Avocado-Derived Biomass as a Source of Bioenergy and Bioproducts
Abstract
:Featured Application
Abstract
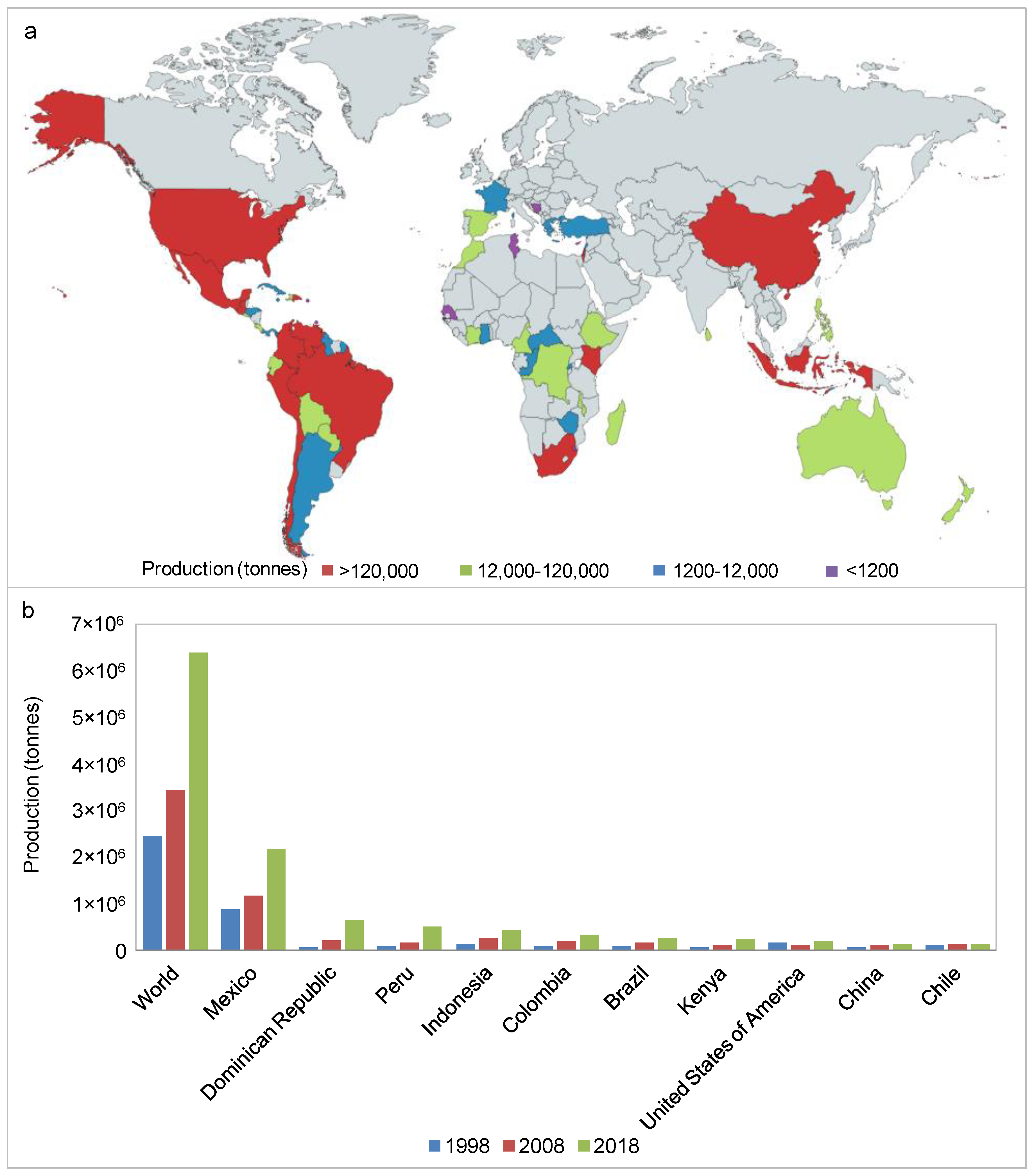
1. Introduction
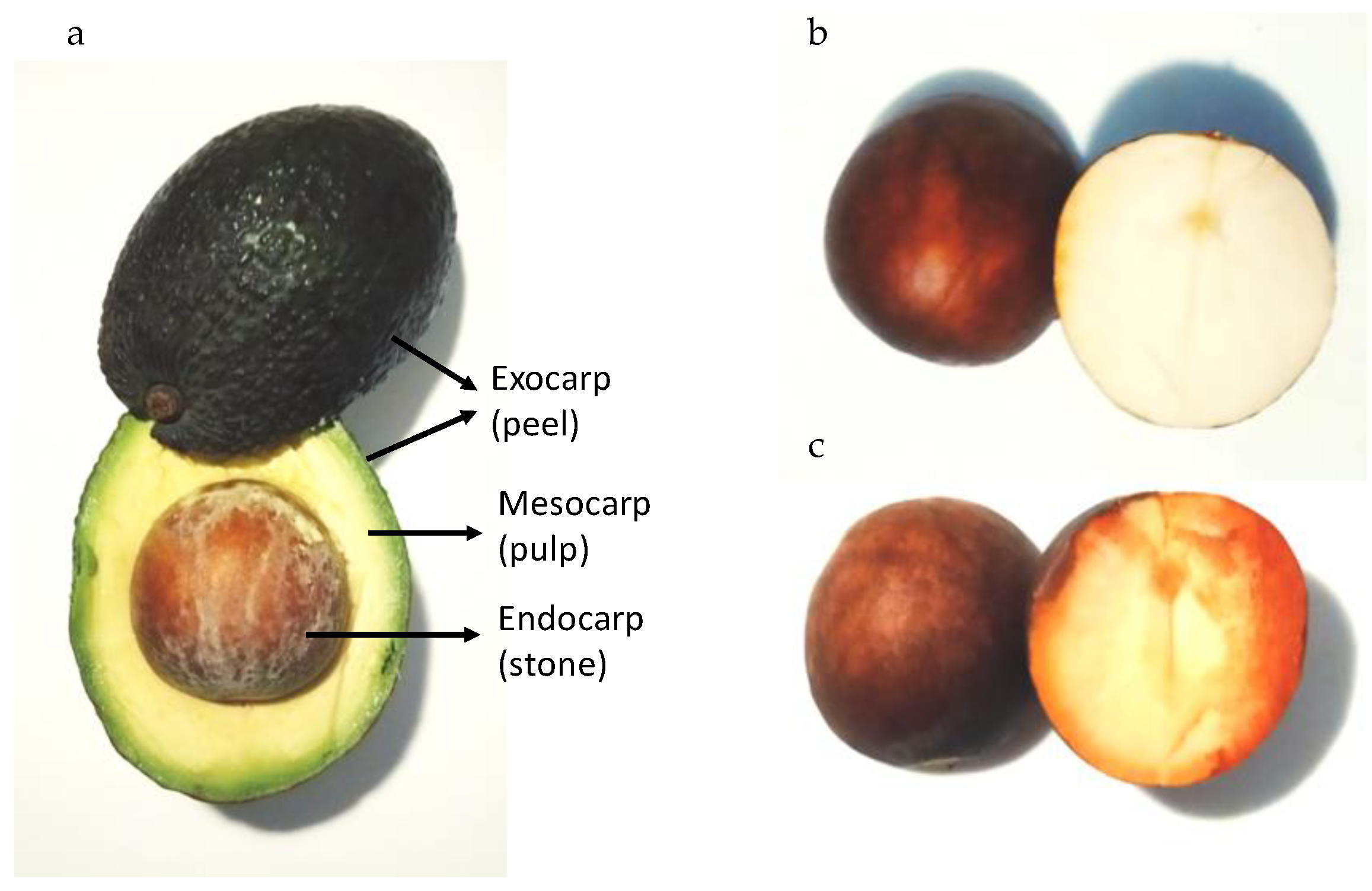
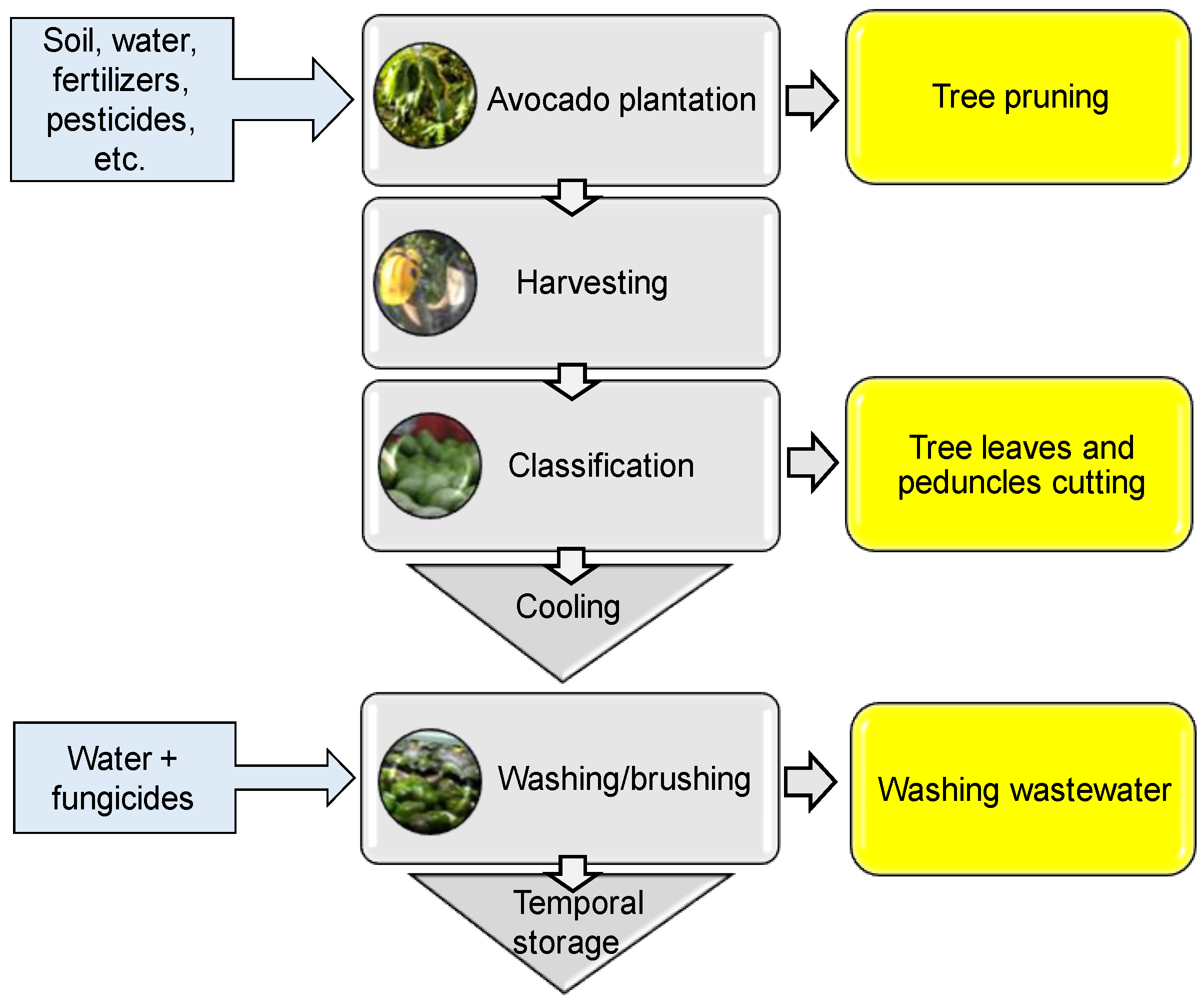
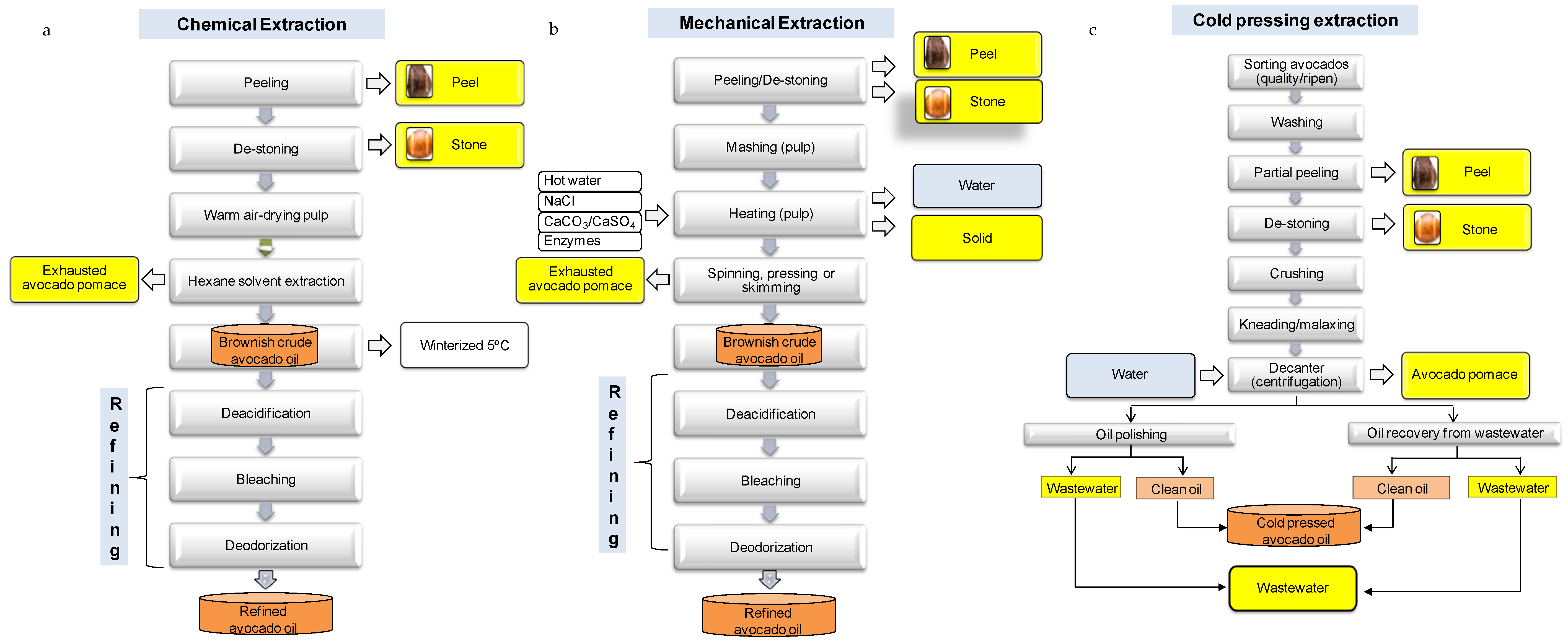
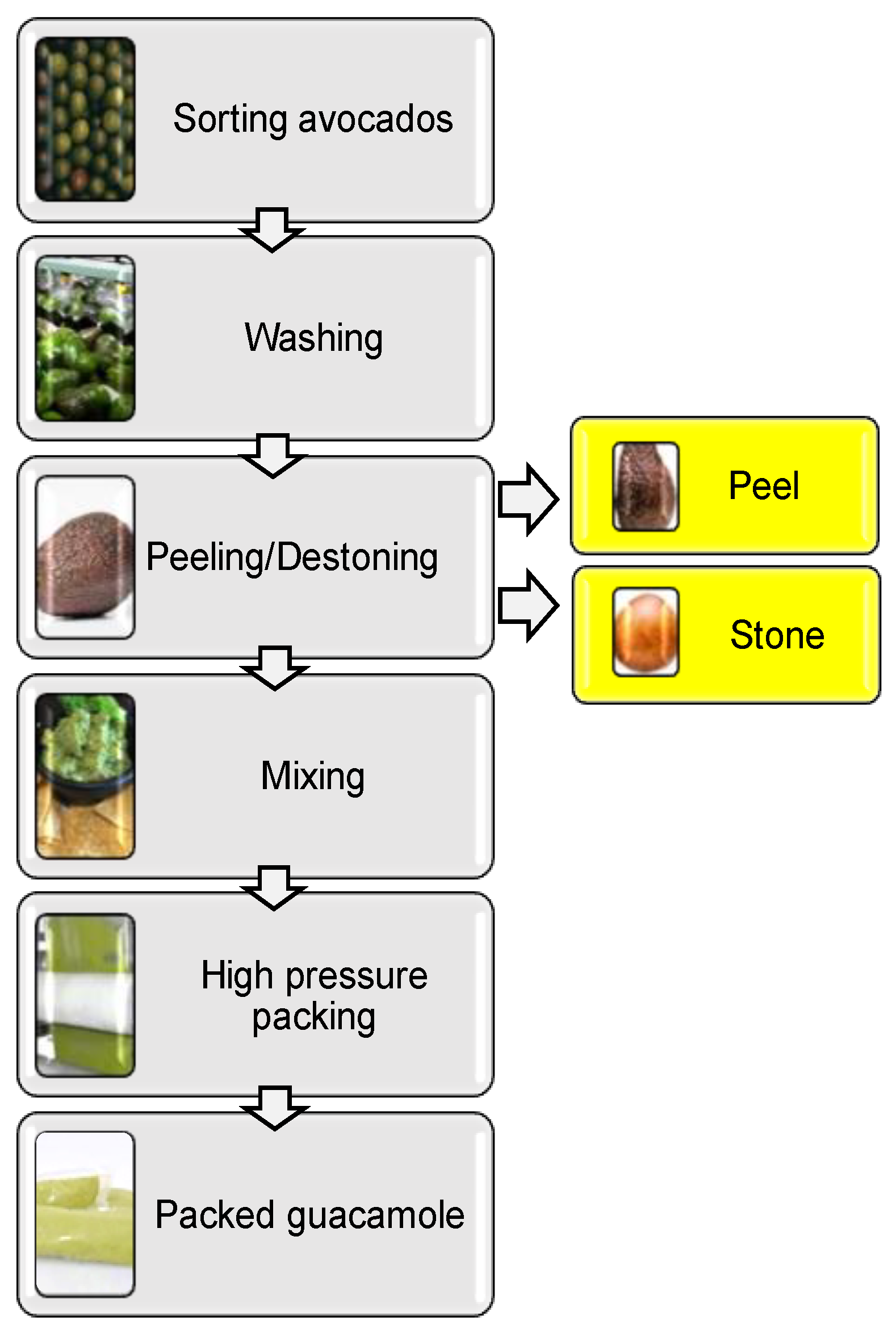
2. Avocado Processing and By-Products
3. Chemical Composition of Avocado Waste
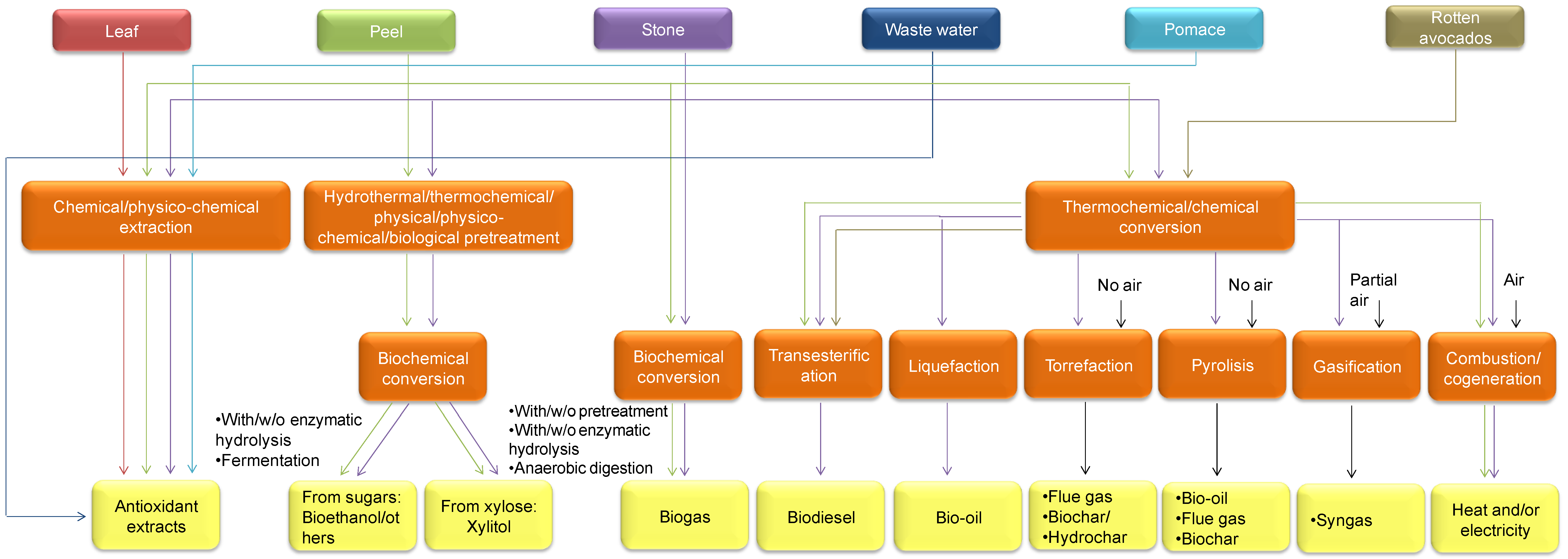
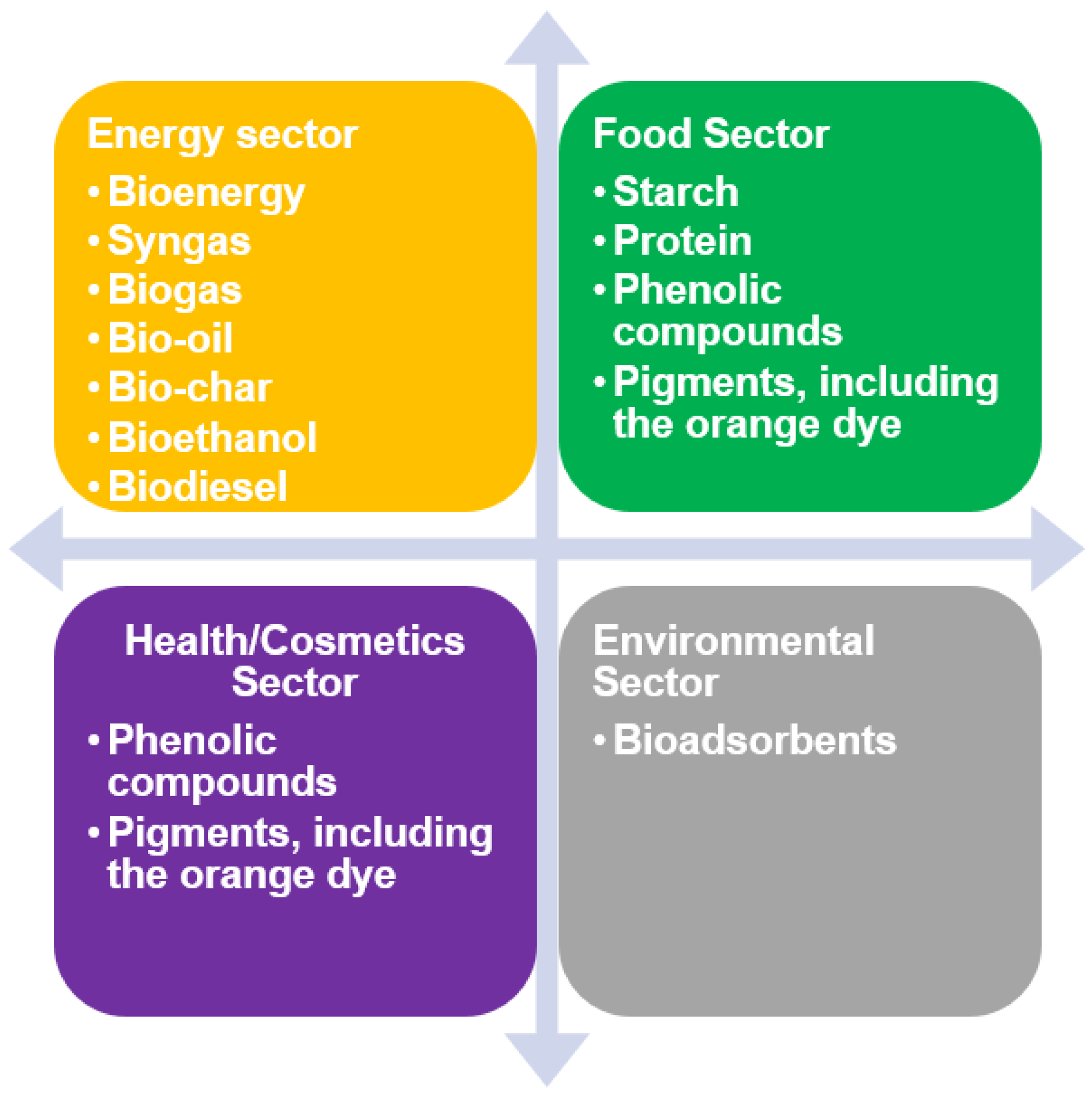
4. Valorization Technologies of Avocado-Derived Biomass through Obtaining Bioenergy and Biofuels
4.1. Bioenergy
4.2. Biogas through Biochemical Conversion
4.3. Biofuels through Thermochemical Methods: Gasification, Pyrolysis, Torrefaction, and Liquefaction
4.4. Biodiesel
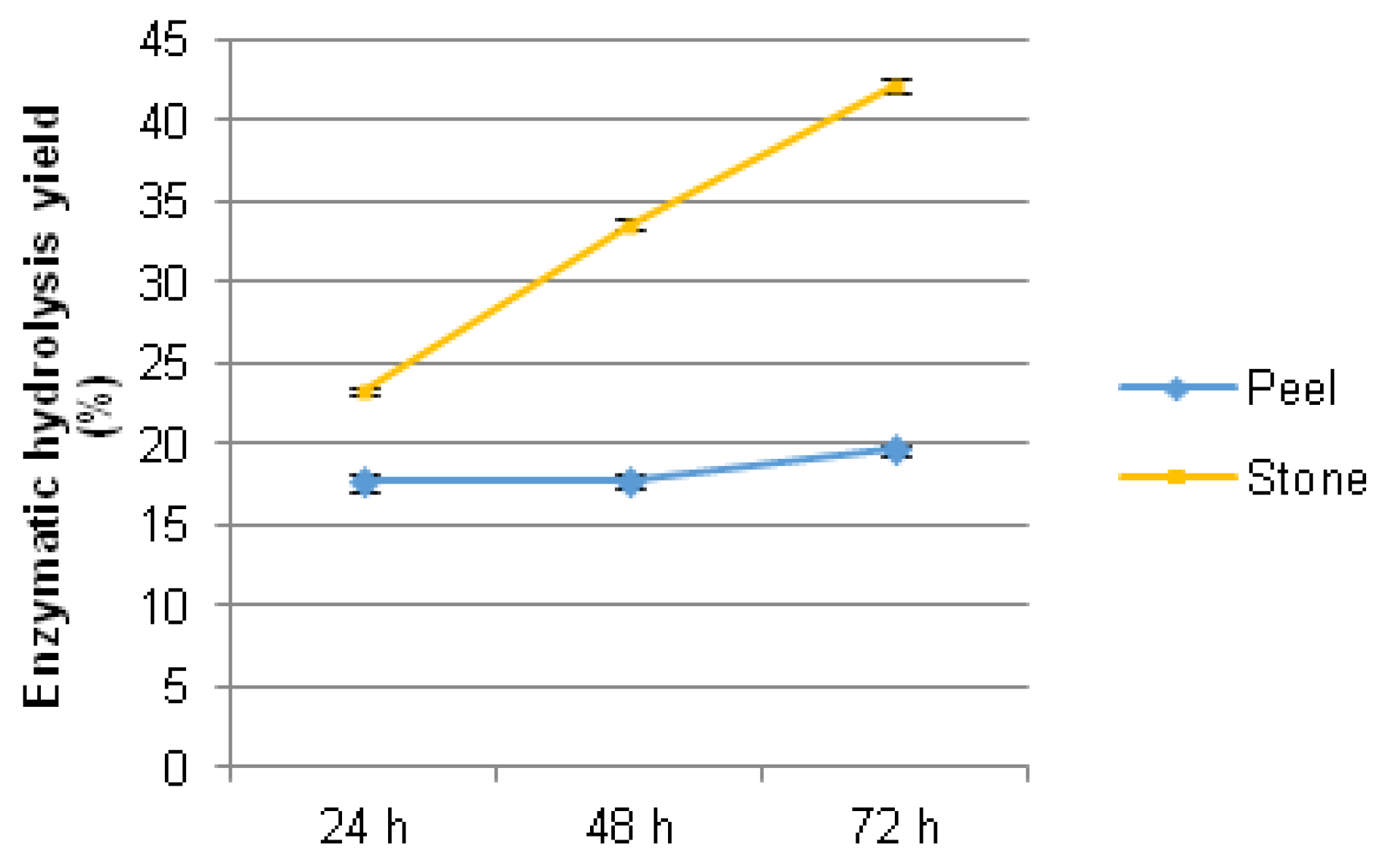
4.5. Fermentable Sugars and Bioethanol
5. Other Valorization Ways through Obtaining Starch and Protein
6. Valorization Technologies for Avocado-Derived Biomass through Obtaining Bioactive Compounds
