Abstract
Extracellular matrix (ECM) turnover is characterized by a unique balance between matrix metalloproteinases’ degradation activity and their natural inhibition by collagen specific tissue inhibitors. Human uterine ECM is a complex structure, majorly consisting of proteins as fibrillar collagen types I and III, fibronectin, and laminin. Collagenases are enzymes from the matrix metalloproteinases’ family, which are predominantly involved in fibrillar collagen types I and III degradation. They are mainly represented by matrix metalloproteinase-1, -13 (MMP-1, -13), naturally inhibited by tissue inhibitors (TIMP-1, -2). The collagen structure of the uterus has been shown to be impaired in women with preeclampsia. This is a result of MMPs/TIMPs dysregulation interplay. This review article summarizes the actual available research data in the literature about the role of MMP-1, MMP-13 and TIMP-1, and TIMP-2 in collagen types I and III turnover in healthy and complicated pregnancy. Their potential use as circulating markers for diagnosis, prognosis, and monitoring of the development of preeclampsia is discussed as well.
1. Collagen Type I Characteristics
Collagen type I is a major connective tissue protein. It increases the strength and stability of the cytoskeleton. The exceptional strength of skin, ligaments, tendons, and vessels requires a long protein chain characterized by repeated amino acid residues and a regular secondary structure.
Type I collagen is fibrillar collagen and a major part of the interstitial membrane’s structure. It is the most prevalent type of collagen and a key structural composition of many tissues. It is found practically in all structures involving connective tissue. Type I collagen is the main structural protein of bone, skin, tendon, ligaments, sclera, cornea, and blood vessels, as well as an important component of other tissues. It is gathered in fibers forming a structural-mechanical scaffold (matrix) of bones, skin, tendons, cornea, blood vessel walls, and other connective tissues [1].
The COL1A1 gene produces the pro-alpha1 (I) chain. This chain combines with another pro-alpha1 (I) chain and also with a pro-alpha2 (I) chain (produced by the COL1A2 gene) to make a molecule of type I procollagen. These triple-stranded, rope-like procollagen molecules must be processed by enzymes outside the cell. Once these molecules are processed, they arrange themselves into long, thin fibrils that cross-link to one another in the spaces around cells. The cross-links result in the formation of very strong mature type I collagen fibers.
“Heterotrimers of two α1 (I) and one α2 (I) chains are the dominant isoform of type I collagen. Homotrimers of three α1 (I) chains are found in fetal tissues and some fibrous lesions. The homotrimeric isoform is more resistant to cleavage than collagenases. This may explain its abnormal accumulation and important role in the pathogenesis of tumors and fibrosis” [1].
In vivo, the triple helical fibers are mostly incorporated into a composite containing either type III collagen (in skin and reticular fibers) or type V collagen (in bone, tendon, cornea) [2,3]. Type I collagen provides tensile stiffness in tendons and fascia, while in bone, it defines considerable biomechanical properties concerning load bearing and tensile [4]
2. Collagen Type III Characteristics
Collagen Type III has a unique molecule structure, giving the connective tissue matrix a specific architecture [5]. Collagen molecules contain three identical or similar polypeptide chains called α-chains and contain at least one triplet helix collagen domain with repeating (Gly-X-Y) n sequences. Thus, every third amino acid is a glycine residue with frequently repeated proline and 4-hydroxyproline at the X and Y positions. In addition, all collagen contain non-collagen domains. Collagens type III form fibrils [6]. Type III collagen is a homotrimer of three a1 (III)-chains and is widely distributed in collagen I-containing tissues with the exception of bone [7]. It is an important component of reticular fibers in the interstitial tissue of the lungs, liver, dermis, spleen, and vessels. This homotrimeric molecule also often contributes to mixed fibrils with type I collagen and is also abundant in elastic tissues [8].
“Type III collagen is composed of one collagen α-chain, unlike most other collagens. This is a homotrimer containing three α1 (III) chains overlapped in a right triple helix. Type III collagen is secreted by fibroblasts and other types of mesenchymal cells, thus playing a major role in different inflammatory pathological conditions like lung damage, liver diseases, renal fibrosis, and vascular fibrosis diseases”. Both collagen type III and type I are the main components of extracellular matrix (ECM). Biomarkers of Type III collagen turnover have been actively studied and different laboratory methods have been used for the detection of fibrosis [9].
3. General Features of Matrix Metalloproteinases (MMPs) and Tissue Inhibitors of MMP (TIMPs)
Matrix metalloproteinases are a complex group of endopeptidases. They are a family of proteolytic enzymes with similar functional domains and a mechanism of action associated with the degradation of ECM components. MMPs are zinc-dependent proteases that can be activated by a number of cytokines and growth factors [10]. Metalloproteinases have the properties to attach to components of the extracellular matrix and in particular to collagen and elastin. MMPs are secreted by activated macrophages in the wall of arterial vessels. The ability of MMPs to change tissues is important from the point of view of normal and pathological physiology. Approximately 20 different types of MMPs are known, classified into groups according to the type of proteolytic substrate (component of the extracellular matrix) against which they act and degrade, respectively. The MMP group includes collagenases (MMP-1 and MMP-13), stromelysins such as MMP-3, gelatinases such as MMP-2 and MMP-9, and membrane MMPs. This classification based on the substrate of action was particularly useful years ago. With the accumulation of additional knowledge about the enzymatic activity of MMP, the benefit of this classification is questioned, as the substrate profile of the enzyme is more relative than absolute [11].
TIMPs, consisting of 184–194 amino acids, are inhibitors of MMPs. They are subdivided into an N-terminal and a C-terminal subdomain. Each domain contains three conserved disulfide bonds and the N-terminal domain folds as an independent unit with MMP inhibitory activity. TIMPs inhibit all MMPs tested so far, but TIMP-1 is a poor inhibitor for membrane-type (MT)1-MMP, MT3-MMP, MT5-MMP, and MMP-19 [12].
