli | Algal protein | Chemical mutagenesis Protein conversion Cofactor engineering | ΔglnA, ΔgdhA, ΔluxS, ΔlsrA, ilvCA71S, R76D, S78D, Q110A, yqhDG39I, S40R ilvEEC, ilvAEC, sdabEC, avtaEC, LueDHTI ilvDEC, alsSBS, kivDLL | 0.2 g/L | Unknown | Flask | [65,66] |
| E. coli BLF2 and E. coli AY3 (1:1.5) | Distillers’ grains | Chemical mutagenesis Protein conversion Cofactor engineering Engineered isobutanol pathway Microbial consortium | E. coli BLF2: Δldh ilvCEC, ilvDEC, YqhDEC, alsSBS, kivdLL E. coli AY3: ΔglnA, ΔgdhA, ΔluxS, ΔlsrA, ilvCA71S, R76D, S78D, Q110A, yqhDG39I, S40R ilvEEC, ilvAEC, sdabEC, avtaEC, LueDHTI ilvDEC, alsSBS, kivDLL | 6.5 g/L | 52 h | Tube | [65,67] |
| Bacillus subtilis | Okara wastes | Activation of ilv-leu operonInhibition competition pathwayKeto acid pathway | ΔcodY, ΔbkdB, ΔrelA,LueDHTI,kivDLL, yqhDEC | 0.02 g/L | Unknown | Flask | [68] |
| Bacillus subtilis and Escherichia coli (1:4) | Watermelon rind and Okara waste | Protein conversion Engineered isobutanol pathway Microbial consortium | B. subtilis: ΔcodY, ΔbkdB, LueDHTI, kivDLL, yqhDEC E. coli: ilvCEC, ilvDEC, YqhDEC, alsSBS, kivdLL | 0.88 g/L | 220 h | Flask | [69,70,71,72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Zhang, W.; Zhang, A.; Shao, W. Biorefinery: The Production of Isobutanol from Biomass Feedstocks. Appl. Sci. 2020, 10, 8222. https://doi.org/10.3390/app10228222
Su Y, Zhang W, Zhang A, Shao W. Biorefinery: The Production of Isobutanol from Biomass Feedstocks. Applied Sciences. 2020; 10(22):8222. https://doi.org/10.3390/app10228222
Chicago/Turabian StyleSu, Yide, Weiwei Zhang, Aili Zhang, and Wenju Shao. 2020. "Biorefinery: The Production of Isobutanol from Biomass Feedstocks" Applied Sciences 10, no. 22: 8222. https://doi.org/10.3390/app10228222
APA StyleSu, Y., Zhang, W., Zhang, A., & Shao, W. (2020). Biorefinery: The Production of Isobutanol from Biomass Feedstocks. Applied Sciences, 10(22), 8222. https://doi.org/10.3390/app10228222