6.1. Phenolic Compounds
6.2. Other Bioactive Compounds
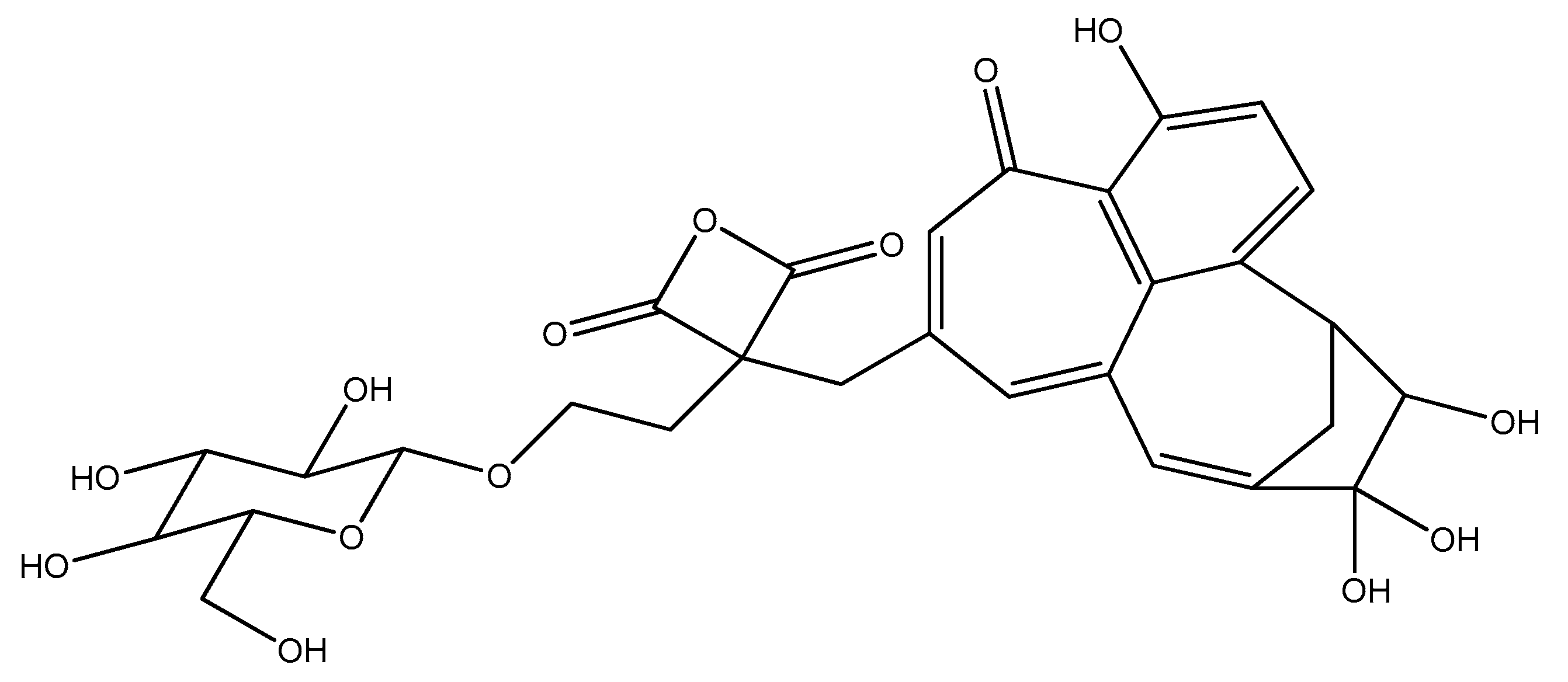
7. Extraction of an Orange Colorant
8. Production of Biosorbents
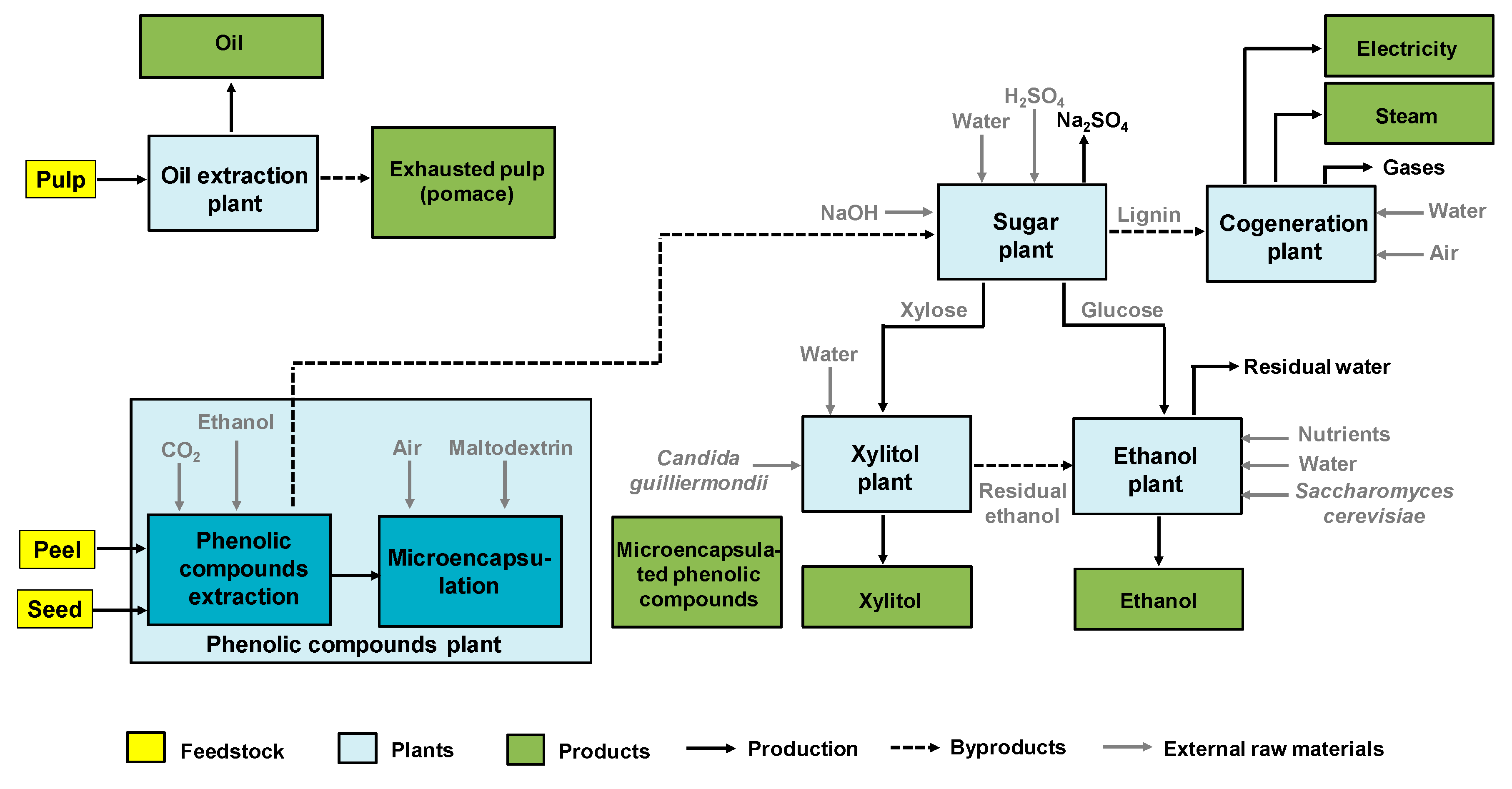
9. Biorefinery Approaches, Techno-Economic, and Environmental Impact Analyses
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Subsecretaría de Fomento a los Agronegocios. Monografía de Cultivos; SAGARPA Mexico DF: Mexico City, Mexico, 2011. [Google Scholar]
- Zhang, Z.; Huber, D.J.; Rao, J. Antioxidant systems of ripening avocado (Persea americana Mill.) fruit following treatment at the preclimacteric stage with aqueous 1-methylcyclopropene. Postharvest Biol. Technol. 2013, 76, 58–64. [Google Scholar] [CrossRef]
- CONABIO. Qué Nos Aportan Los Aguacates. Available online: https://www.biodiversidad.gob.mx/diversidad/alimentos/que-nos-aportan/N_aguacate (accessed on 10 September 2020).
