Featured Application
Anticancer Drug Activity and Underlying Mechanisms.
Abstract
Previous studies regarding malloapelta B (malB), a natural compound isolated from the Vietnamese medicinal plant, showed a strong NF-κB inhibitory effect, making it a promising source for the development of novel anticancer drugs. However, similar to many other natural compounds from plants, malB has several disadvantages for clinical applications, including high toxicity and low solubility. To improve its bioavailability, malB was conjugated into nanoliposomes, which are ideal drug carriers. The formulations with 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, mPEG-cholesterol, malB, with or without cholesterol exhibited nanoliposomes with an average diameter of approximately 76.98 nm, PDI of 0.28, zeta potential of −5.53 mV, and the highest encapsulation efficiency of 78.73% ± 9.5%. These malB-nanoliposomes inhibited the survival of all lung cancer cell lines examined with IC50 values ranging from 11.86 to 13.12 µM. Moreover, malB-nanoliposomes showed stronger inhibition of A549 colony-forming activity compared to that of the free compound. The effects of malB and its nanoliposomal formulation may be mediated through activation of apoptosis by the significant induction of caspase 3 activity. The nanoliposomal formulations also showed potential to inhibit tumor growth (37.03%) and prolong survival (32.20 days) of tumor-bearing mice compared with the unloaded drug (p < 0.05). The improved antitumor activity of malB-nanoliposomes suggests their promising clinical applications.
1. Introduction
Lung cancer is the leading cause of cancer-related death worldwide (18.4%), and is especially prevalent in male smokers [1]. The most common type of lung cancer is non-small cell lung cancer (NSCLC), accounting for 85% of cases of lung cancer [2]. Approximately 40% of lung cancers are adenocarcinomas, and most cases of lung cancer in smokers are of this type. Several therapeutic methods are available for treatment of lung adenocarcinomas, including surgery, radiofrequency ablation, radioactive therapy, chemotherapy, and immunotherapy, alone or in various combinations. However, these therapeutic options are not only expensive but also insufficiently effective. Therefore, there is an increasing need for new, safer, and more effective clinical treatments. Our previous study showed that 1-(5,7-dimetoxy-2,2dimetyl-2H-cromen-8-yl)-but-2-en-1-on (malloapelta B), isolated from Mallotus apelta, is a potential active anticancer compound [3]. This compound, malloapelta B (malB), inhibits the activation of nuclear factor kappa B (NF-κB) with an IC50 value of 5.0 μM. This IC50 value is much lower than that of parthenolide (PTN, 6.66 ± 0.07 µM) [4,5]. The compound was also shown to downregulate genes that contribute to inflammatory mechanisms, such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and Il-1β [6]. Additionally, as reported, the enone side chain may play an important role in the activity of this molecule [4]. However, the potential effects of malB against lung carcinomas in vitro and in vivo have not been studied in detail. In addition, its high toxicity and low solubility represent barriers to the development of malB as an anticancer drug for use in chemotherapy. Nanoliposomes have recently emerged as ideal drug carriers with a number of beneficial characteristics, including minimal immune response, biocompatibility, biodegradability, reduction of drug toxicity, etc. [7]. Nanoliposomes can be used to encapsulate hydrophobic drugs as a means of improving their solubility in water. Due to these advantages, a number of nanoliposomal formulations incorporating different anticancer drugs are available commercially, including daunorubicin (DaunoXome), doxorubicin in PEG-liposomes (Doxil), vincristine (Marqibo), topotecan (INX-0076), nystatin (Nyotran), and paclitaxel (LEP-ET) [8]. Therefore, in the present study, malB was entrapped in nanoliposomes at different concentrations, and anticancer activities of these malB-nanoliposomal formulations against lung carcinomas were examined as well as their anti-malignant potential in Lewis lung carcinoma (LLC) tumor-bearing mice.
2. Materials and Methods
2.1. Chemicals
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) was purchased from Avanti Polar Lipids (Alabaster, AL, USA); cholesterol was produced at Acros Organics, a part of Thermo Fisher Scientific, (Merelbeke, Belgium), and mPEG-cholesterol was provided by Dr. Chun-Liang Lo, National Yang-Ming National University, Taipei, Taiwan. The compound malloapelta B (malB) was provided by the MienTrung Institute for Scientific Research, Vietnam Academy of Science and Technology (Hue, Vietnam). Other chemicals and cell culture reagents were from Sigma Chemical Co. (St. Louis, MO, USA) and Invitrogen (Carlsbad, CA, USA).