4. Matrix Metalloproteinases and Tissue Inhibitors of MMPs in Preeclampsia
4.1. MMP-1 Structure and Function
Collagenases (MMP-1, MMP-8, and MMP-13) cleave interstitial collagens I, II, and III into characteristic 3/4 and 1/4 fragments, but they can digest other ECM molecules and soluble proteins [13,14]. MMP-1, also known as collagenase-1, was the first MMP identified by Gross and Lapiere in 1962 [15]. “Humans express MMP-1 while rodents have two MMP-1 isoforms—namely, MMP-1a and -1b. MMP-1 cleaves both ECM and non-ECM substrates such as collagen, gelatin, laminin, complement C1q, IL-1β, and TNF-α, suggesting a crucial role in inflammatory and fibrotic responses” [16]. MMP-1 can also activate MMP-2 and -9, initiating an activation cascade. MMP-1 is an important member of MMP family, which particularly degrades interstitial collagen and is abundant in tissues of the placenta and decidua. TIMP-1 is a natural inhibitor of MMP-1 [17,18]. “The invasive capacity of trophoblasts has been associated with their secretion of MMP-1. The zymolytes of MMP-1 are collagen and metagelatin, which play major roles in trophoblast invasion” [13].
4.2. MMP-13 Structure and Function
MMP-13 plays a role in the degradation of extracellular matrix proteins including fibrillar collagen and fibronectin. It cleaves triple helical collagens, including type I, type II, and type III collagen, but has the highest activity with soluble type II collagen. “Can also degrade collagen type IV, type XIV and type X. Plays a role in wound healing, tissue remodeling, may play a role in cell migration and in tumor cell invasion” [19]. MMP-13 also play role in tissue repair and in progression of diseases such as cancer, arthritis, atherosclerosis, and aneurysm.
4.3. TIMP-1 Structure and Function
TIMP-1 is a glycoprotein, member of the TIMPs family [20]. It is expressed by several tissues [21]. This protein serves as natural inhibitor of the matrix metalloproteinases, which are involved in extracellular matrix degradation [14]. While TIMP-1 potently inhibits the activity of most MMPs, with the exception of MMP-2 and MT1-MMP, TIMP-2 is a potent inhibitor of most MMPs, except MMP-9 [22].
4.4. TIMP-2 Structure and Function
TIMP-2 inhibits specific types of MMPs, thus involving degradation of the extracellular matrix. TIMP-2 has ability to directly suppress the proliferation of endothelial cells. This leads to critical possibility for the encoded protein in the maintenance of tissue homeostasis by suppressing the proliferation of quiescent tissues in response to angiogenic factors, and by inhibiting protease activity in tissues undergoing remodeling of the extracellular matrix [23]. While TIMP-1 inhibits MMP-7, MMP-9, MMP-1, and MMP-3 better than TIMP-2, TIMP-2 inhibits MMP-2 more effectively than other TIMPs.
5. Collagen Type I and III Turnover in Normal Pregnancy
Collagen types I and III are the main proteins involved in the structure of the uterine wall. As the uterus grows during pregnancy, there is an intensified collagen turnover. It is well known that the uterine collagen structure has been shown to be disturbed in women with pre-eclampsia. Amino-terminal and carboxy-terminal propeptides of collagen type I and III play a central role in this process [24]. The human uterus is composed of a fibrous tissue framework consisting mainly of collagen types I and III [25]. It is, therefore, possible that in hypertensive disorders in pregnancy these collagens (which are mainly responsible for the coherence and supportive strength of the uterus) could be affected. Controlled collagenolysis and/or changes in collagen cross-linking will be needed to meet the demand of the growing uterine content to expand. As the uterus grows during pregnancy there is a high production and turnover of collagen proteins [26].
Collagen types I and III are major components of human cervical uterine connective tissue. During pregnancy, a remodeling of the cervical connective tissue takes place, with decreases in the concentrations of collagen and proteoglycans concomitant with an increase in the collagenolytic activity [27,28,29]. 70% decrease in the amount of collagen and the change in its organization is observed [30,31]. In abnormal conditions such as preeclampsia and gestational hypertension, the blood flow to both placenta and foetus is disturbed, favouring a microcirulatory ischaemia. Altered extracellular matrix turnover with MMP/TIMP dysbalance play a crucial role in these pathological processes [32,33].
6. Impaired Collagen Type I and III Turnover in Preeclampsia—The Role of MMP/TIMP Complex Dysregulation
Several studies suggest that a key trait of preeclampsia is “the impaired capacity of the trophoblast to invade the uterine spiral arteries resulting in a poorly perfused fetoplacental unit. As a result various factors inducing endothelial dysfunction could be found in circulation of women with preeclampsia” [34,35]. Changes in circulatory concentrations and activity of MMPs as well as their endogenous inhibitors, TIMPs, majorly involved in collagen metabolism, led to the consideration of these proteases as key mediators in the pathological features of preeclampsia [36].
Preeclampsia (PE) is one of the most widely seen hypertensive disorders of pregnancy. Data show that preeclampsia complicates about 2–8% of pregnancies worldwide [37]. PE is characterized by the new onset of hypertension (≥140/90 mmHg) and proteinuria (0.3 g in a 24 h urine sample) occurring after 20 weeks of gestation [38]. PE is a major cause of maternal and perinatal morbidity and mortality. Despite that fact, it has not been fully studied yet.
Preeclampsia is an important pregnancy complication and one of the most common pregnancy disorders. Preeclampsia is characterized by a high blood pressure of mother, often with proteinuria. Fetal growth restriction is also generally seen. There is evidence that, extracellular matrix (ECM) metabolism is altered in the human uterus during preeclampsia. However, little is known about the change in the composition of the collagen in the human uterus during preeclampsia [39,40].