- Ding, H.; Chin, Y.W.; Kinghorn, A.D.; D’Ambrosio, S.M. Chemopreventive characteristics of avocado fruit. Semin. Cancer Biol. 2007, 17, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Ranade, S.S.; Thiagarajan, P. A review on Persea americana Mill. (Avocado)—Its fruit and oil. Int. J. PharmTech Res. 2015, 8, 72–77. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO) FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 10 September 2020).
- Leite, J.J.G.; Brito, É.H.S.; Cordeiro, R.A.; Brilhante, R.S.N.; Sidrim, J.J.C.; Bertini, L.M.; De Morais, S.M.; Rocha, M.F.G. Chemical composition, toxicity and larvicidal and antifungal activities of Persea americana (avocado) seed extracts. Rev. Soc. Bras. Med. Trop. 2009, 42, 110–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yahia, E.M.; Woolf, A.B. Avocado (Persea americana Mill.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Woodhead Publishing Limited: Sawston, CA, USA, 2011; Volume 2, pp. 125–185. [Google Scholar]
- Shahbandeh, M.U.S. Consumers’ Main Drivers for Buying Avocados. 2019. Available online: https://www.statista.com/statistics/317753/us-consumers-main-drivers-for-buying-avocados/ (accessed on 10 September 2020).
- Camila, D.B.; Waiga, L.H.; Soltovski De Oliveira, C.; Lacerda, L.G.; Schnitzler, E. Morphological and thermoanalytical study of modified avocado seeds starch with lactic acid. Chem. J. Mold. 2017, 12, 13–18. [Google Scholar] [CrossRef]
- Krumreich, F.D.; Borges, C.D.; Mendonça, C.R.B.; Jansen-Alves, C.; Zambiazi, R.C. Bioactive compounds and quality parameters of avocado oil obtained by different processes. Food Chem. 2018, 257, 376–381. [Google Scholar] [CrossRef]
- García-Vargas, M.C.; del Contreras, M.; Gómez-Cruz, I.; Romero-García, J.M.; Castro, E. Avocado-derived biomass: Chemical composition and antioxidant potential. Proceedings. 2020. Available online: https://sciforum.net/paper/view/7750 (accessed on 18 November 2020).