2.2. Animals
Male and female albino BALB/c mice (8–10 weeks old) were received from the Institute of Biotechnology, Vietnam Academy of Science and Technology (VAST, Hanoi, Vietnam). All mice were caged in a temperature-controlled room on a 12-h light/12-h dark cycle with food and water ad libitum. Experiments were performed in accordance with Vietnamese Ethical Laws, European Communities Council Directives of 24 November 1986 (86/609/EEC) guidelines and Approval from the Scientific Council of Institute of Biotechnology, Vietnam Academy of Science and Technology, for the care and use of laboratory animals.
2.3. MalB-Nanoliposome Preparation
2.3.1. Bangham Thin Film Method
Liposome production was carried out according to the Bangham thin film method [9] with some modifications. Briefly, a complex including lipids and malB was diluted in dichloromethane (DCM) solvent (Table 1). A magnetic stirrer was used to dissolve the complex at 200 rpm, room temperature (RT) for 20 min. DCM was then removed by a rotary evaporator for the formation of thin film. The solvent was thoroughly dispatched by nitrogen gas flushing. Subsequently, the thin film was hydrated with PBS (pH = 7.2) at 60 °C. To obtain nanoliposomes, the hydrated complex was then immediately subjected to a probe sonicator that directly inserted the probed head into the solution with an ultrasonic frequency of 2 atm, 20 s of ultrasound and 10 s of rest, repeated five times. The ultrasonic solution was shaken at 60 °C and 500 rpm for one hour before large size nanoliposomes were removed by filtering with a 0.22 μm polyvinylidene difluoride (PVDF) membrane. The filtered nanoliposomes were washed three times with PBS (pH = 7.2).

Table 1.
Lipid components including 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), mPEG-cholesterol, with/without cholesterol and malloapelta B (malB) compound ratio.
2.3.2. Nanoliposomal Characterization
The size (z-average), polydispersity indexes (PDIs), and zeta potential of the nanoparticles were measured using a dynamic light scattering instrument (DLS, Horiba Instruments Inc., Irvine, CA, USA). The malB-nanoliposomes were also stained with 4% uranyl acetate (UR 4%) dye on the surface of a thin carbon-coated copper plate to a thickness of 200–500 A° and dried at RT. Then, the plates were magnified 200× to capture the morphology of the prepared nanoparticles using high-solution transmission electronic microscopy (TEM) (Jeol 1200EX TEM, Jeol Company, Tokyo, Japan).
2.3.3. Encapsulated Efficiency
The encapsulated efficiency (EE) of the loaded malB was calculated by UV–spectrophotometry method. The pure malB compound was dissolved in DMSO (100%) as a standard curve with a two time diluted concentration range which was started from0.25 mg/mL. The malB-nanoliposomes were re-diluted in DMSO (100%) to release all packed malB. Then, 100 μL of both the standard curve and the nanoliposomal samples were placed into a 96-well plate, repeated three times to ensure accuracy. The optical density values (ODs) were measured at 280 nm on a ThermoScientific™ Varioskan™ Flash Multimode Reader. The standard curve drawn using Microsoft Office Excel 2016 software was used to calculate the amount of active ingredient that was packed into the nanoliposomes. The encapsulated efficiency (EE) of the process was determined using the following equation:
EE(%) = 100 × (weight of conjugated malB)/(weight of initial malB)
2.4. Cell Lines and Cell Culture
A549, SK-LU-1, CL-141, and LLC lung cancer cell lines were provided by Prof. Chi-Ying Huang, Institute of Biopharmaceutical Sciences, National Yang Ming University, Taipei, Taiwan and Prof. J Meier, Milan University, Milan, Italy. Cells were cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS), 100 μg/mL streptomycin, 100 IU/mL penicillin, and 2mM L-glutamine. All cells were maintained in a humidified incubator with 5% CO2 at 37 °C, and harvested with trypsin-EDTA.
2.5. Cytotoxic Activities of malB-Conjugated Nanoliposomes
Cells were seeded in 96-well plates at a density of 2000 cells per well in triplicate for 24 h before sample exposure. Cells were then treated with different unloaded malB concentrations and corresponding malB-nanoliposomes of indicated treatments for 48 h. Cytotoxicity was assessed by using the sulforhodamine B (SRB) assay [10]. Briefly, the medium was discarded, and adherent cells were fixed by 100 μL/well of cold 10% trichloroacetic acid (w/v) for 1 h at 4 °C. After fixation, cells were stained with 0.4% SRB solution (w/v in 1% acetic acid) for 30 min at RT, and then washed twice with 1% acetic acid. After air-drying, 100 μL of 20 mM Tris-base were added to each well and the absorbance was measured at 540 nm. Cytotoxicity is expressed as the percent of cells relative to the number of cells in the solvent only as control (set to 100%). Each experiment was performed independently at least 3 times.