The human uterine wall consists mainly of collagen types I and III. These proteins play a central role in the stability of tissue structure and the regulation of cell growth and differentiation. The processes of collagen types I and III synthesis and degradation in ECM of human uterus are dynamic and reflect not only healthy, but also complicated pregnancy [41]. Collagen turnover has been shown to be impaired in women with preeclampsia [42]. Early preeclampsia detection is paramount for risk stratification and prevention of further complications.
Collagen structure of the uterus has been shown to be impaired in women with preeclampisa. This is a result of MMPs/TIMPs dysregulation interplay. Collagenases matrix metalloproteinase-1, -13 (MMP-1, -13), naturally inhibited by tissue inhibitors (TIMP-1, -2) are involved in this process. This leads to pathological changes in uterine structure and abnormal uterine remodeling in preeclampsia (Figure 1).
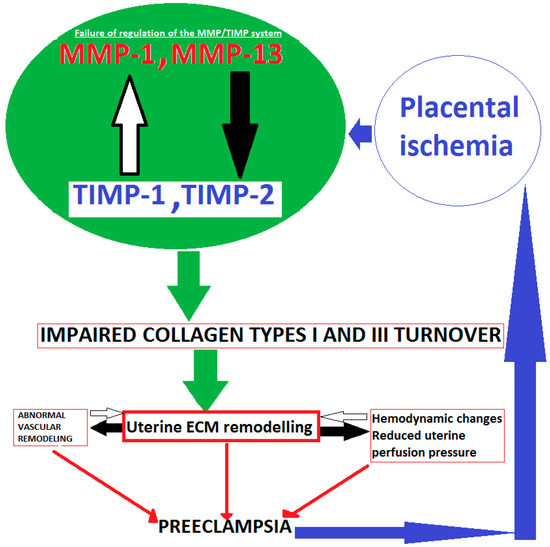
Figure 1.
Schematic presentation of impaired collagen I and III turnover in pathological pregnancy.
Placental ischemia could be a key point in preeclampsia development [43]. In result, MMP/TIMP dysregulation interplay occurs. Since the human uterine ECM majorly consists of collagen types I and III, balance between degradation activity of collagenases MMP-1, -13, and their tissue inhibitors TIMP-1 and -2 is disturbed. Hence, collagen type I and III turnover is impaired. This may lead to reduced uterine perfusion pressure, abnormal vascular, and uterine ECM remodeling at the maternal fetal interface. These processes lead to the over deposition of collagen, which may affect the remodeling of uterine spiral artery, and it may be an important factor in the pathogenesis of preeclampsia [44].
There is a growing data for matrix metalloproteinases and their tissue inhibitors’ key role in the pathogenesis of hypertensive disorders of pregnancy. Karthikeyan et al. [45] have shown that plasma and genetic alterations in the MMP/TIMP system is associated with hypertensive disorder of pregnancy (HDP). They have concluded that failure of regulation of the MMP/TIMP system in controlling the extracellular matrix remodeling may lead to diverse pathology, such as gestational hypertension and preeclampsia. In previous years, vascular remodeling disorders of the uterine and placenta and placenta hypoperfusion have been generally recognized [46]. Studies have shown the role of matrix metalloproteinases in the pathogenesis of hypertensive disorders in pregnancy [47,48,49,50,51,52].
6.1. MMP-1 Dysregulation
MMP 1 is a member of the MMP family. The zymolytes of MMP 1 are collagen and metagelatin, which play major roles in trophoblast invasion [53]. TIMP 1 is a natural inhibitor of MMP 1 [54,55]. Different methods may be used to determine their circulatory levels.
Guadalupe Estrada-Gutierrez et al., in 2011, have shown an increased expression of matrix metalloproteinase-1 in systemic vessels of women with preeclampsia. Vessel expression of MMP-1 and circulating MMP-1 levels were increased in women with preeclampsia, whereas vascular expression of collagen or tissue inhibitor of metalloproteinase-1 were down-regulated or unchanged [56].
Gupta M et al., 2016, compared serum values of MMP-1, TIMP-1, and their ratio in the second and third trimester of normal and pre-eclamptic pregnancy. In that study, 30 females progressing to normal pregnancy were compared with 16 females who developed preeclampsia. MMP-1 and TIMP-1 concentrations were measured in serum samples (2nd and 3rd trimester) of the females by enzyme linked immuno-sorbent assay. There was no significant difference in the levels of MMP-1, TIMP-1, and ratio of MMP-1 and TIMP-1 in pre-eclamptic and normal pregnancy females. There is a lack of alteration in the levels of MMP-1, TIMP-1, and their ratio during the progression of pre-eclampsia when compared with normal pregnancy [57].
Wei Li et al., 2017, tested the hypothesis that placental ischemia could target vascular and uteroplacental MMPs through an inflammatory cytokine-mediated mechanism. “MMP levels and distribution were measured in the aorta, uterus, and placenta of normal pregnant (Preg) rats and pregnant rats with reduced uterine perfusion pressure (RUPP). Gelatin zymography showed prominent uterine MMP-2 and MMP-9 activity that was dependent on the amount of loaded protein. This study showed that placental ischemia, possibly through the release of TNF-α, causes increases in the levels of matrix metalloproteinase MMP-1 and MMP-7, which could alter collagen deposition and cause inadequate uteroplacental and vascular remodeling in hypertension in pregnancy. The data suggest that targeting MMP-1 and MMP-7 and their upstream modulators, such as TNF-α, could provide a new approach in the management of hypertension in pregnancy and preeclampsia” [58].
Chun-Lei Deng et al., 2015, measured levels of MMP-1 in maternal umbilical serum of women with preeclampsia and compared the concentrations with those of normotensive pregnant females. MMP-1 levels were decreased in maternal umbilical serum of patients with preeclampsia [59]. Table 1 summarizes the available literature data for studies measuring levels of MMP-1 in samples of patients with hypertensive disorders of pregnancy.