- Rodríguez-Carpena, J.G.; Morcuende, D.; Estévez, M. Avocado by-products as inhibitors of color deterioration and lipid and protein oxidation in raw porcine patties subjected to chilled storage. Meat Sci. 2011, 89, 166–173. [Google Scholar] [CrossRef]
- Chel-Guerrero, L.; Barbosa-Martín, E.; Martínez-Antonio, A.; González-Mondragón, E.; Betancur-Ancona, D. Some physicochemical and rheological properties of starch isolated from avocado seeds. Int. J. Biol. Macromol. 2016, 86, 302–308. [Google Scholar] [CrossRef]
- Gutierrez-Contreras, M.; Lara-Chavez, M.B.N.; Guillen-Andrade, H.C.-B. Agroecoloy of the avocado-producing belt area in Michoacan, Mexico/Agroecologia de la Franja Aguacatera en Michoacan, Mexico/Agroecologia da Faixa de Abacateiros em Michoacan, Mexico. Interciencia 2010, 35, 647–653. [Google Scholar]
- del Mar Contreras, M.; Lama-Muñoz, A.; Manuel Gutiérrez-Pérez, J.; Espínola, F.; Moya, M.; Castro, E. Protein extraction from agri-food residues for integration in biorefinery: Potential techniques and current status. Bioresour. Technol. 2019, 280, 459–477. [Google Scholar] [CrossRef]
- Medfai, W.; Contreras, M.D.M.; Lama-Muñoz, A.; Mhamdi, R.; Oueslati, I.; Castro, E. How Cultivar and Extraction Conditions Affect Antioxidants Type and Extractability for Olive Leaves Valorization. ACS Sustain. Chem. Eng. 2020, 8, 5107–5118. [Google Scholar] [CrossRef]
- del Mar Contreras, M.; Lama-Muñoz, A.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Valorization of olive mill leaves through ultrasound-assisted extraction. Food Chem. 2020, 314, 126218. [Google Scholar] [CrossRef] [PubMed]
- Macías, A.M. México en el mercado internacional de aguacate. Rev. Cienc. Soc. 2011, 17, 517–532. [Google Scholar] [CrossRef]
- Permal, R.; Chang, W.L.; Seale, B.; Hamid, N.; Kam, R. Converting industrial organic waste from the cold-pressed avocado oil production line into a potential food preservative. Food Chem. 2020, 306, 125635. [Google Scholar] [CrossRef] [PubMed]
- Costagli, G.; Betti, M.; Laval, A.; Market, S.; Food, U.; Oil, O.; Val, T. Avocado oil extraction processes: Method for cold-pressed high-quality edible oil production versus traditional production. J. Agric. Eng. 2015, 46, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.; Ashton, O.; Mcghie, T.; Requejo-Jackman, C.; Eyres, L.; Sherpa, N.; Woolf, A. Avocado Oil—The Colour of Quality. ACS Symp. Ser. 2008, 983, 328–349. [Google Scholar]
- Tan, C.X. Virgin avocado oil: An emerging source of functional fruit oil. J. Funct. Foods 2019, 54, 381–392. [Google Scholar] [CrossRef]
- Ramtahal, G.; Akingbala, J.O.; Baccus-Taylor, G.S. Laboratory preparation and evaluation of Pollock variety avocado (Persea americana Mill) guacamole. J. Sci. Food Agric. 2007, 1243, 1237–1243. [Google Scholar] [CrossRef]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Pasini, F.; Caboni, M.F.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-ESI-QTOF-MS and HPLC-FLD-MS as valuable tools for the determination of phenolic and other polar compounds in the edible part and by-products of avocado. LWT-Food Sci. Technol. 2016, 73, 505–513. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Pintado, M.M.E.; Aguilar, C.N. Avocado by-products: Nutritional and functional properties. Trends Food Sci. Technol. 2018, 80, 51–60. [Google Scholar] [CrossRef]
- Saavedra, J.; Córdova, A.; Navarro, R.; Díaz-Calderón, P.; Fuentealba, C.; Astudillo-Castro, C.; Toledo, L.; Enrione, J.; Galvez, L. Industrial avocado waste: Functional compounds preservation by convective drying process. J. Food Eng. 2017, 198, 81–90. [Google Scholar] [CrossRef]
- Pratywi, C.D.; Marantika, S.; Dwijananti, P. Masturi Characterization of starch degradation during simple heating for bioethanol production from the avocado seed. IOP Conf. Ser. Mater. Sci. Eng. 2018, 432, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Perea-Moreno, A.J.; Aguilera-Ureña, M.J.; Manzano-Agugliaro, F. Fuel properties of avocado stone. Fuel 2016, 186, 358–364. [Google Scholar] [CrossRef]
- Eliyahu, D.; Yosef, E.; Weinberg, Z.G.; Hen, Y.; Nikbachat, M.; Solomon, R.; Mabjeesh, S.J.; Miron, J. Composition, preservation and digestibility by sheep of wet by-products from the food industry. Anim. Feed Sci. Technol. 2015, 207, 1–9. [Google Scholar] [CrossRef]
- Dávila, J.A.; Rosenberg, M.; Castro, E.; Cardona, C.A. A model biorefinery for avocado (Persea americana mill.) processing. Bioresour. Technol. 2017, 243, 17–29. [Google Scholar] [CrossRef]
- Barbosa-Martín, E.; Chel-Guerrero, L.; González-Mondragón, E.; Betancur-Ancona, D. Chemical and technological properties of avocado (Persea americana Mill.) seed fibrous residues. Food Bioprod. Process. 2016, 100, 457–463. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Schmidt, E.M.; Bonafe, E.G.; Eberlin, M.N.; Sawaya, A.C.; Visentainer, J.V. Antioxidant activity, phenolics and UPLC-ESI(-)-MS of extracts from different tropical fruits parts and processed peels. Food Res. Int. 2015, 77, 392–399. [Google Scholar] [CrossRef]
- García, R.; Pizarro, C.; Lavín, A.G.; Bueno, J.L. Characterization of Spanish biomass wastes for energy use. Bioresour. Technol. 2012, 103, 249–258. [Google Scholar] [CrossRef]
- Gaurav, N.; Sivasankari, S.; Kiran, G.S.; Ninawe, A.; Selvin, J. Utilization of bioresources for sustainable biofuels: A Review. Renew. Sustain. Energy Rev. 2017, 73, 205–214. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, Q.; Bartocci, P.; Wei, H.; Liu, S.S.; Wu, Z.; Zhou, H.; Yang, H.; Fantozzi, F.; Chen, H. Bioenergy in China: Evaluation of domestic biomass resources and the associated greenhouse gas mitigation potentials. Renew. Sustain. Energy Rev. 2020, 127, 109842. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Yaashikaa, P.R. Sources and Operations of Waste Biorefineries. In Refining Biomass Residues for Sustainable Energy and Bioproducts; Kumar, R.P., Gnansounou, E., Raman, J.K., Baskar, G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 111–133. [Google Scholar]
- Rincon, L.; Puri, M.; Kojakovic, A.; Maltsoglou, I. The contribution of sustainable bioenergy to renewable electricity generation in Turkey: Evidence based policy from an integrated energy and agriculture approach. Energy Policy 2019, 130, 69–88. [Google Scholar] [CrossRef]
- Di Bitonto, L.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Pastore, C. Residual Mexican biomasses for bioenergy and fine chemical production: Correlation between composition and specific applications. Biomass Convers. Biorefin. 2020. [Google Scholar] [CrossRef]
- Telmo, C.; Lousada, J.; Moreira, N. Proximate analysis, backwards stepwise regression between gross calorific value, ultimate and chemical analysis of wood. Bioresour. Technol. 2010, 101, 3808–3815. [Google Scholar] [CrossRef] [PubMed]
- del Mar Contreras, M.; Romero, I.; Moya, M.; Castro, E. Olive-derived biomass as a renewable source of value-added products. Process Biochem. 2020, 97, 43–56. [Google Scholar] [CrossRef]
- Domínguez, M.P.; Araus, K.; Bonert, P.; Sánchez, F.; San Miguel, G.; Toledo, M. The Avocado and Its Waste: An Approach of Fuel Potential/Application. In Environment, Energy and Climate Change II, 1st ed.; Lefebvre, G., Jiménez, E., Cabañas, B., Eds.; Springer: New York, NY, USA, 2016; Volume 34, pp. 199–223. [Google Scholar]
- Xue, J.; Chellappa, T.; Ceylan, S.; Goldfarb, J.L. Enhancing biomass + coal Co-firing scenarios via biomass torrefaction and carbonization: Case study of avocado pit biomass and Illinois No. 6 coal. Renew. Energy 2018, 122, 152–162. [Google Scholar] [CrossRef]
- Perea-Moreno, M.A.; Samerón-Manzano, E.; Perea-Moreno, A.J. Biomass as renewable energy: Worldwide research trends. Sustainability 2019, 11, 863. [Google Scholar] [CrossRef] [Green Version]
- Rashama, C.; Ijoma, G.; Matambo, T. Biogas generation from by-products of edible oil processing: A review of opportunities, challenges and strategies. Biomass Convers. Biorefin. 2019, 9, 803–826. [Google Scholar] [CrossRef]
- Dhanya, B.S.; Mishra, A.; Chandel, A.K.; Verma, M.L. Development of sustainable approaches for converting the organic waste to bioenergy. Sci. Total Environ. 2020, 723, 138109. [Google Scholar] [CrossRef]
- Kenasa, G.; Kena, E. Optimization of Biogas production from avocado fruit peel wastes codigestion with animal manure collected from juice vending house in Gimbi Town, Ethiopia. Ferment. Technol. 2019, 8, 1000153. [Google Scholar] [CrossRef]
- Global Bioenergy Statistics. World Bioenergy Association. 2019. Available online: https://worldbioenergy.org/global-bioenergy-statistics (accessed on 9 October 2020).