2.6. Clonogenic Inhibition of malB-Conjugated Nanoliposomes
Clonogenic assay was performed to determine the ability of a single cell to grow into a colony under the compound treatment. In detail, 800 cells were seeded in each well of 6-well plates at 37 °C, 5% CO2. The addition of drugs was manipulated after 24 h of seeding and triplication of experiments was carried out. The medium including drugs was changed each 3 days. The cells were harvested after 8 days of treatment. After the treatment, the cells were washed with 1 mL phosphate-buffered saline (PBS), and fixed with 1mL mixture of methanol and acetic acid (3:1) in 15 min at RT. Subsequently, the colonies were stained with 0.5 mL of 0.5% crystal violet in methanol for 15 min. Then, crystal violet was discarded and the plate were rinsed under tap water. Only colonies consisting of approximately 50 cells were counted. The percentage of cell survival after drug treatment was expressed as a percentage of the control-colony efficiency.
2.7. Caspase 3 Inducible Activities of malB-Conjugated Nanoliposome
Caspase 3 activity was performed using a Caspase-3 Colorimetric Assay Kit (BioVision Inc., USA). According to the assaying protocol of the kit manufacturer, 1 × 106 cells treated with malB or malB-nanoliposome at different concentrations for 24h were lysed with chilled lysis buffer. The lysates were then centrifuged to collect the supernatant. After measurement of protein content using Bradford reagent, caspase 3 activity was specified by mixing 50 µL of cell lysis supernatant with 50 µL of 10 mM dithiothreitol (DTT) and 5 µL of the 4 mM DEVD-p-nitroaniline (DEVD-pNA) substrate (from the kit). The caspase 3 activity, which corresponds with the presence of the chromophore pNA, a product formed from the cleavage of the DEVD-pNA substrate in enzyme reaction, can be detected by using a spectrophotometer at 405 nm.
2.8. In Vivo Antitumor Activity
The experiment was carried out using 36 healthy BALB/c mice at 20–25 g weight. Mice were subcutaneously injected with 1 × 106 LLC cells to induce tumors. After 5 days, tumorized mice were randomly distributed to 6 groups (n = 6). Group 1 served as the negative control that received normal saline. Group 2 was treated with blank nanoliposome (with lipid components only). Groups 3 and 4 received malB-nanoliposome at doses of 5 mg/kg and 2.5 mg/kg body weight (b.w.) by intraperitoneal injection (i.p.) every 2 days for 14 days continuously. Group 5 was i.p. injected with unconjugated malB compound at a dose of 5 mg/kg b.w. every 2 days for the same duration. Group 6 was treated with the reference control (doxorubicin 5 mg/kg b.w. i.p. injection). The tumor size of each mouse was measured every 7 days for 28 days and tumor volume was calculated by the following equation:
where V is the volume of the tumor, W is the width of the tumor, and L is the length of the tumor.
V = (W2 × L)/2,
The survival time of tumorized mice in all experimental groups was also determined. It was calculated from the day of LLC cell inoculation to the day of death and percentage increase in average life span (ILS) was calculated by the following equation:
where A is the survival time of the treated group, B is the mean survival time of the control group, and ILS is the increase in the average life span group.
% ILS = (A/B − 1) × 100,
2.9. Statistical Analyses
All data were expressed as means ± standard error of the mean (SEM). Statistical differences were analyzed by two-tailed paired Student’s t-test or one-way analysis of variance (one-way ANOVA). A value of p < 0.05 was considered a statistically significant difference.
3. Results
3.1. Preparation and Morphology of malB-Encapsulated Nanoliposomes
The main components used to produce drug-encapsulating nanoliposomes included malB compound at different concentrations, DPPC, mPEG-cholesterol, with or without cholesterol. The physicochemical characteristics of the nanoliposomes thus formed were assessed by determining the mean diameter (nm), polydispersity index (PDI), zeta potential, and encapsulation efficiency (EE).
Based on the results shown in Table 2, all of the nanoliposomal formulations had not only a mean diameter <200 nm, but also PDI <0.3, and carried a low negative charge. However, the formulations without cholesterol as a component showed significantly higher packing efficiency (p < 0.01). The EE of formulations A and B containing cholesterol were only about 10%, whereas the EEs of the cholesterol-free structures were >50%. The EE was also dependent on the malB concentration; a lower malB concentration of 0.25 mg was associated with higher EE (78.73%), whereas a higher concentration of 0.5 mg showed a lower EE (51.33%). Due to the highest efficient effect, structure C was selected for further studies.

Table 2.
Characteristics of obtained malB-loaded nanoliposomes.
The morphology of the malB-nanoliposomal formulation C was examined by transmission electron microscopy (TEM) (Figure 1). The malB-nanoliposomes were small unilamellar vesicles, likely spherical in shape, and fairly homogeneous in size with an average diameter of 76.98 ± 9.57 nm (Table 2). Based on morphological assessment, the mal B-nanoliposomal formulation C was suitable to manipulate in the further experiments for anticancer activities.