Table 1.
Levels of matrix metalloproteinase 1 (MMP-1) in samples of patients with hypertensive disorders of pregnancy.
6.2. MMP-13 Dysregulation
In 2017, Marzena Laskowska investigated whether maternal serum matrix metalloproteinases 2, 3, 9, and 13 levels differ in early- and late-onset preeclampsia and uncomplicated pregnancies. “Maternal serum MMP-2 levels in both the groups of preeclamptic women were significantly higher than those in the controls. Levels of MMP-3 were significantly higher in preeclamptic patients with early-onset disease; however, the MMP-3 levels in patients with late-onset preeclampsia were similar to those observed in the control subjects. MMP-9 levels were lower whereas the levels of MMP-13 were higher in both preeclamptic groups of pregnant women than in the healthy controls, but these differences were statistically insignificant. One important finding of the present study was that MMP-3 appears to be involved solely in early-onset preeclampsia, but not in late-onset preeclampsia. Higher levels of MMP-2 and MMP-13 and lower levels of MMP-9 seem to be related to both early- and late-onset severe preeclampsia” [60].
6.3. TIMP-1 and TIMP-2 Dysregulation
According to Gupta M et al., 2016, there was no significant difference in the levels of MMP-1, TIMP-1, and ratio of MMP-1 and TIMP-1 in pre-eclamptic and normal pregnancy females. There is a lack of alteration in the levels of MMP-1, TIMP-1, and their ratio during the progression of pre-eclampsia when compared with normal pregnancy [57].
MMP-9, TIMP-1 polymorphism, plasma TIMP-1 levels, and antihypertensive therapy responsiveness in hypertensive disorders of pregnancy are examined by Luizon MR et al., 2014. Plasma MMP-9 and TIMP-1 levels were measured by ELISA. Gestational hypertension patients with the GG genotype for the TIMP-1 polymorphism had lower MMP-9 levels and MMP-9/TIMP-1 ratios than those with the TT genotype. Preeclampsia patients with the TG genotype had higher TIMP-1 levels [61].
Tayebjee MH et al., 2005, investigated circulating MMP-9 and TIMP-1 and -2 levels in women with gestational hypertension, normotensive women with normal pregnancies and healthy nonpregnant control subjects. Plasma MMP-9, TIMP-1, and TIMP-2 were measured by ELISA. Levels of circulating MMP-9, TIMP-1 and TIMP-2, and the MMP-9/TIMP-1 and MMP-9/TIMP-2 ratios were significantly different among the three groups. Plasma TIMP-1 in non-pregnant was 197 ‡ (ng/mL) vs. normotensive pregnant 180 ‡ (ng/mL) vs. gestational hypertension 195 (ng/mL) (p = 0.006). Plasma TIMP-2 in non-pregnant was 140 ‡ (ng/mL) vs. normotensive pregnant 138§ (ng/mL) vs. gestational hypertension 180 ‡§ (ng/mL) (p = 0.007) Authors concluded that “altered MMP/TIMP ratios in maternal blood during gestational hypertension. These observations suggest pregnancy-related changes in ECM breakdown and turnover. Given the importance of changes in ECM composition to vascular and cardiac structure in hypertension, investigators suggest that these observations may be related to the pathophysiology of human gestational hypertension” [62].
Gupta M et al., 2016 [57], compared the serum values of MMP-1, TIMP-1, and their ratio in the second and third trimester of normal and preeclamptic pregnancy. ELISA was used for determination of MMP-1 and TIMP-1. There is a lack of alteration in the levels of MMP-1, TIMP-1, and their ratio during the progression of preeclampsia when compared with normal pregnancy.
Myers JE et al., 2005, examined MMP-2 and-9 levels, TIMP-1 and TIMP-2 in the plasma of women who subsequently develop preeclampsia. Plasma samples were taken from women whose pregnancies were subsequently complicated by preeclampsia and from normal pregnant women at 22 and 26 weeks and at delivery or diagnosis. “Following equal protein loading, MMP-2 and 9 and TIMP-1 and 2 were quantified using zymography and Western blot analysis, respectively. TIMP-1 levels were significantly reduced in the preeclampsia group at 26 weeks (p = 0.0002), but TIMP-2 levels were not quantifiable. Authors conclude that at all three gestational time points an imbalance in the MMP-2/TIMP-1 ratio was found in patients who subsequently developed preeclampsia” [63].
In a cross-sectional study, Palei et al., 2008, compared circulating levels of Pro-MMP-2, Pro-MMP-9, TIMP-1, TIMP-2, and the Pro-MMP-9/TIMP-1 and Pro-MMP-2/TIMP-2 ratios in women with preeclampsia, gestational hypertension, normotensive pregnancies and healthy nonpregnant women. Pro-MMP and TIMP concentrations were measured in plasma samples by gelatin zymography and ELISA. Higher TIMP-1 levels were found in PE compared with GH and normotensive pregnant women. Nonpregnant women had lower TIMP-2 levels than those found in normotensive pregnant controls, gestational hypertension and preeclampsia [64].
Palei et al., 2012, examined whether two functional polymorphisms (g.-1306 C > T and g.-735 C > T) in MMP-2 gene are associated with preeclampsia or gestational hypertension, and whether they modify MMP-2 or TIMP-2 plasma concentrations in these hypertensive disorders of pregnancy. Plasma MMP-2 and TIMP-2 concentrations were measured by ELISA. “The main findings were that pregnant women with preeclampsia had higher plasma MMP-2 and TIMP-2 concentrations than healthy pregnant (p < 0.05), although the MMP-2/TIMP-2 ratios were similar (p > 0.05). Moreover, pregnant women with gestational hypertension had elevated plasma MMP-2 levels and MMP-2/TIMP-2 ratios compared to healthy pregnant (p < 0.05)” [65].