- Wang, S.; Jena, U.; Das, K.C. Biomethane production potential of slaughterhouse waste in the United States. Energy Convers. Manag. 2018, 173, 143–157. [Google Scholar] [CrossRef]
- Colombo, R.; Papetti, A. Avocado (Persea americana Mill.) by-products and their impact: From bioactive compounds to biomass energy and sorbent material for removing contaminants. A review. Int. J. Food Sci. Technol. 2019, 54, 943–951. [Google Scholar] [CrossRef]
- Streitwieser, D.A.; Cadena, I.A. Preliminary study of biomethane production of organic waste based on their content of sugar, starch, lipid, protein and fibre. Chem. Eng. Trans. 2018, 65, 661–666. [Google Scholar] [CrossRef]
- Vintila, T.; Ionel, I.; Rufis Fregue, T.T.; Wächter, A.R.; Julean, C.; Gabche, A.S. Residual biomass from food processing industry in Cameroon as feedstock for second-generation biofuels. BioResources 2019, 14, 3731–3745. [Google Scholar] [CrossRef]
- Deressa, L.; Libsu, S.; Chavan, R.B.; Manaye, D.; Dabassa, A. Production of Biogas from Fruit and Vegetable Wastes Mixed with Different Wastes. Environ. Ecol. Res. 2015, 3, 65–71. [Google Scholar] [CrossRef]
- Olivado. How Our Factory in Kenya Thrives on Sustainable Practices. Available online: https://www.olivado.com/factory-kenya-sustainable-practices (accessed on 10 September 2020).
- Aysu, T.; Durak, H. Assessment of avocado seeds (Persea americana) to produce bio-oil through supercritical liquefaction. Biofuel. Bioprod. Biorefin. 2012, 6, 246–256. [Google Scholar] [CrossRef]
- Sánchez, F.; Araus, K.; Domínguez, M.P.; Miguel, G.S. Thermochemical transformation of residual avocado seeds: Torrefaction and carbonization. Waste Biomass Valorization 2017, 8, 2495–2510. [Google Scholar] [CrossRef]
- Huang, Y.F.; Chiueh, P.T.; Lo, S.L. A review on microwave pyrolysis of lignocellulosic biomass. Sustain. Environ. Res. 2016, 26, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Foong, S.Y.; Liew, R.K.; Yang, Y.; Cheng, Y.W.; Yek, P.N.Y.; Wan Mahari, W.A.; Lee, X.Y.; Han, C.S.; Vo, D.V.N.; Van Le, Q.; et al. Valorization of biomass waste to engineered activated biochar by microwave pyrolysis: Progress, challenges, and future directions. Chem. Eng. J. 2020, 389, 124401. [Google Scholar] [CrossRef]
- Elsayed, M.; Ran, Y.; Ai, P.; Azab, M.; Mansour, A.; Jin, K.; Zhang, Y.; Abomohra, A.E.-F. Innovative integrated approach of biofuel production from agricultural wastes by anaerobic digestion and black soldier fly larvae. J. Clean. Prod. 2020, 263, 121495. [Google Scholar] [CrossRef]
- Rachimoellah, H.M.; Resti, D.A.; Zibbeni, A.; Susila, I.W. Production of biodiesel through transesterification of avocado (Persea gratissima) seed oil using base catalyst. J. Tek. Mesin 2009, 11, 85–90. [Google Scholar] [CrossRef]
- Aga, T. Production and Characterization of Biodiesel from Avocado Peel Oil (Apo). Master’s Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2018. [Google Scholar]
- Avhad, M.R.; Sánchez, M.; Bouaid, A.; Martínez, M.; Aracil, J.; Marchetti, J.M. Modeling chemical kinetics of avocado oil ethanolysis catalyzed by solid glycerol-enriched calcium oxide. Energy Convers. Manag. 2016, 126, 1168–1177. [Google Scholar] [CrossRef]
- Paul, A.A.L.; Adewale, F.J. Data on physico-chemical, performance, combustion and emission characteristics of Persea americana Biodiesel and its blends on direct-injection, compression-ignition engines. Data Brief 2018, 21, 1533–1540. [Google Scholar] [CrossRef]
- Paul, A.A.L.; Adewale, F.J. Data on optimization of production parameters on Persea americana (Avocado) plant oil biodiesel yield and quality. Data Brief 2018, 20, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, A.G.; Ighalo, J.O.; Adeoye, A.S.; Onifade, D.V. Modelling and optimisation of biodiesel production from Euphorbia lathyris using ASPEN Hysys. SN Appl. Sci. 2019, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.M.; Oh, J.I.; Kwon, D.; Park, Y.K.; Zhang, M.; Lee, J.; Kwon, E.E. Synthesis of fatty acid methyl esters via non-catalytic transesterification of avocado oil with dimethyl carbonate. Energy Convers. Manag. 2019, 195, 1–6. [Google Scholar] [CrossRef]
- Romero-García, J.M.; Niño, L.; Martínez-Patiño, C.; Álvarez, C.; Castro, E.; Negro, M.J. Biorefinery based on olive biomass. State of the art and future trends. Bioresour. Technol. 2014, 159, 421–432. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “top 10” revisited. Green Chem. 2010, 12, 539–555. [Google Scholar] [CrossRef]
- Redwood, M.D.; Orozco, R.L.; Majewski, A.J.; Macaskie, L.E. An integrated biohydrogen refinery: Synergy of photofermentation, extractive fermentation and hydrothermal hydrolysis of food wastes. Bioresour. Technol. 2012, 119, 384–392. [Google Scholar] [CrossRef] [Green Version]
- del Mar Contreras, M.; Lama-Muñoz, A.; Romero-García, J.M.; García-Vargas, M.; Romero, I.; Castro, E. Production of renewable products from brewery spent grains. In Waste Biorefinery; Pandey, A., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 305–347. [Google Scholar]
- Cuevas, M.; Saleh, M.; García-Martín, J.F.; Sánchez, S. Acid and enzymatic fractionation of olive stones for ethanol production using Pachysolen tannophilus. Processes 2020, 8, 195. [Google Scholar] [CrossRef] [Green Version]
- Cornelia, M.; Christianti, A. Utilization of modified starch from avocado (Persea americana Mill.) seed in cream soup production. IOP Conf. Ser. Earth Environ. Sci. 2018, 102. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodríguez-Jasso, R.M.; Ruiz, H.A.; Govea-Salas, M.; Rosas-Flores, W.; Aguilar-González, M.A.; Pintado, M.E.; Lopez-Badillo, C.; Luevanos, C.; Aguilar, C.N. Hydrothermal-microwave processing for starch extraction from Mexican avocado seeds: Operational conditions and characterization. Processes 2020, 8, 759. [Google Scholar] [CrossRef]
- Tesfaye, T.; Gibril, M.; Sithole, B.; Ramjugernath, D.; Chavan, R.; Chunilall, V.; Gounden, N. Valorisation of avocado seeds: Extraction and characterisation of starch for textile applications. Clean Technol. Environ. Policy 2018, 20, 2135–2154. [Google Scholar] [CrossRef]
- Manzanares, P.; Ballesteros, I.; Negro, M.J.; González, A.; Oliva, J.M.; Ballesteros, M. Processing of extracted olive oil pomace residue by hydrothermal or dilute acid pretreatment and enzymatic hydrolysis in a biorefinery context. Renew. Energy 2020, 145, 1235–1245. [Google Scholar] [CrossRef]
- López-Linares, J.C.; Romero, I.; Moya, M.; Cara, C.; Ruiz, E.; Castro, E. Pretreatment of olive tree biomass with FeCl3 prior enzymatic hydrolysis. Bioresour. Technol. 2013, 128, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Wang, A.B.; Zang, X.P.; Tan, L.; Xu, B.Y.; Chen, H.H.; Jin, Z.Q.; Ma, W.H. Physicochemical, functional and emulsion properties of edible protein from avocado (Persea americana Mill.) oil processing by-products. Food Chem. 2019, 288, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Segovia, F.J.; Hidalgo, G.I.; Villasante, J.; Ramis, X.; Almajano, M.P. Avocado seed: A comparative study of antioxidant content and capacity in protecting oil models from oxidation. Molecules 2018, 23, 2421. [Google Scholar] [CrossRef] [Green Version]
- Daiuto, É.R.; Tremocoldi, M.A.; De Alencar, S.M.; Vieites, R.L.; Minarelli, P.H. Chemical composition and antioxidant activity of the pulp, peel and by products of avocado ‘hass. Rev. Bras. Frutic. 2014, 36, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I.C.R.F. Bioactive characterization of Persea americana Mill. by-products: A rich source of inherent antioxidants. Ind. Crop Prod. 2018, 111, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Tremocoldi, M.A.; Rosalen, P.L.; Franchin, M.; Massarioli, A.P.; Denny, C.; Daiuto, É.R.; Paschoal, J.A.R.; Melo, P.S.; De Alencar, S.M. Exploration of avocado by-products as natural sources of bioactive compounds. PLoS ONE 2018, 13, e192577. [Google Scholar] [CrossRef] [Green Version]
- Araújo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Govea-Salas, M.; Pintado, M.E.; Aguilar, C.N. Process optimization of microwave-assisted extraction of bioactive molecules from avocado seeds. Ind. Crop Prod. 2020, 154, 112623. [Google Scholar] [CrossRef]