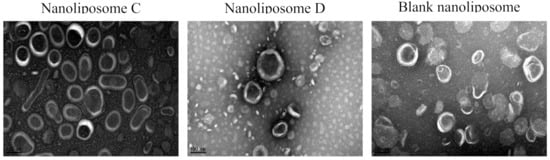
Figure 1.
TEM morphological images of malB-loaded nanoliposomes: nanoliposome C (malB entrapped in nanoliposomes at 0.25 mg, EE = 78.73% ± 9.52%); nanoliposome D (malB entrapped in nanoliposomes at 0.50 mg, EE = 51.33% ± 16.53%). Blank nanoliposomal particles.
3.2. Compound Encapsulation by Nanoliposomes and Their Cytotoxicity
To determine the cytotoxicity of free malB and malB-nanoliposomes on non-small cell lung cancer cell lines, the viability of different cell lines was examined by sulforhodamine B (SRB) assay with malB treatment at several concentrations. As shown in Figure 2 and Table 3, both free compound and malB-nanoliposomes inhibited cell growth in a dose-dependent manner and significantly compared with the control (p < 0.005). High doses of malB and malB-nanoliposomes (7.5 µM and 15 µM) remarkably inhibited viability of all tested lung cancer cell lines. However, the cytotoxic effects of malB and malB-nanoliposomes on all cell lines were decreased at a dose of 3.75 µM, and minimal effects were observed at a dose of 1.875 µM. Based on the IC50 values, malB-nanoliposomes exhibited the strongest cytotoxicity against the A549 cell line. Free malB showed stronger cytotoxic activity than that of the malB-nanoliposomal formulation on all examined cell lines. However, the difference was insignificant (p > 0.05). Blank nanoliposomes showed no significant effects on cell growth (cell survival >90%). Therefore, the IC50 value of blank liposomes could not be determined.
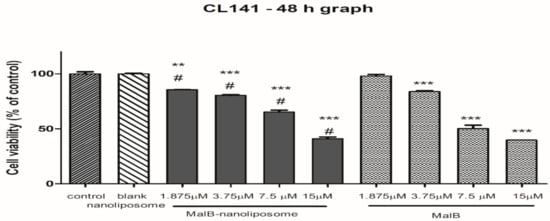
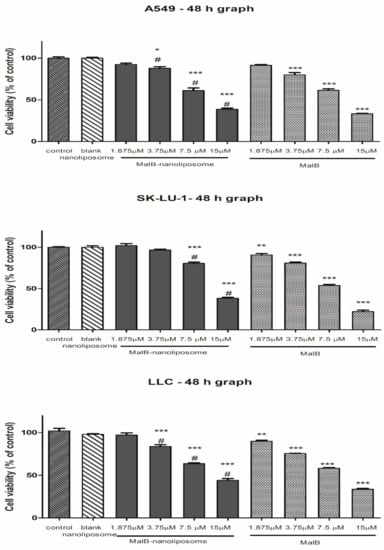
Figure 2.
Cell viability after 48 h under the treatment of malB-nanoliposome C or free compound on different lung cancer cell lines. Cultured cells (2 × 103 cells/well) were treated with different concentrations of either malB-nanoliposomal or free malB. Normal saline served as the negative control. Each value represents the mean ± SEM. One-way ANOVA was used for analyzing statistical differences between groups. * p < 0.05, ** p < 0.01, *** p < 0.001 when compared with negative control. # p < 0.01 when compared with blank liposome.

Table 3.
Cytotoxic activities of malB-nanoliposomes and untrapped compound malB on different lung cancer cell lines.
3.3. Inhibition of Colony Formation malB–Nanoliposome Complex
One of the most important characteristics of cancer is self-renewal ability, which allows tumor cells to proliferate and grow from a single cancer stem cell (CSC). To evaluate the effects of malB and malB-nanoliposomes on cellular self-renewal, a colony-forming assay was performed on the A549 cell line, cells of which were able to form colonies in vitro. The outcome after 8 days of treatment showed that both malB-liposomes and free malB decreased the numbers of colonies derived from A549 cells (Figure 3). The number of colonies decreased markedly at 5 µM and 2.5 µM malB in comparison to the controls. However, a lower concentration of malB had a minimal effect on the number of colonies. In addition, malB-nanoliposome C also inhibited colony formation by >50% at concentrations of 5 µM and 2.5 µM. The IC50 values of malB and malB-nanoliposomes were calculated as 2.28 µM and 1.75 µM, respectively. These inhibitory activities of the compound in both forms were significant compared to the control (p < 0.001). The results suggested that malB encapsulated in nanoliposomes had a stronger inhibitory effect on the formation of A549 lung adenocarcinomas than the free form. In addition, the effects of free malB and malB-nanoliposomes were different as the concentration increased. In detail, at the same dose of 0.75 µM, malB-nanoliposomes inhibited the formation of A549 colonies by up to 40.66%, whereas the free malB showed only 17.21% inhibition of A549 colony formation. The same was also true at a dose of 1.25 µM.