In a case-control study, Ab Hamid et al., 2012, examined the total levels of MMP-9 and TIMP-1 and 2 in women with gestational hypertension and normotensive pregnant women. ELISA was used for evaluation of these biomarkers. TIMP-1 and TIMP-2 levels showed low levels of expression in the gestational hypertension group. Weak positive correlations were found on correlation analysis between maternal age and TIMP-1 in the gestational hypertension group and between gestational age and TIMP-2 in the control group [66].
In 2014, Early Pregnancy Prediction of Preeclampsia in Nulliparous Women, Combining Clinical Risk and Biomarkers The Screening for Pregnancy Endpoints (SCOPE) International Cohort Study investigated 47 biomarkers involved in the pathogenesis of preeclampsia. These biomarkers were measured in plasma sampled at 14 to 16 weeks’ gestation from 5623 women. Considering TIMPs—higher levels of TIMP-1—101 ng/mL compared with the no preeclampsia group 91 ng/mL (p = 0.01) were reported [67].
Montagnana, M. et al., 2009, evaluated MMP-2 and -9, along with their inhibitors TIMP-1 and -2 in preeclamptic compared with normotensive pregnant and non-pregnant women. A sandwich enzyme-linked immunosorbent assay was used to measure MMPs and TIMPs. The serum levels of TIMP-1 were significantly higher in preeclamptic compared with both non-pregnant and normotensive pregnant women. TIMP-2 values were higher in normotensive pregnant and preeclamptic women compared with non-pregnant women. Results of the present investigation confirm that MMP-2 and TIMP-1 values are significantly higher in preeclampsia [68]. Table 2 and Table 3 summarize the available literature data for studies measuring levels of TIMP-1 and TIMP-2 in samples of patients with hypertensive disorders of pregnancy.

Table 2.
Levels of tissue inhibitor of matrix metalloproteinase 1 (TIMP-1) in samples of patients with hypertensive disorders of pregnancy.

Table 3.
Levels of tissue inhibitor of matrix metalloproteinase 2 (TIMP-2) in samples of patients with hypertensive disorders of pregnancy.
7. Conclusions
The unique balance between the degradation activity of collagen specific MMPs and their natural tissue inhibitors indicates that TIMPs plays an important role in normal structural uterine changes during healthy pregnancy. Dysregulation of balance between MMPs and their TIMPs occurs in preeclampisa, leading to impaired fibrillar collagen type I and III turnover. This results in pathological changes in uterine structure and abnormal uterine ECM remodeling. MMP-1, MMP-13, TIMP-1, and TIMP-2 are biomolecules, tightly involved in these processes. MMP-1, MMP-13, TIMP-1, and TIMP-2 potential as preeclampsia biomarkers is very promising and possible clinical applications can hopefully be introduced soon.
8. Future Directions
According to the above studies, matrix metalloproeinase-1, -13 (MMP-1, -13) and their tissue inhibitors (TIMPs-1, -2) have promising scope favoring future clinical applications of these biomolecules as serological markers for the diagnosis, prognosis, and monitoring of the development of preeclampsia. Despite that, there are some controversial findings and limitations for their routine use. Several important critical remarks make gaps in evidence. Changes in collagen type I and type III turnover are likely to occur at a very early stage. Many studies were undertaken in women with established preeclampsia [56,57,59,62,64,65,66]; therefore, it cannot be determined whether the change in MMPs and TIMPs levels were a cause or a consequence of the disease. To more precisely determine the extent of the association of these markers with concurrent preeclampsia, it would be necessary for healthy pregnant women with family history of preeclampsia to be closely monitored and tested for changes in collagen type I and III turnover biomarkers, prior to preeclampsia development. Different studies examine different patient groups including women with wide range of hypertensive disorders of pregnancy, such as women with gestational hypertension [61,62,64,66] vs. preeclampsia [56,57,59,68]. Moreover, some studies investigate patients with early onset preeclampsia ≤34 gestational week, while others focus on late onset of preeclampsia ≥34 gestational week. This makes it very difficult to compare the results. Whether collagen metabolism markers give enough information like lone indicators, or clinicians need a combination model adding other known biomarkers remains to be determined. For example, pregnancy associated plasma protein A (PAPA), placental growth factor (PlGF), or soluble FLT-1 (also known as soluble VEGF receptor 1 or sFLT-1) have been previously explored as preeclampsia diagnostic and prognostic biomarkers. Probably, the complex use of an integrative model combining different types of markers looks more reasonable than just one indicator. Considering the commented investigations in this review, some important differences can be observed between the methods for detection of serum markers of collagen type I and III turnover. Some researchers use zymography for detection of biomolecules’ activity [58,63], other use ELISA—for measuring biomarkers’ concentration [57,59,61,62,65,66,68]. Studies [56,64] use both Zymography and ELISA for diagnosis, prognosis, and monitoring of the development of preeclampsia.
Analyzing the above mentioned studies, differences can be observed between the type of samples used for detection of markers of collagen type I and III turnover, serum [57,66,68] vs. plasma [56,61,62,63,64,65,67]. Not only absolute MMPs and TIMPs levels are important, but also the relationship between their ratios [57,61,63,65] could play a key role in uterine vascular remodeling in hypertensive disorders of pregnancy. Probably, MMPs/TIMPs ratios should be tested along with MMP and TIMP levels lone. Clinical severity of preeclampsia should also be considered. It is important to take into account mild vs. severe preeclampsia cases. It can influence the concentration of circulating collagen biomarkers. Cross-sectional studies report levels of biomarkers only at one certain point. These markers should however be determined in serial measurements at various time points. This calls the need for larger and longitudinal investigations to be accomplished. Early pregnancy prediction of term preeclampsia remains too poor to be of clinical use. That is why the SCOPE study [67] suggests a concept of 2-stage screening with screening in early pregnancy for early-onset disease and screening for term preeclampsia in the third trimester, despite the fact that it is not fully investigating collagen type I and III biomarkers. It has not been completely established whether a serum biomarker is representative for the tissue level concentration. A method to correlate the serum levels of the biomarkers with uterine remodeling involves immunohistochemistry and histology of uterine biopsies, but it cannot be performed in cross-sectional studies.
