Impact of Aperture, Depth, and Acoustic Clutter on the Performance of Coherent Multi-Transducer Ultrasound Imaging
Abstract
1. Introduction
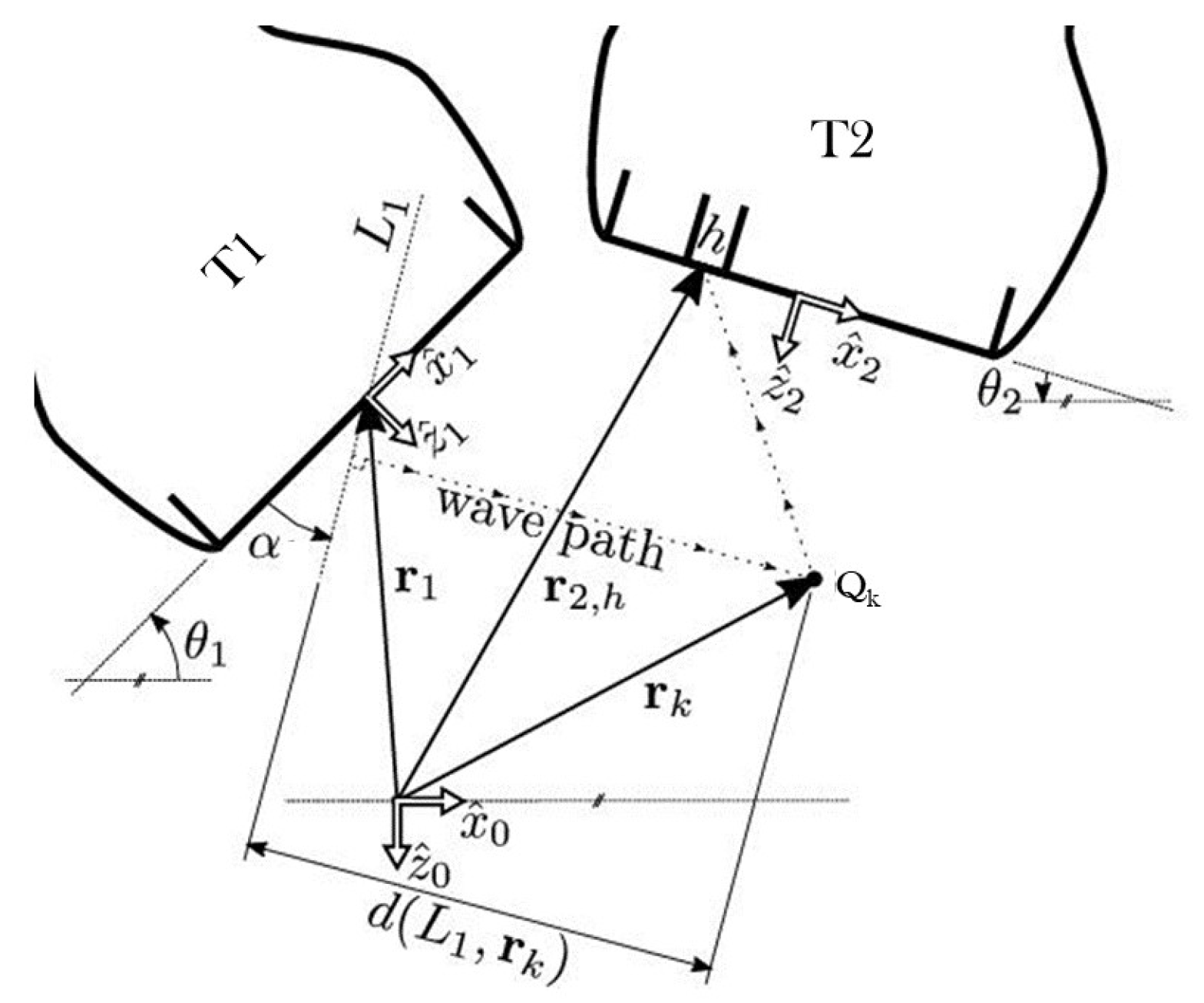
2. Theory: Coherent Multi-Transducer Ultrasound Imaging