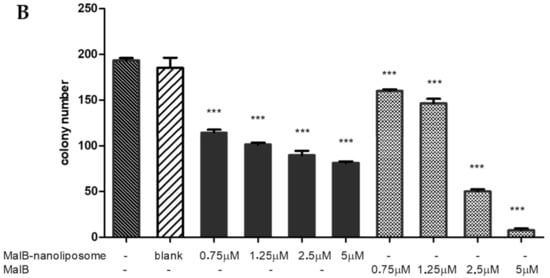
Figure 3.
Antitumorigenic activity of malB-nanoliposome and free compound on A549 cells. (A) Colony images after treatment of malB-nanoliposome and free-compound at different concentrations compared to the negative control (normal saline) and blank liposome on day 8. (B) Colony number after treated with blank nanoliposome, malB-nanoliposome, and malB compared to the negative control. Each value represents the mean ± SEM. *** p < 0.001.
3.4. Caspase 3 Inductive Activities of malB and malB-Nanoliposome
Caspase 3 is a typical enzyme related to the apoptosis of mammalian cells. To determine the apoptosis-inducing activity of malB and malB-liposomes, caspase 3 activity was measured under conditions of treatment with the free compound or its nanoliposomes using a caspase 3 colorimetric kit. As shown in Figure 4, malB and structure C malB-nanoliposomes significantly increased the changes in caspase 3 activity at doses of 10 µM and 5 µM in comparison with the control (p < 0.01 and p < 0.05, respectively). However, no significant differences in caspase 3 induction were observed between malB and malB-nanoliposomes.
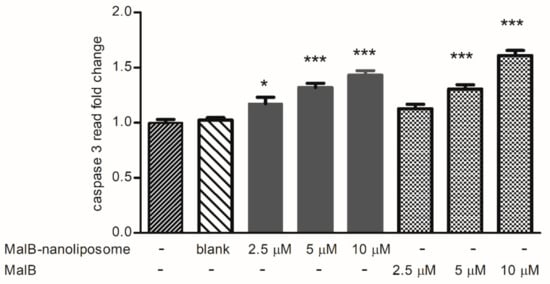
Figure 4.
Caspase 3 inducible effects of unconjugated malB and malB-nanoliposomes at different treated concentrations ranging from 2.5 to 10 µM on the A549 cells after 24 h of incubation. Normal saline served as the negative control. Each value represents the mean ± SEM. *** p < 0.001 and * p < 0.05 compared to negative control.
3.5. Tumor Inhibition Activity
The antitumor activity of malB-liposomes was examined in the LLC tumor model in BALB/c mice. MalB and malB-liposome had no significant change in body weight of mice at both initial time and the end time of treatment (Figure 5). As shown in Figure 6, animals treated with free malB at a concentration of 5 mg/kg body weight (b.w.) did not show a significant reduction in tumor size compared to negative controls. However, malB-nanoliposomes significantly inhibited tumor growth at the same concentration (5 mg/kg b.w.) (p < 0.05). After 28 days of treatment with malB-nanoliposomes, the tumor size was decreased by 37.03% compared to the saline-treated negative control, whereas the free form reduced the tumor size by only 13.09%. When the concentration was decreased, the tumor suppression effect of malB-nanoliposomes was markedly reduced and was not significant compared with the control. Moreover, blank liposome did not affect the tumors’ growth at any tested time points.
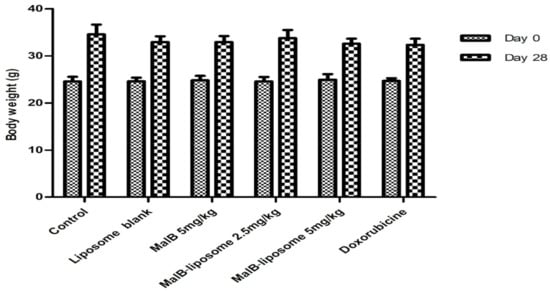
Figure 5.
Effects of malB or malB-nanoliposomes on body weight of BALB/c mice harboring a malignant tumor induced by LLC cells (n = 6). Error bars represent standard error of the mean (SEM).
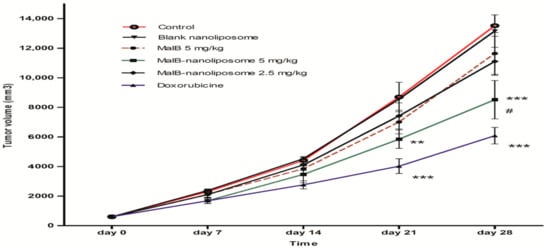
Figure 6.