Author Contributions
Conceptualization, A.N. and N.P.; methodology, I.H.; writing—original draft preparation, A.N.; writing—review and editing, A.N.; visualization, I.H.; supervision, A.N. and N.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Henriksen, K.; Karsdal, M.A. Type I Collagen. In Biochemistry of Collagens, Laminins and Elastin Structure, Function and Biomarkers, 1st ed.; Karsdal, M.A., Ed.; Academic Press: Cambridge, MA, USA, 2016; Chapter 1; pp. 1–11. [Google Scholar]
- Fleischmajer, R.; Macdonald, E.D.; Perlish, J.S.; Burgeson, R.E.; Fisher, L.W. Dermal collagen fibrils are hybrids of type I and type III collagen molecules. J. Struct. Biol. 1990, 105, 162–169. [Google Scholar] [CrossRef]
- Niyibizi, C.; Eyre, D.R. Bone type V collagen: Chain composition and location of a trypsin cleavage site. Connect. Tissue Res. 1989, 20, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Gelse, K. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J.; Kivirikko, K.I. Collagens: Molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 1995, 64, 403–434. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.; Bateman, J.F. A new FACIT of the collagen family: COL21A. FEBS Lett. 2001, 505, 275–280. [Google Scholar] [CrossRef]
- Rossert, J.; Decrombrugghe, B. Type I collagen structure, synthesis, and regulation. In Principles of Bone Biology; Bilezkian, J., Raisz, J.P., Rodan, L.G., Eds.; Elsevier BV: Amsterdam, The Netherlands, 2002; Volume 1, pp. 189–210. [Google Scholar]
- Von Der Mark, K. Localization of collagen types in tissues. Int. Rev. Connect. Tissue Res. 1981, 9, 265–324. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G. Matrix metalloproteinases: Regulation and dysregulation in the failing heart. Circ. Res. 2002, 90, 520–530. [Google Scholar] [CrossRef]
- Spinale, F.G. Myocardial Matrix remodeling and the Matrix metalloproteinases: Influence on cardiac form and function. Physiol. Rev. 2007, 87, 1285–1342. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Woessner, J.F.; Nagase, H. Matrix Metalloproteinases and TIMPs: Protein Profile; Oxford Univ. Press: Oxford, UK, 2000; pp. 8–13. [Google Scholar]
- Gross, J.; Lapiere, C.M. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc. Natl. Acad. Sci. USA 1962, 48, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Padmanahan Iyer, R.; de Castro Brás, L.E.; Toba, H.; Yabluchanskiy, A. Cross talk between inflammation and extracellular matrix following myocardial infarction. In Inflammation in Heart Failure; Academic Press: Cambridge, MA, USA, 2015; Chapter 4; pp. 67–79. [Google Scholar]
- Itoh, Y.; Seiki, M. MT1-MMP: A potent modifier of pericellular microenvironment. J. Cell. Physiol. 2006, 206, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Murphy, G. Matrix metalloproteinases. In Encyclopedia of Biological Chemistry, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 90–97. [Google Scholar]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta (BBA) Bioenerg. Mol. Cell Res. 2010, 1803, 55–71. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, S.-H.; Kang, J.-G.; Ko, J.-H. Expression level and glycan dynamics determine the net effects of TIMP-1 on cancer progression. BMB Rep. 2012, 45, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Creemers, E.E.; Cleutjens, J.P.; Smits, J.F.; Daemen, M.J. Matrix metalloproteinase inhibition after myocardial infarction, a new approach to prevent heart failure? Circ. Res. 2001, 389, 201–210. [Google Scholar] [CrossRef]
- Morgunova, E.; Tuuttila, A.; Bergmann, U.; Tryggvason, K. Structural insight into the complex formation of latent matrix metalloproteinase 2 with tissue inhibitor of metalloproteinase. Proc. Natl. Acad. Sci. USA 2002, 99, 7414–7419. [Google Scholar] [CrossRef]
- Bourboulia, D.; Stetler-Stevenson, W.G. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin. Cancer Biol. 2010, 20, 161–168. [Google Scholar] [CrossRef]
- Amaral, L.M.; Wallace, K.; Owens, M.; Lamarca, B. Pathophysiology and current clinical management of preeclampsia. Curr. Hypertens. Rep. 2017, 19, 61. [Google Scholar] [CrossRef]
- Poon, L.C.; Nicolaides, K.H. Early prediction of preeclampsia. Obstet. Gynecol. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Pulkkinen, M.; Lehto, M.; Jalkanen, M.; Näntö-Salonen, K. Collagen types and fibronectin in the uterine muscle of normal and hypertensive pregnant patients. Am. J. Obstet. Gynecol. 1984, 149, 711–717. [Google Scholar] [CrossRef]
- Sahay, A.S.; Sundrani, D.P.; Joshi, S.R. Regional changes of placental vascularization in preeclampsia: A review. IUBMB Life 2015, 67, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Wallis, R.M.; Hillier, K. Regulation of collagen dissolution in the human cervix by oestradiol-17 beta and progesterone. J. Reprod. Fertil. 1981, 62, 55–61. [Google Scholar] [CrossRef]