- Weremfo, A.; Adulley, F.; Adarkwah-Yiadom, M. Simultaneous Optimization of Microwave-Assisted Extraction of Phenolic Compounds and Antioxidant Activity of Avocado (Persea americana Mill.) Seeds Using Response Surface Methodology. J. Anal. Methods Chem. 2020, 2020. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Comprehensive characterization of phenolic and other polar compounds in the seed and seed coat of avocado by HPLC-DAD-ESI-QTOF-MS. Food Res. Int. 2018, 105, 752–763. [Google Scholar] [CrossRef]
- Páramos, P.R.; Granjo, J.F.; Corazza, M.L.; Matos, H.A. Extraction of high value products from avocado waste biomass. J. Supercrit. Fluids 2020, 165. [Google Scholar] [CrossRef]
- Jiménez-Velázquez, P.; Valle-Guadarrama, S.; Alia-Tejacal, I.; Salinas-Moreno, Y.; García-Cruz, L.; Pérez-López, A.; Guerra-Ramírez, D. Separation of bioactive compounds from epicarp of ‘Hass’ avocado fruit through aqueous two-phase systems. Food Bioprod. Process. 2020, 123, 238–250. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Escalona-Buendía, H.B.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Pedroza-Islas, R.; Ponce-Alquicira, E. Optimization of the antioxidant and antimicrobial response of the combined effect of nisin and avocado byproducts. LWT-Food Sci. Technol. 2016, 65, 46–52. [Google Scholar] [CrossRef]
- Kosińska, A.; Karamać, M.; Estrella, I.; Hernández, T.; Bartolomé, B.; Dykes, G.A. Phenolic compound profiles and antioxidant capacity of Persea americana mill. peels and seeds of two varieties. J. Agric. Food Chem. 2012, 60, 4613–4619. [Google Scholar] [CrossRef]
- Castro-López, C.; Bautista-Hernández, I.; González-Hernández, M.D.; Martínez-Ávila, G.C.G.; Rojas, R.; Gutiérrez-Díez, A.; Medina-Herrera, N.; Aguirre-Arzola, V.E. Polyphenolic profile and antioxidant activity of leaf purified hydroalcoholic extracts from seven Mexican Persea americana cultivars. Molecules 2019, 24, 173. [Google Scholar] [CrossRef] [Green Version]
- Zaki, S.A.E.-H.; Ismail, F.A.E.-A.; Abdelatif, S.H.; El-Mohsen, N.R.A.; Helmy, S.A. Bioactive compounds and antioxidant activities of avocado peels and seeds. Pak. J. Biol. Sci. 2020, 23, 345–350. [Google Scholar] [CrossRef]
- Soldera-Silva, A.; Seyfried, M.; Campestrini, L.H.; Zawadzki-Baggio, S.F.; Minho, A.P.; Molento, M.B.; Maurer, J.B.B. Assessment of anthelmintic activity and bio-guided chemical analysis of Persea americana seed extracts. Vet. Parasitol. 2018, 251, 34–43. [Google Scholar] [CrossRef]
- Wang, W.; Bostic, T.R.; Gu, L. Antioxidant capacities, procyanidins and pigments in avocados of different strains and cultivars. Food Chem. 2010, 122, 1193–1198. [Google Scholar] [CrossRef]
- Villacís-Chiriboga, J.; Elst, K.; Van Camp, J.; Vera, E.; Ruales, J. Valorization of byproducts from tropical fruits: Extraction methodologies, applications, environmental, and economic assessment: A review (Part 1: General overview of the byproducts, traditional biorefinery practices, and possible applications). Compr. Rev. Food Sci. Food Saf. 2020, 19, 405–447. [Google Scholar] [CrossRef]
- Alissa, K.; Hung, Y.C.; Hou, C.Y.; Lim, G.C.W.; Ciou, J.Y. Developing new health material: The utilization of spray drying technology on avocado (Persea americana mill.) seed powder. Foods 2020, 9, 139. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Gámez, M.d.M.; Gómez-Caravaca, A.M. Underutilized Sources of Carotenoids. In Carotenoids: Properties, Processing and Applications; Galanakis, C.M., Ed.; Academic Press: Burlington, MA, USA, 2020; pp. 107–147. [Google Scholar]
- Permal, R.; Chang, W.L.; Chen, T.; Seale, B.; Hamid, N.; Kam, R. Optimising the spray drying of avocado wastewater and use of the powder as a food preservative for preventing lipid peroxidation. Foods 2020, 9, 1187. [Google Scholar] [CrossRef]
- Dabas, D.; Elias, R.J.; Lambert, J.D.; Ziegler, G.R. A Colored avocado seed extract as a potential natural colorant. J. Food Sci. 2011, 76, 1335–1341. [Google Scholar] [CrossRef]
- Arlene, A.A.; Prima, K.A.; Utama, L.; Anggraini, S.A. The preliminary study of the dye extraction from the avocado seed using ultrasonic assisted extraction. Procedia Chem. 2015, 16, 334–340. [Google Scholar] [CrossRef] [Green Version]
- Dabas, D.; Ziegler, G.R.; Lambert, J.D. Anti-Inflammatory Properties of a Colored Avocado Seed Extract. Abbr. Adv. Food Technol. Nutr. Sci. Open J. 2019, 5, 8–12. [Google Scholar] [CrossRef]
- Shegog, R.M. Characterization of Perseoranjin a natural orange pigment found in Hass avocado (Persea americana) seed and its uses as a natural food colorant. Master’s Thesis, The Pennsylvania State University, University Park, PA, USA, 2015. [Google Scholar]
- Hatzakis, E.; Mazzola, E.P.; Shegog, R.M.; Ziegler, G.R.; Lambert, J.D. Perseorangin: A natural pigment from avocado (Persea americana) seed. Food Chem. 2019, 293, 15–22. [Google Scholar] [CrossRef]
- Aranda-García, E.; Cristiani-Urbina, E. Effect of pH on hexavalent and total chromium removal from aqueous solutions by avocado shell using batch and continuous systems. Environ. Sci. Pollut. Res. 2019, 26, 3157–3173. [Google Scholar] [CrossRef]
- Díaz-Muñoz, L.L.; Bonilla-Petriciolet, A.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I. Sorption of heavy metal ions from aqueous solution using acid-treated avocado kernel seeds and its FTIR spectroscopy characterization. J. Mol. Liq. 2016, 215, 555–564. [Google Scholar] [CrossRef]
- Leite, A.B.; Saucier, C.; Lima, E.C.; dos Reis, G.S.; Umpierres, C.S.; Mello, B.L.; Shirmardi, M.; Dias, S.L.P.; Sampaio, C.H. Activated carbons from avocado seed: Optimisation and application for removal of several emerging organic compounds. Environ. Sci. Pollut. Res. 2018, 25, 7647–7661. [Google Scholar] [CrossRef] [Green Version]
- Palma, C.; Lloret, L.; Puen, A.; Tobar, M.; Contreras, E. Production of carbonaceous material from avocado peel for its application as alternative adsorbent for dyes removal. Chin. J. Chem. Eng. 2016, 24, 521–528. [Google Scholar] [CrossRef]
- Aiyesanmi, A.F.; Adebayo, M.A.; Arowojobe, Y. Biosorption of lead and cadmium from aqueous solution in single and binary systems using avocado pear exocarp: Effects of competing ions. Anal. Lett. 2020, 53, 2868–2885. [Google Scholar] [CrossRef]
- Zetterholm, J.; Bryngemark, E.; Ahlström, J.; Söderholm, P.; Harvey, S.; Wetterlund, E. Economic evaluation of large-scale biorefinery deployment: A framework integrating dynamic biomass market and techno-economic models. Sustainability 2020, 12, 7126. [Google Scholar] [CrossRef]
- Han, D.; Yang, X.; Li, R.; Wu, Y. Environmental impact comparison of typical and resource-efficient biomass fast pyrolysis systems based on LCA and Aspen Plus simulation. J. Clean. Prod. 2019, 231, 254–267. [Google Scholar] [CrossRef]
- European Commission, Environment. Available online: https://ec.europa.eu/environment/ecolabel/ (accessed on 7 October 2020).

| By-Product | Moisture | Ash | Lipids | CP | Ext | Hem 3 | Cel/Glu 4 | Lignin |
|---|---|---|---|---|---|---|---|---|
| Exhausted pulp/pomace | 81.4–82.8 | 4.5–7.0 | 9.3 | 14.7–12.8 | 18.2 1 | 8.5 | 29.8 | 26.9 |
| Peel | 70.9–75.3 | 1.0–8.7 | 6.3–10.4 | 6.0–9.3 | 43.7 1/20.5–34.4 2 | 11.5–25.3 | 12.1–27.6 | 4.4–35.3 |
| Stone | 35.2–57.0 | 0.9–2.9 | 2.3–6.0 | 3.7–5.3 | 21.0–35.9 2 | 3.0–47.9 | 6.5–40.9 | 1.8–15.8 |
| Wastewater | 88.3 | 17.9 | 53.8 | 10.3 | ND | ND | ND | ND |
| Element (%) | Avocado Peel | Avocado Stone | Olive Stone | Vegetal Coal |
|---|---|---|---|---|
| C | 49.8 | 42.1–48.0 | 46.6 | 79.3 |
| H | 5.7 | 5.6–5.8 | 6.33 | 2.7 |
| N | 1.0 | 0.5–0.7 | 1.81 | 0.7 |
| O | 42.2 | 43.0–50.8 | 45.20 | 17.0 |
| S | ND | ND–0.1 | 0.11 | 0.3 |
| By-Product | Volatile Matter (%) | Fixed Carbon (%) | HHV (MJ/kg) | LHV (MJ/kg) |
|---|---|---|---|---|
| Avocado branches | ND | ND | 16.4 | ND |
| Avocado leaves | 70.0 | 22.7 | 18.0 | ND |
| Avocado peel | 82.0 | 14.9 | 18.7 | ND |
| Avocado stone | 72.0 | 26.0 | 15.2–19.2 | 17.9 |
| Olive stone | 78.3 | 20.4 | 17.9–20.5 | 19.2 |
| Vegetal coal | 26.0 | 68.1 | 29.7 | ND |