Anti-tumor effects of malB or malB-nanoliposomes on BALB/c mice harboring a malignant tumor induced by LLC cells (n = 6). Liposome-conjugated malB at the dose 5.0 mg/kg b.w. significantly inhibited tumor growth after 21 and 28 days compared with the negative control (normal saline treated) (** p < 0.01 and *** p < 0.005, respectively). # p < 0.05 compared to the free malB at the same dose. Error bars represent standard error of the mean (SEM).
In addition, the administration of malB-nanoliposomes prolonged the survival of the mice but still insignificantly (p > 0.05) (Table 4). The median survival of mice treated with the malB-nanoliposomal formulation was 32.20 ± 0.97 days, which was 6.62% longer than that of control mice. Survival was also increased slightly (31.00 ± 0.84 days) in the free malB-treated group at the treated dose.

Table 4.
Effect of malB-nanoliposome and free malB on survival time of LLC-induced tumor-bearing mice from different experimented groups (mean ± SEM) (n = 6).
4. Discussion
In addition to novel treatments for cancer, such as antibodies and gene therapy, a number of drugs based on natural bioactive compounds have been shown to be efficacious in chemotherapy and the prevention of cancer. Therefore, there is much interest in identifying potential new anticancer compounds, such as the large-scale programs of the National Cancer Institute for the discovery and screening of natural products for development as anticancer drugs [11]. These studies have identified a number of categories of novel compounds with anticancer activities. However, the use of these compounds has been hampered by their toxicity and poor solubility, problems that have also prevented the development of malB for clinical use. Previous reports regarding its promising anticancer activities suggested that malB was a promising candidate for drug development. Microarray analysis demonstrated that this compound has anticancer capacity in vitro by regulating gene expression similar to the commercial anticancer drug, withanolide A [12]. This compound was also shown to inhibit the activation of NF-κB with an IC50 value in the range of 3.5–5.0 µM [3,5]. The compound affected NF-κB activation by inhibiting the activation of IκB kinase (IKK) [6]. However, malB shows high toxicity and is insoluble, which has limited its pharmaceutical applications. The use of nanoliposomal carriers for drug design to reduce toxicity and enhance the bioavailability of natural compounds is appropriate for anticancer drug development. Drugs packed into liposomes show improved blood circulation activity, promotion of deposition in tumors, protection from metabolism, direct distribution of the drug into tumors, as well as enhanced uptake in adenocarcinoma, mononuclear macrophages, and the intracellular lattice system (liver, spleen, bone marrow). Furthermore, liposomal-encapsulated drugs show reduced uptake in the kidneys, myocardium, and brain tissues [13,14].
In the present study, malB was experimentally incorporated into liposomal nanocarriers. The nanoliposomes containing malB were small unilamellar vesicles <200 nm in diameter with a slight negative charge and low PDI. Magin reported that nanoliposomes in the size range of 50–200 nm would be the most suitable for liposomal stability in the body [15,16,17]. However, liposomes containing cholesterol had much lower EE in comparison with the non-cholesterol formulations. Similar results were reported previously for some lipophilic drugs, such as ciprofloxacin [18] and dexamethasone [19], as well as natural compounds, such as ascorbic acid [20]. Cholesterol molecules are normally located in the space between lipid bilayer membranes. Therefore, it was supposed that cholesterol competed out malB molecules and some positions in the bilayers were occupied, preventing successful incorporation of the test compound. Furthermore, cholesterol makes the bilayer more rigid, which would make the incorporation of malB molecules difficult. In this study, the addition of cholesterol caused a noticeable decrease in EE, which was taken to indicate that EE depends on the normal structure of the liposomal bilayer [21].
The investigation of liposomal stability in this study showed that malB-nanoliposomes were stable at 4 °C in PBS for approximately 30 days, as the average size of the liposomes was still <200 nm and they had a slight negative charge. However, the PDI of liposomes was significantly increased at around 30 days to almost twice that on day 1 (data not shown). The PDI parameter is an indicator of whether the formed liposomes are monodispersed and have an average size distribution. The PDI should be as low as possible, as higher PDI indicates a broader size distribution of nanoparticles. Thus, the results of the present study suggested that malB-liposomes exhibited an appropriate PDI and this characteristic seems to change after 30 days of storage at 4 °C in PBS.