- Sato, T.; Ito, A.; Mori, Y.; Yamashita, K.; Hayakawa, T.; Nagase, H. Hormonal regulation of collagenolysis in uterine cervical fibroblasts. Modulation of synthesis of procollagenase, prostromelysin and tissue inhibitor of metalloproteinases (TIMP) by progesterone and oestradiol-17 beta. Biochem. J. 1991, 275, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Uldbjerg, N.; Forman, A.; Petersen, L. Biochemical changes of the uterus and cervix during pregnancy. In Medicine of the Fetus and Mother; Reece, E.A., Hobbins, J.C., Mahoney, M.J., Petrie, R.H., Eds.; JB Lippincott Co.: Philadelphia, PA, USA, 1992; pp. 849–868. [Google Scholar]
- Burrows, T.D.; King, A.; Lok, Y.W. European society for human reproduction and embryology trophoblast migration during human placental implantation. Hum. Reprod. Update 1996, 2, 307–321. [Google Scholar] [CrossRef]
- Zhou, Y.; Damsky, C.H.; Chiu, K.; Roberts, J.M.; Fisher, S.J. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J. Clin. Investig. 1993, 91, 950–960. [Google Scholar] [CrossRef]
- Goldman-Wohl, D.S.; Yagel, S. Examination of distinct fetal and maternal molecular pathways suggests a mechanism for the development of preeclampsia. J. Reprod. Immunol. 2007, 76, 54–60. [Google Scholar] [CrossRef]
- Goldman-Wohl, D.; Yagel, S. Regulation of trophoblast invasion: From normal implantation to pre-eclampsia. Mol. Cell. Endocrinol. 2002, 187, 233–238. [Google Scholar] [CrossRef]
- Merchant, S.J.; Davidge, S.T. The role of matrix metalloproteinases in vascular function: Implications for normal pregnancy and pre-eclampsia. BJOG Int. J. Obstet. Gynaecol. 2004, 111, 931–939. [Google Scholar] [CrossRef]
- Danforth, D. The fibrous nature of the human cervix, and its relation to the isthmic segment in gravid and nongravid uteri. Am. J. Obstet. Gynecol. 1947, 53, 541–560. [Google Scholar] [CrossRef]
- Kitamura, K.; Ito, A.; Mori, Y.; Hirakawa, S. Glycosaminoglycans of human uterine cervix: Heparan sulfate increase with reference to cervical ripening. Biochem. Med. 1980, 23, 159–166. [Google Scholar] [CrossRef]
- Harmon, A.C.; Cornelius, D.C.; Amaral, L.M.; Faulkner, J.L.; Cunningham, M.W., Jr.; Wallace, K.; LaMarca, B. The role of inflammation in the pathology of preeclampsia. Clin. Sci. (Lond.) 2016, 130, 409–419. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet. Gynecol. 2013, 122, 1122–1130. [Google Scholar]
- Eiland, E.; Nzerue, C.; Faulkner, M. Preeclampsia 2012. J. Pregnancy 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Pulkkinen, M.O.; Kivikoski, A.I.; Nevalainen, T.J. Group I and group II phospholipase A2 in serum during normal and pathological pregnancy. Gynecol. Obstet. Invest. 1993, 36, 96–101. [Google Scholar] [CrossRef]
- Henriksen, K.; Karsdal, M.A. Type I Collagen. In Biochemistry of Collagens, Laminins and Elastin Structure, Function and Biomarkers, 2nd ed.; Karsdal, M.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 1; pp. 1–12. [Google Scholar]
- Nielsen, M.J.; Karsdal, M.A. Type III Collagen. In Biochemistry of Collagens, Laminins and Elastin Structure, Function and Biomarkers, 2nd ed.; Academic Press: Cambridge, MA, USA, 2019; Chapter 3; pp. 21–30. [Google Scholar]
- Shi, J.-W.; Lai, Z.-Z.; Yang, H.-L.; Yang, S.-L.; Wang, C.-J.; Ao, D.; Ruan, L.-Y.; Shen, H.-H.; Zhou, W.-J.; Mei, J.; et al. Collagen at the maternal-fetal interface in human pregnancy. Int. J. Biol. Sci. 2020, 16, 2220–2234. [Google Scholar] [CrossRef]
- Lim, K.H.; Zhou, Y.; Janatpour, M.; McMaster, M.; Bass, K.; Chun, S.H.; Fisher, S.J. Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am. J. Pathol. 1997, 151, 1809–1818. [Google Scholar]
- Karthikeyan, V.J.; A Lane, D.; Beevers, D.G.; Lip, G.Y.H.; Blann, A.D. Matrix metalloproteinases and their tissue inhibitors in hypertension-related pregnancy complications. J. Hum. Hypertens. 2012, 27, 72–78. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, S.; Liu, C.; Zhao, B.; Pei, K.; Tian, L.; Ma, X. Expressional and epigenetic alterations of placental matrix metalloproteinase 9 in preeclampsia. Gynecol. Endocrinol. 2010, 26, 96–102. [Google Scholar] [CrossRef]
- Merchant, S.J.; Narumiya, H.; Zhang, Y.; Guilbert, L.J.; Davidge, S.T. The effects of preeclampsia and oxygen environment on endothelial release of matrix metalloproteinase. Hypertens. Pregnancy 2004, 23, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Galewska, Z.; Bańkowski, E.; Romanowicz, L.; Jaworski, S. Pre-eclampsia (EPH-gestosis)-induced decrease of MMP-s content in the umbilical cord artery. Clin. Chim. Acta 2003, 335, 109–115. [Google Scholar] [CrossRef]
- Narumiya, H.; Zhang, Y.; Fernandez-Patron, C.; Guilbert, L.J.; Davidge, S.T. Matrix metalloproteinase-2 is elevated in the plasma of women with preeclampsia. Hypertens. Pregnancy 2001, 20, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Mahameed, S.; Goldman, S.; Gabarin, D.; Weiss, A.; Shalev, E. The effect of serum from women with preeclampsia on JAR (trophoblast-like) cell line. J. Soc. Gynecol. Investig. 2005, 12, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Lian, I.A.; Toft, J.H.; Olsen, G.D. Matrix metalloproteinase 1 in pre-eclampsia and fetal growth restriction: Reduced gene expression in decidual tissue and protein expression in extravillous trophoblasts. Placenta 2010, 31, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Hara, T.; Hayama, T.; Obara, M.; Yoshizato, K.; Ohama, K. Membrane-type 1 matrix metalloproteinase is induced in decidual stroma without direct invasion by trophoblasts. Mol. Hum. Reprod. 2001, 7, 271–277. [Google Scholar] [CrossRef]