3. Materials and Methods
3.1. Simulations
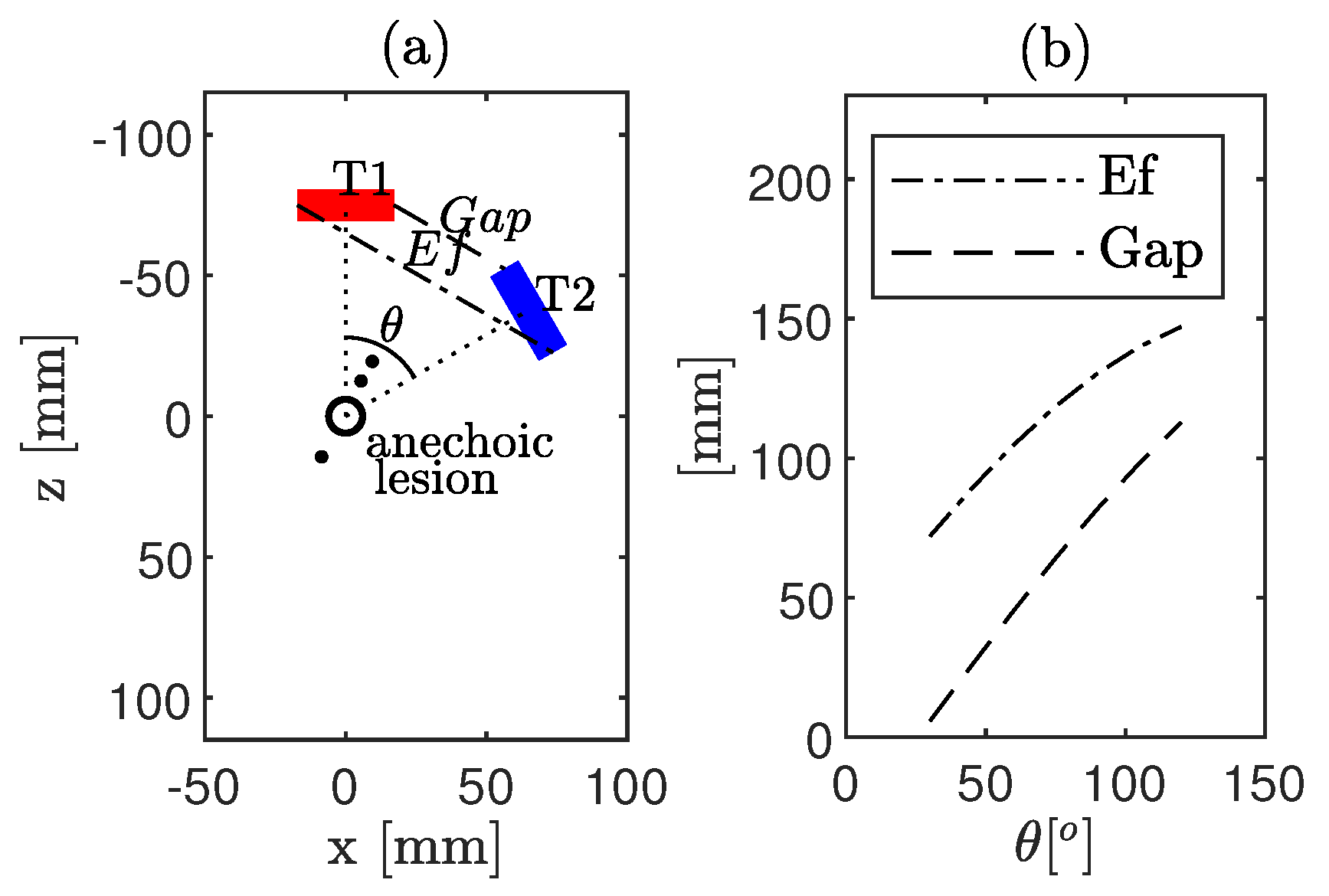
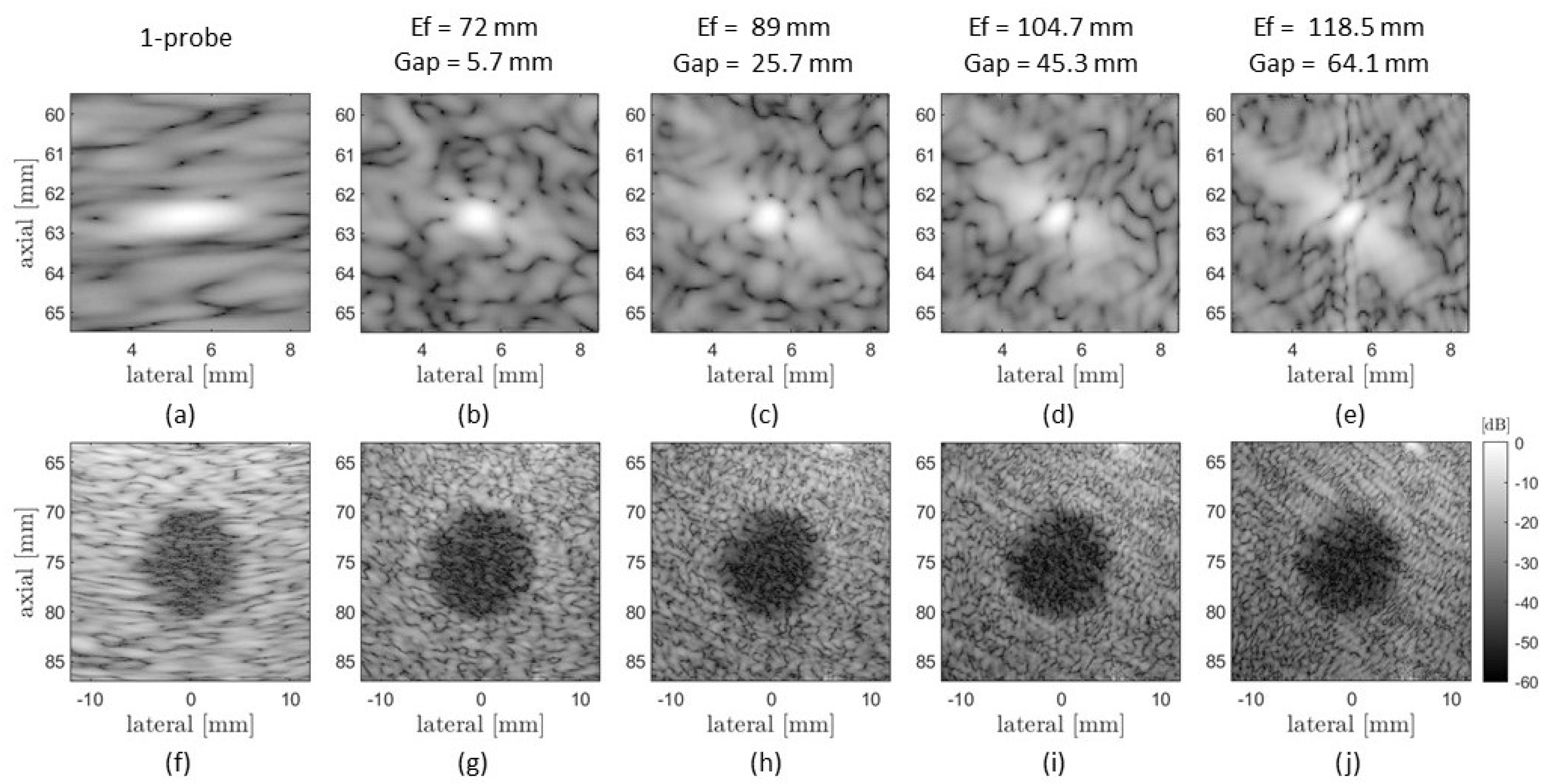