With regard to assessment of bioactivity, malB encapsulated in nanoliposomes still showed strong anticancer activities in vitro that were comparable to those of the free form. Indeed, the nanoliposomal formulation inhibited cancer cell survival after 48 h with IC50 values ranging from 11.86 to 13.12 µM, which was slightly higher than that of free malB (8.67 − 10.35 µM). However, the anti-clonogenic activity of malB-nanoliposomes was more obvious than that of the free compound in the A549 colony-forming assay. In addition, malB and malB-nanoliposomes affected the activity of caspase 3, a crucial protease that mediates apoptosis. This caspase catalyzes the specific cleavage of many key cellular proteins, and plays an important role in apoptotic chromatin condensation and DNA fragmentation [22,23]. The significant caspase 3 induction and activation by malB and malB-nanoliposomes, which contributed to both intrinsic and extrinsic apoptotic pathways, were reported here for the first time.
Furthermore, the improved cytotoxic and antitumorigenic effects of malB-nanoliposomes were also verified in tumor-bearing mice. The malB-nanoliposomes showed the ability to effectively inhibit tumor growth in comparison with negative controls as well as with free malB at the same concentration of 5 mg/kg. There have been many studies on loading of potential anticancer agents into nanoliposomes to improve their activities. Those studies showed that the encapsulation of drugs into liposomes reduces toxicity, improves bioactivity, and increases the circulation time of drugs in the body, leading to increased effectiveness of these drugs in clinical treatment [24]. The intraperitoneal (i.p.) administration of liposomes also has advantages, such as reduction of local toxicity [25]. In addition, when administered by the i.p. route, the drug is absorbed into the organs through the peritoneum or the lymphatic system [26]. Drugs packaged in liposomes could be stable for longer period in the abdominal cavity or in the lymph vessels, thus improving the effectiveness of treatment for peritoneal carcinomas, such as ovarian cancer and liver cancer [27]. However, the uptake of the drug into the blood via the abdominal cavity also depends on the size of the liposomes. Feng reported that liposomes approximately 100 nm in diameter show a high concentration in blood equivalent to intravenous (i.v.) administration, and could therefore be used to treat cancer far from the peritoneum [28]. On the other hand, PEGylation of liposomes plays a central role in prolonging their circulation in the blood. PEGylation helps protect drug-nanoliposomes from mononuclear phagocytes by impeding the absorption of opsonin protein in the circulation on the liposome surface, which would minimize clearance of the drug by these cells [29,30]. The characteristics of PEGylated liposomes allow them to circulate longer in the bloodstream than free drugs, leading to increased effectiveness in cancer treatment. In the present study, the PEG-cholesterol components in the malB-nanoliposome complex may have enhanced the antitumor activity in comparison with the free drug.
5. Conclusions
The results of this study confirmed that malB has the potential for development as an anticancer drug after nanoliposomal encapsulation. The malB-encapsulated formulation had typical characteristics of nanoliposomes, such as size <200 nm, negative charge, and PDI <0.3. The free form of malB and malB-nanoliposomes showed activities against all lung cancer cell lines examined. Both malB forms significantly inhibited colony formation and increased caspase 3 activity of A549 cancer cells. The malB-nanoliposomes showed significantly stronger tumor growth inhibition compared with the free form. The malB-nanoliposomes could slightly prolong the survival period of tumor-bearing mice in comparison with the untreated group.
Author Contributions
Conceptualization, T.T.D.; Methodology, T.N.N., T.P.D., T.C.N., H.P.T., P.T.T.V., and T.A.H.L.; Software, validation, formal analysis, investigation, resources, and data curation, T.N.N. and P.T.T.V.; Writing—original draft preparation: P.T.T.V. and T.N.N.; Writing—review and editing, T.T.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106.02-2017.20.