- Hurskainen, T.; Seiki, M.; Apte, S.S.; Syrjäkallio–Ylitalo, M.; Sorsa, T.; Oikarinen, A.; Autio–Harmainen, H. Production of membrane-type matrix metalloproteinase-1 (MT-MMP-1) in early human placenta: A possible role in placental implantation? J. Histochem. Cytochem. 1998, 46, 221–229. [Google Scholar] [CrossRef]
- Lian, I.A.; Løset, M.; Mundal, S.B.; Fenstad, M.H.; Johnson, M.P.; Eide, I.P.; Bjørge, L.; Freed, K.A.; Moses, E.K.; Austgulen, R. Increased endoplasmic reticulum stress in decidual tissue from pregnancies complicated by fetal growth restriction with and without pre-eclampsia. Placenta 2011, 32, 823–829. [Google Scholar] [CrossRef]
- Raffetto, J.D.; Khalil, R.A. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem. Pharmacol. 2008, 75, 346–359. [Google Scholar] [CrossRef]
- Estrada-Gutierrez, G.; Cappello, R.; Mishra, N.; Romero, R.; Strauss, J.; Walsh, S. Increased Expression of Matrix Metalloproteinase-1 in Systemic Vessels of Preeclamptic Women A Critical Mediator of Vascular Dysfunction. Am. J. Pathol. 2011, 178, 451–459. [Google Scholar] [CrossRef]
- Gupta, M.; Chari, S. Assessment of matrix metalloproteinase-1 and its tissue inhibitor of metalloproteinase-1 in pre-eclampsia. Int. J. Sci. Study 2016, 3, 70–73. [Google Scholar]
- Li, W.; Cui, N.; Mazzuca, M.Q.; Mata, K.M.; Khalil, R.A. Increased vascular and uteroplacental matrix metalloproteinase-1 and-7 levels and collagen type I deposition in hypertension in pregnancy: Role of TNF-α. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H491–H507. [Google Scholar] [CrossRef]
- Deng, C.-L.; Ling, S.-T.; Liu, X.-Q.; Zhao, Y.-J.; Lv, Y.-F. Decreased expression of matrix metalloproteinase-1 in the maternal umbilical serum, trophoblasts and decidua leads to preeclampsia. Exp. Ther. Med. 2015, 9, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Laskowska, M. Altered maternal serum matrix metalloproteinases MMP-2, MMP-3, MMP-9, and MMP-13 in severe early-and late-onset preeclampsia. BioMed Res. Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Luizon, M.R.; Palei, A.C.T.; Sandrim, V.C.; Amaral, L.M.; Machado, J.S.R.; Lacchini, R. Tissue inhibitor of metalloproteinase (TIMP)-1 is a major endogenous inhibitor of matrix metalloproteinase (MMP)-9, which may affect the responsiveness to therapy in hypertensive disorders of pregnancy. Pharmacogenom. J. 2014, 14, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Tayebjee, M.; Karalis, I.; Nadar, S.; Beevers, D.J.; MacFadyen, R.; Lip, G.Y.H. Circulating matrix metalloproteinase–9 and tissue inhibitors of metalloproteinases–1 and –2 levels in gestational hypertension. AJH 2005, 18, 325–329. [Google Scholar] [CrossRef]
- Myers, J.E.; Merchant, S.J.; MacLeod, M.; Mires, G.J.; Baker, P.N.; Davidge, S.T. MMP-2 levels are elevated in the plasma of women who subsequently develop preeclampsia. Hypertens. Pregnancy 2005, 24, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Palei, A.C.; Sandrim, V.C.; Cavalli, R.D.C.; Tanus-Santos, J.E. Comparative assessment of matrix metalloproteinase (MMP)-2 and MMP-9, and their inhibitors, tissue inhibitors of metalloproteinase (TIMP)-1 and TIMP-2 in preeclampsia and gestational hypertension. Clin. Biochem. 2008, 41, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Palei, A.C.; Sandrim, V.C.; Amaral, L.M.; Machado, J.S.R.; Cavalli, R.D.C.; Duarte, G.; Tanus-Santos, J.E. Association between matrix metalloproteinase (MMP)-2 polymorphisms and MMP-2 levels in hypertensive disorders of pregnancy. Exp. Mol. Pathol. 2012, 92, 217–221. [Google Scholar] [CrossRef]
- Ab Hamid, J.; Mohtarrudin, N.; Osman, M.; Asri, A.A.; Hassan, W.H.W.; Aziz, R. Matrix metalloproteinase-9 and tissue inhibitors of metalloproteinases 1 and 2 as potential biomarkers for gestational hypertension. Singap. Med. J. 2012, 53, 681–683. [Google Scholar]
- Kenny, L.C.; Black, M.A.; Poston, L.; Taylor, R.; Myers, J.E.; Baker, P.N.; McCowan, L.M.; Simpson, N.A.B.; Dekker, G.A.; Roberts, C.T.; et al. Early pregnancy prediction of preeclampsia in nulliparous women, combining clinical risk and biomarkers the screening for pregnancy endpoints (SCOPE) international cohort study. Hypertension 2014, 64, 644–652. [Google Scholar] [CrossRef]
- Montagnana, M.; Lippi, G.; Albiero, A.; Scevarolli, S.; Salvagno, G.L.; Franchi, M.; Guidi, G.C. Evaluation of metalloproteinases 2 and 9 and their inhibitors in physiologic and pre-eclamptic pregnancy. J. Clin. Lab. Anal. 2009, 23, 88–92. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).