| Sample Type | Conditioning | Oil Extraction | Procedure a | Heating Value (MJ/kg) | Density at 15 °C (kg/m3) | Viscosity at 40 °C) (mm2/s) | Flash Point (°C) | Ester Content (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Biodiesel (stone oil) | Size reduction (120 mesh) and drying (110 °C) | Hexane | Methanolysis with NaOH (60 °C; 60 min; r, 6:1 1% catalyst) | 41.3 | 877.7 | 5.0 | 184.0 | 84.6 | [60] |
| Biodiesel (peel oil) | Size reduction (2.6 mm) and air drying | Hexane (Soxhlet) | Methanolysis with NaOH (60 °C; 67.5 min; r: 6:1; 1.21% catalyst) | 40.0 | 910.0 | 4.2 | 161.0 | 95.2 | [61] |
| Biodiesel (avocado oil) | - | - | Ethanolysis glycerol-enriched CaO (75 °C; 1 h; r: 9:1; 7% catalyst) | ND | ND | 4.9 | ND | 91.2 | [62] |
| Biodiesel (avocado oil) | - | - | Methanolysis with NaOH (65 °C; 2 h; r: 6:1; 1% catalyst) | 40.1 | ND | 3.8 | 148.0 | ND | [63,64] |
| Biodiesel (avocado oil) | Size reduction and drying (85 °C) | Hexane (Soxhlet) | Methanolysis with KOH (60 °C; 2 h; r: 6:1; 5% catalyst) | ND | ND | ND | ND | 61.2 | [66] |
| Biodiesel (avocado oil) | Size reduction and drying (85 °C) | Hexane (Soxhlet) | Dimethyl carbonate with KOH (85 °C; 2 h; r: 9:1; 8.5% catalyst) | ND | ND | ND | ND | 7.3 | [66] |
| Biodiesel (avocado oil) | Size reduction and drying (85 °C) | Hexane (Soxhlet) | Methanolysis non-catalyzed (380 °C) with silica | 90.1 | [66] | ||||
| Biodiesel (avocado oil) | Size reduction and drying (85 °C) | Hexane (Soxhlet) | Dimethyl carbonate non-catalyzed with silica (380 °C) with silica (r, 8:1) | 93.0 | [66] | ||||
| Avocado oil | 39.5 | 34.0 | 186.0 | [63] | |||||
| Diesel | 45.3 | 3.0 | 58.0 | [63] |
| Biomass | Cultivar | Conditioning | Extraction Method | Solvent | TPC | TEAC | FRAP | Reference |
|---|---|---|---|---|---|---|---|---|
| In terms of biomass weight | ||||||||
| Peel | Hass | Air-drying and milling | Soxhlet extraction | Water | 4.13 1 | 17.5 1 | 15.2 1 | [12] |
| Stone | Hass | Air-drying and milling | Soxhlet extraction | Water | 0.31 1 | 1.7 1 | 1.3 1 | [12] |
| Stone | Hass | Cutting, sun-dried and milling | Microwave-assisted extraction | 58.3% Ethanol | 8.4 1 | ND | ND | [83] |
| Stone | Hass A | Drying, grinding and sieving | Soxhlet extraction | Ethanol | 2.0 1 | ND | ND | [85] |
| Stone | Hass B | Drying, grinding and sieving | Soxhlet extraction | Ethanol | 3.0 1 | 46.3 1 | ND | [85] |
| Peel | Hass B | Drying, grinding and sieving | Soxhlet extraction | Ethanol | 10.7 1 | 111.2 1 | ND | [85] |
| Stone | Hass B | Drying, grinding and sieving | Supercritical fluid extraction | CO2 with ethanol | 5.1 1 | ND | ND | [85] |
| Peel | Hass B | Drying, grinding and sieving | Supercritical fluid extraction | CO2 with ethanol | 4.7 1 | ND | ND | [85] |
| In terms of extract weight | ||||||||
| Peel | Hass | Air-drying and milling | Soxhlet extraction | Water | 26.6 2 | 112.2 2 | 97.8 2 | [12] |
| Stone | Hass | Air-drying and milling | Soxhlet extraction | Water | 1.8 2 | 9.9 2 | 7.7 2 | [12] |
| Stone | - | - | Homogenization | 70% Acetone | 6.1 2 | ND | ND | [13] |
| Peel | - | - | Homogenization | 70% Acetone | 9.0 2 | ND | ND | [13] |
| Stone | Hass | Freeze-drying | Homogenization | 50% Methanol → 70% acetone | 8.1 2 | ND | 30.8 2 | [20] |
| Peel | Hass | Freeze-drying | Homogenization | 50% Methanol → 70% acetone | 13.7 2 | ND | 54.7 2 | [20] |
| Pomace | Hass | Freeze-drying | Homogenization | 50% Methanol → 70% acetone | 3.6 2 | ND | 16.0 2 | [20] |
| Wastewater | Hass | Freeze-drying/spray-drying | 1.6–5.1 2,3 | ND | 8.0–21.6 2,3 | [20] | ||
| Stone | Hass | Chopping and drying | Homogenization | 70% Acetone | 4.1 2 | ND | ND | [27] |
| Peel | Hass | Cutting and drying | Homogenization | 70% Acetone | 5.1 2 | ND | ND | [27] |
| Stone | Hass | Milling, lyophilization | Stirring in bath | Methanol | 2.5 2 | 12.4 2 | 31.7 2 | [78] |
| Stone | Hass | Milling, lyophilization | Stirring in bath | 50% Ethanol | 3.1 2 | 26.4 2 | 43.9 2 | [78] |
| Stone | Hass | Lyophilization, milling | Ultrasound-assisted extraction | 80% Ethanol | 5.7 2 | 64.6 2 | ND | [79] |
| Peel | Hass | Lyophilization, milling | Ultrasound-assisted extraction | 80% Ethanol | 6.4 2 | 79.2 2 | ND | [79] |
| Stone | Hass | Cutting and freeze-drying | Microwave-assisted extraction | 70% Acetone | 30.7 2 | 242.5 2 | ND | [82] |
| Stone | Hass | Cutting and freeze-drying | Microwave-assisted extraction | 58.5% Ethanol | 25.4 2 | 206.2 2 | ND | [82] |
| Stone | Hass | Oven-drying and milling | Accelerated solvent extraction | 50% Ethanol | ND | 30.0 2 | ND | [84] |
| Pomace | Hass | Freeze-drying and milling | Aqueous two-phase systems | Sodium citrate–PEG4000 or magnesium sulfate–PEG4000 | 3.3 2 | ND | ND | [86] |
| Stone | Hass | Grinding and drying | Boiled, stirred and filtered | Water | 0.6 2 | ND | 3.8 2 | [87] |
| Peel | Hass | Grinding and drying | Boiled, stirred and filtered | Water | 2.0 2 | ND | 9.2 2 | [87] |
| Stone | Shepard | Lyophilization and milling | Thermostatic shaking water bath | 80% Methanol | 1.3 4,5 | 9.1 4 | ND | [88] |
| Peel | Shepard | Lyophilization and milling | Thermostatic shaking water bath | 80% Methanol | 1.6 4,5 | 11.2 4 | ND | [88] |
| Stone | Hass | Lyophilization and milling | Thermostatic shaking water bath | 80% Methanol | 1.0 4,5 | 9.4 4 | ND | [88] |
| Peel | Hass | Lyophilization and milling | Thermostatic shaking water bath | 80% Methanol | 2.5 4,5 | 16.1 4 | ND | [88] |
| Compound | By-Product | Compound | By-Product |
|---|---|---|---|
| Phenolic alcohol derivatives | Flavan-3-ols | ||
| Hydroxytyrosol glucoside | S | (+)-Catechin | P/S/SC |
| Tyrosol-glucoside | S | (-)-Epicatechin | P/S/SC |
| Tyrosol-glucosyl-pentoside | S/SC | (Epi)gallocatechin | S/SC |
| 3-hydroxytyrosol | P/S | Isomers 1 and 2 of (epi)catechin glucopyranoside | S/SC |
| Oleuropein | P/S | (Epi)catechin gallate | SC |
| Flavonols | B-Type (epi)catechin dimer, including B1 or B2 | P/S/SC | |
| Quercetin | P/S/SC/L | B-Type (epi)catechin trimer | P/S/SC |
| Quercetin-pentoside-hexoside | P | B-Type (epi)catechin tetramer | P/S/SC |
| Quercetin-glucoronide | P | B-Type (epi)catechin pentamer | P/SC |
| Quercetin-3-O-glucoside or other hexoside | P/S/SC | B-Type (epi)catechin hexamer | P |
| Quercetin-hexoside | P | A-Type (epi)catechin dimer | S/SC |
| Quercetin-dihexoside | P | A-Type (epi)catechin trimer | S/SC |
| Quercetin-rhamnoside-pentoside | P | A-Type (epi)catechin tetramer | S/SC |
| Quercetin-3-β-glucoside | Other phenolic compounds | ||
| Isomers 1 and 2 of quercetin-diglucoside | SC | Catechol | P/S/SC |
| Isorhametin-glucuronide | P | Ellagic acid | P/S |
| Rutin | P/S | Dimethyl ellagic acid hexoside | L |
| Kaempferol | P/S | Pyrogallol or 1,2,3-trihydroxybenzene | P/S/SC |
| Kaempferol-glucuronide | P | Vanillin | P/S/SC |
| Flavanones | Flavalignan isomers | S/SC | |
| Naringin | P/S | Non-phenolic compounds | |
| Hesperidin | P/S | Quinic acid | S/SC |
| Hesperetin | P/S | Citric acid | S/SC |
| Naringenin | P/S/SC | Citric acid isomer | S/SC |
| Sakuranetin | S/SC | Malic acid | S/SC |
| Flavone | Succinic acid | S/SC | |
| Acacetin neorutinoside | P/S | Penstemide acid | SC |
| Apigenin | P/S/L | Hydroxyabscisic acid glucoside | SC |
| Apigenin-C-hexoside-pentoside | L | Perseitol | SC/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Vargas, M.C.; Contreras, M.d.M.; Castro, E. Avocado-Derived Biomass as a Source of Bioenergy and Bioproducts. Appl. Sci. 2020, 10, 8195. https://doi.org/10.3390/app10228195
García-Vargas MC, Contreras MdM, Castro E. Avocado-Derived Biomass as a Source of Bioenergy and Bioproducts. Applied Sciences. 2020; 10(22):8195. https://doi.org/10.3390/app10228195
Chicago/Turabian StyleGarcía-Vargas, Minerva C., María del Mar Contreras, and Eulogio Castro. 2020. "Avocado-Derived Biomass as a Source of Bioenergy and Bioproducts" Applied Sciences 10, no. 22: 8195. https://doi.org/10.3390/app10228195
APA StyleGarcía-Vargas, M. C., Contreras, M. d. M., & Castro, E. (2020). Avocado-Derived Biomass as a Source of Bioenergy and Bioproducts. Applied Sciences, 10(22), 8195. https://doi.org/10.3390/app10228195