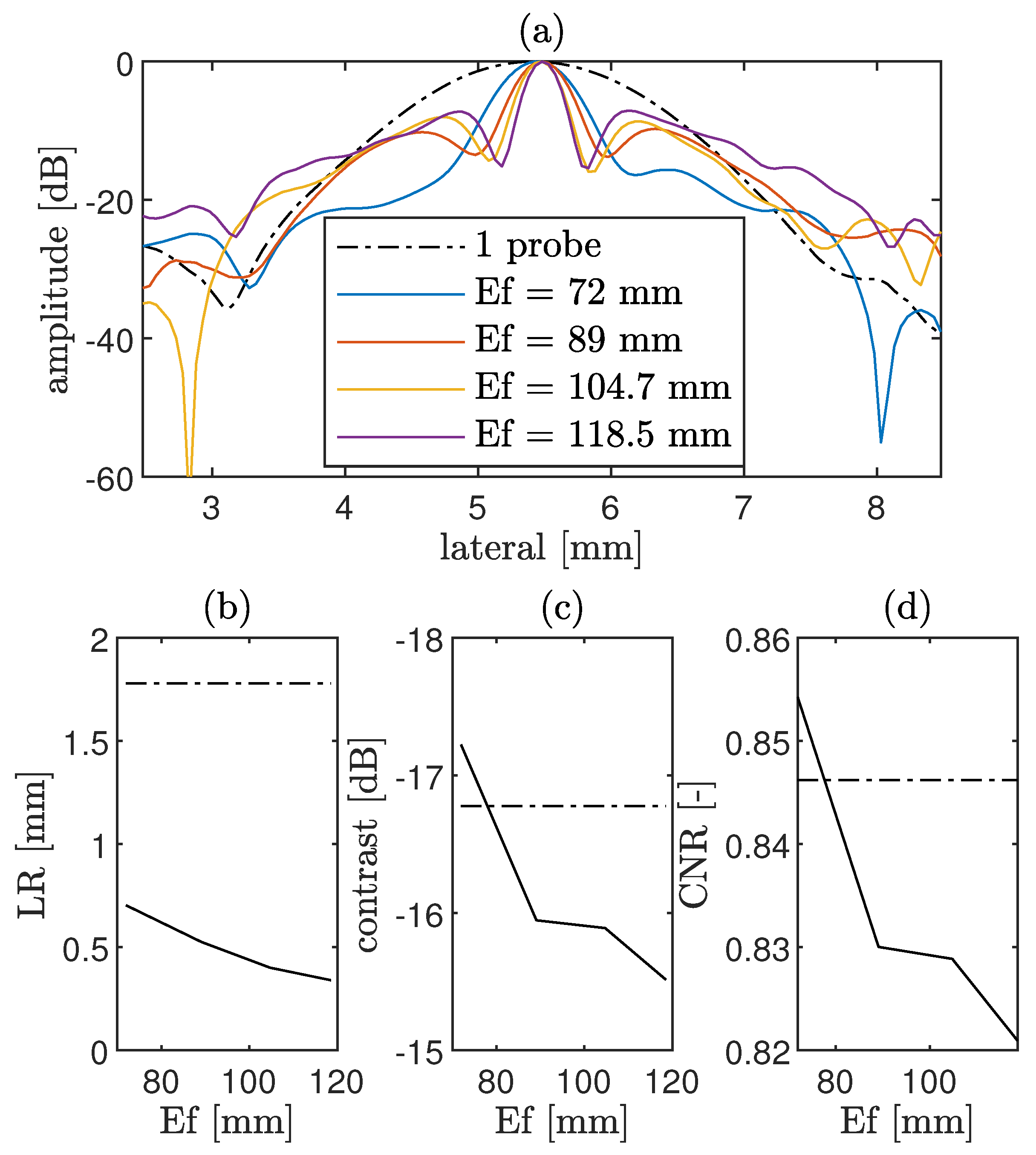
3.1.1. CoMTUS Discontinuous Effective Aperture