Acknowledgments
The authors wish to thank Chi-Ying Huang, Lu-Yi Yu, Chun-Liang Lo, Department of Biomedical Engineering, National Yang-Ming University, Taipei, Taiwan, for their kind guidance and their support with all equipment, especially the Jeol 1200EX Transmission Electronic Microscope (TEM system) (Jeol Ltd., Japan).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Kim, J.S.; Cho, M.S.; Nam, J.H.; Kim, H.-J.; Choi, K.-W.; Ryu, J.-S. Prognostic impact of EGFR mutation in non-small-cell lung cancer patients with family history of lung cancer. PLoS ONE 2017, 12, e0177015. [Google Scholar] [CrossRef]
- Van Kiem, P.; Dang, N.H.; Bao, H.V.; Huong, H.T.; Van Minh, C.; Lee, J.J.; Kim, Y.H. New cytotoxic benzopyrans from the leaves ofMallotus apelta. Arch. Pharm. Res. 2005, 28, 1131–1134. [Google Scholar] [CrossRef]
- Van Luu, C.; Van Chau, M.; Lee, J.-J.; Jung, S.-H. Exploration of essential structure of malloapelta B for the inhibitory activity against TNF induced NF-κB activation. Arch. Pharmacal Res. 2006, 29, 840–844. [Google Scholar] [CrossRef]
- Nam, N.H.; Dang, N.H.; Van Kiem, P.; Van Chinh, L.; Binh, P.T.; La Dinh, M.; Van Minh, C. Study on benzopyrans and other isolated compounds from Mallotus apelta. J. Chem. 2007, 45, 111–121. [Google Scholar]
- Ma, J.; Shi, H.; Mi, C.; Li, H.L.; Lee, J.J.; Jin, X. Malloapelta B suppresses LPS-induced NF-κB activation and NF-κB-regulated target gene products. Int. J. Immunopharmacol. 2015, 24, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.-H.; Qasim, M.; Kim, J.-H. Nanoparticle-mediated combination therapy: Two-in-one approach for cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef] [PubMed]
- ElBayoumi, T.A.; Torchilin, V.P. Current trends in liposome research. Methods Mol. Biol. 2010, 605, 1–27. [Google Scholar]
- Bangham, A.D.; Horne, R. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. Adv. Drug Deliv. Rev. 1964, 8, 660-IN10. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural compounds for cancer treatment and prevention. Pharmacol. Res. 2009, 59, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Thao, D.T.; Phuong, D.T.; Trang, N.T.; Nga, N.T.; Chi, H.Y. Study the anticancer mechanism of the promissing compound 2B2D by using microarray technique. Vietnam J. Sci. Technol. 2012, 50, 267. [Google Scholar]
- Lomis, N.; Westfall, S.; Farahdel, L.; Malhotra, M.; Shum-Tim, D.; Prakash, S. Human serum albumin nanoparticles for use in cancer drug delivery: Process optimization and in vitro characterization. J. Nanomater. 2016, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Prathyusha, K.; Muthukumaran, M.; Krishnamoorthy, B. Liposomes as targetted drug delivery systems present and future prospectives: A review. J. Drug Deliv. Ther. 2013, 3, 195–201. [Google Scholar] [CrossRef]
- Lin, C.-M.; Li, C.-S.; Sheng, Y.-J.; Wu, D.T.; Tsao, H.-K. Size-dependent properties of small unilamellar vesicles formed by model lipids. Langmuir 2012, 28, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, T.O.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A.J.M. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef]
- Magin, R.L.; Hunter, J.M.; Niesman, M.R.; Bark, G.A. Effect of vesicle size on the clearance, distribution, and tumor uptake of temperature-sensitive liposomes. Cancer Drug Deliv. 1986, 3, 223–237. [Google Scholar] [CrossRef]
- Hosny, K.M. Ciprofloxacin as ocular liposomal hydrogel. AASP PharmSciTech 2010, 11, 241–246. [Google Scholar] [CrossRef]
- Tsotas, V.-A.; Mourtas, S.; Antimisiaris, S.G. Dexamethasone incorporating liposomes: Effect of lipid composition on drug trapping efficiency and vesicle stability. Drug Deliv. 2007, 14, 441–445. [Google Scholar] [CrossRef]
- Tabandeh, H.; Mortazavi, S.A. An investigation into some effective factors on encapsulation efficiency of alpha-tocopherol in MLVs and the release profile from the corresponding liposomal gel. Iran J. Pharm. Res. 2013, 12, 21. [Google Scholar]
- Epand, R.M.; Epand, R.F.; Maekawa, S. The arrangement of cholesterol in membranes and binding of NAP-22. Chem. Phys. Lipids 2003, 122, 33–39. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Yang, X. Proteases for Cell Suicide: Functions and Regulation of Caspases. Microbiol. Mol. Biol. Rev. 2000, 64, 821–846. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- De Smet, L.; Ceelen, W.; Remon, J.P.; Vervaet, C. Optimization of drug delivery systems for intraperitoneal therapy to extend the residence time of the chemotherapeutic agent. Sci. World J. 2013, 2013, 720858. [Google Scholar] [CrossRef] [PubMed]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal Route of Drug Administration: Should it Be Used in Experimental Animal Studies? J. Pharm. Res. 2020, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- Mirahmadi, N.; Babaei, M.; Vali, A.; Dadashzadeh, S. Effect of liposome size on peritoneal retention and organ distribution after intraperitoneal injection in mice. Int. J. Pharm. 2010, 383, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Iyer, A.; Seo, Y.; Broaddus, C.; Liu, B.; VanBrocklin, H.; He, J. Effects of size and targeting ligand on biodistribution of liposome nanoparticles in tumor mice. J. Nucl. Med. 2013, 54, 1339. [Google Scholar]
- Singhania, A.; Wu, S.Y.; McMillan, N.A. Effective delivery of PEGylated siRNA-containing lipoplexes to extraperitoneal tumours following intraperitoneal administration. J. Drug Deliv. 2011, 2011, 192562. [Google Scholar] [CrossRef]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).