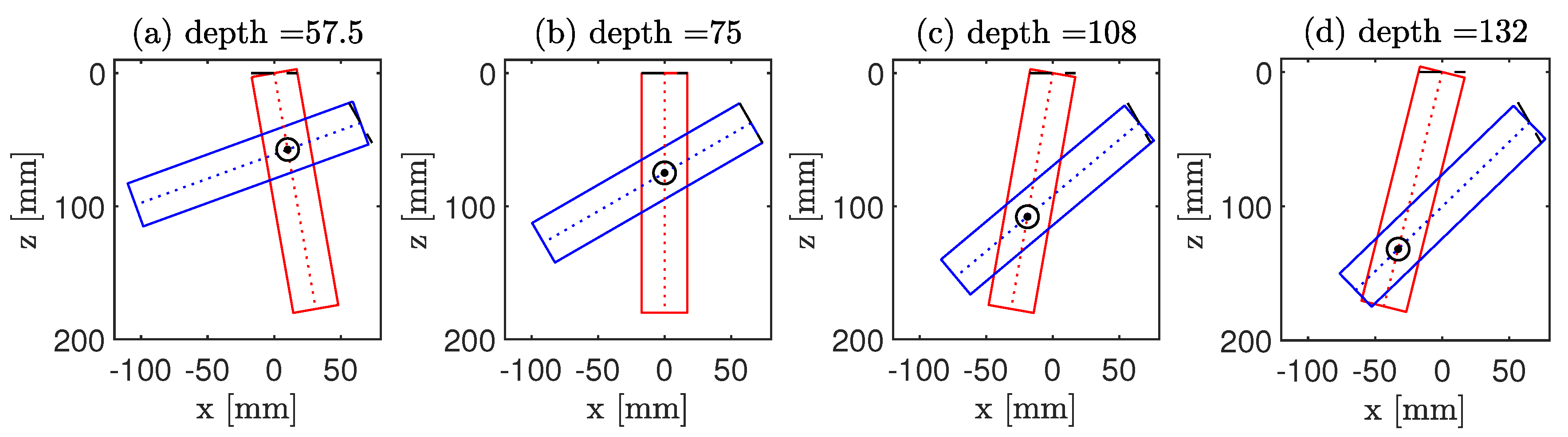
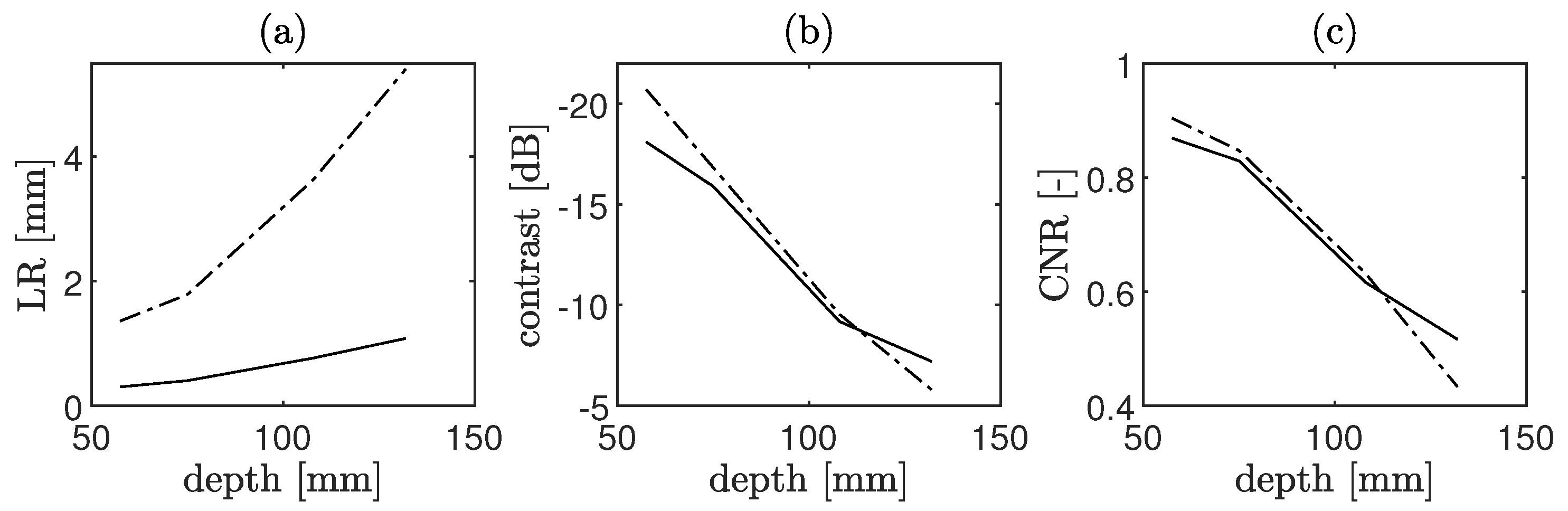
3.1.2. CoMTUS Imaging Depth
3.1.3. CoMTUS through Layered Media
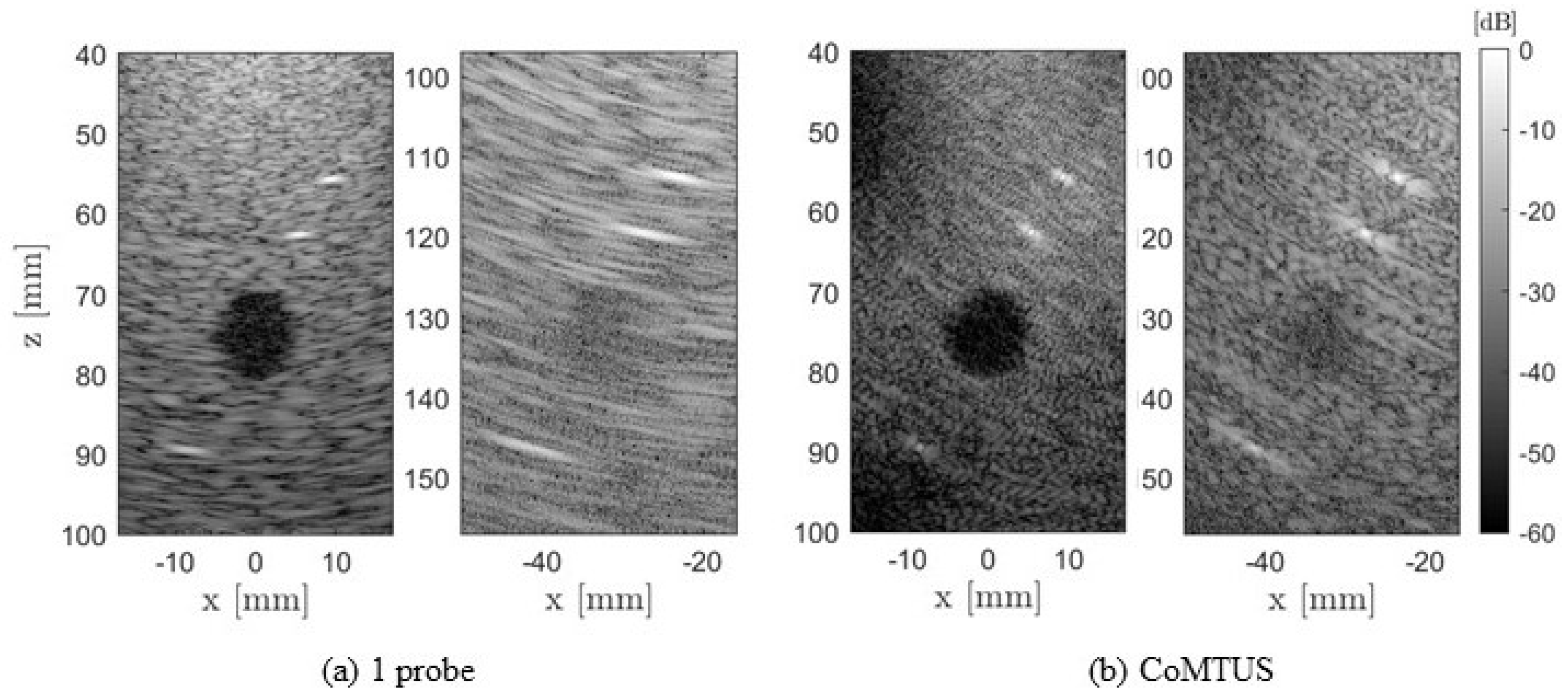
3.2. In Vitro Experiments
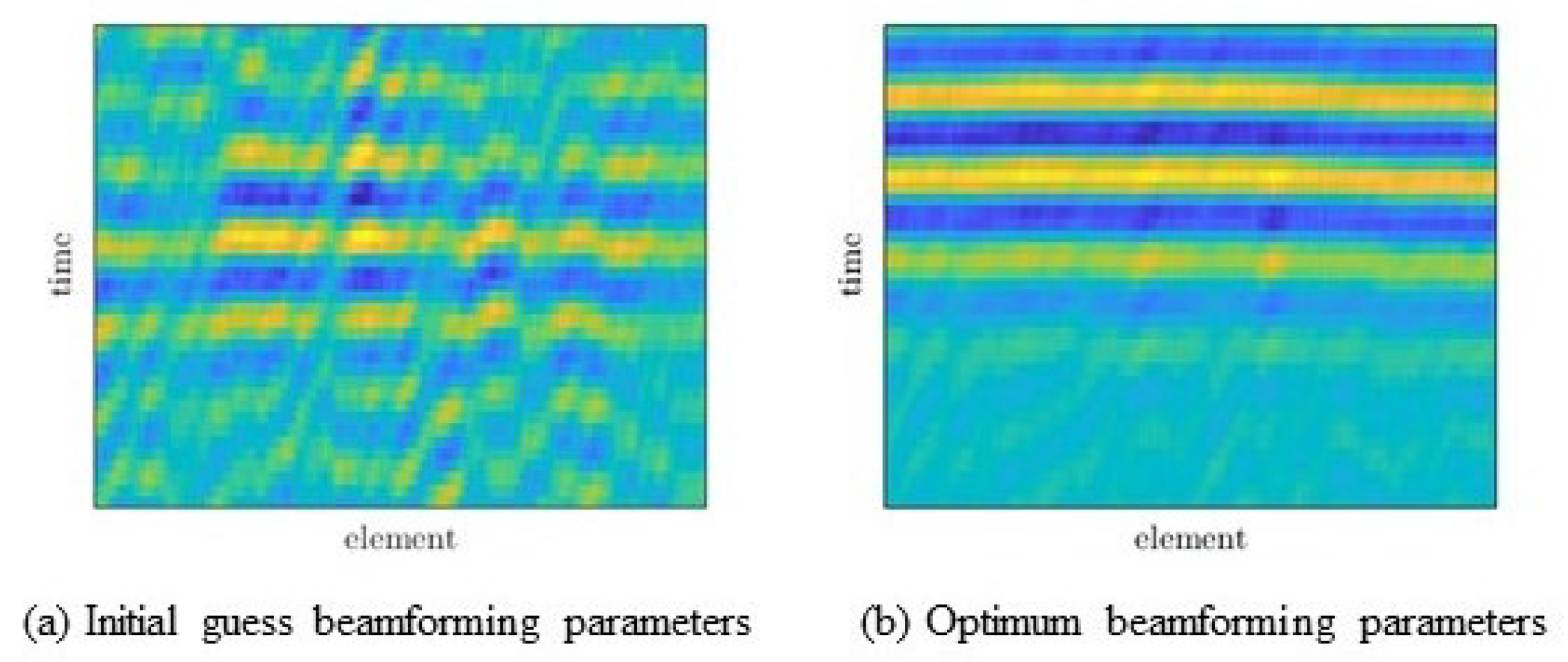
3.3. Data Processing
4. Results
4.1. Simulation Results
4.1.1. Control Case: Conventional Aperture Imaging
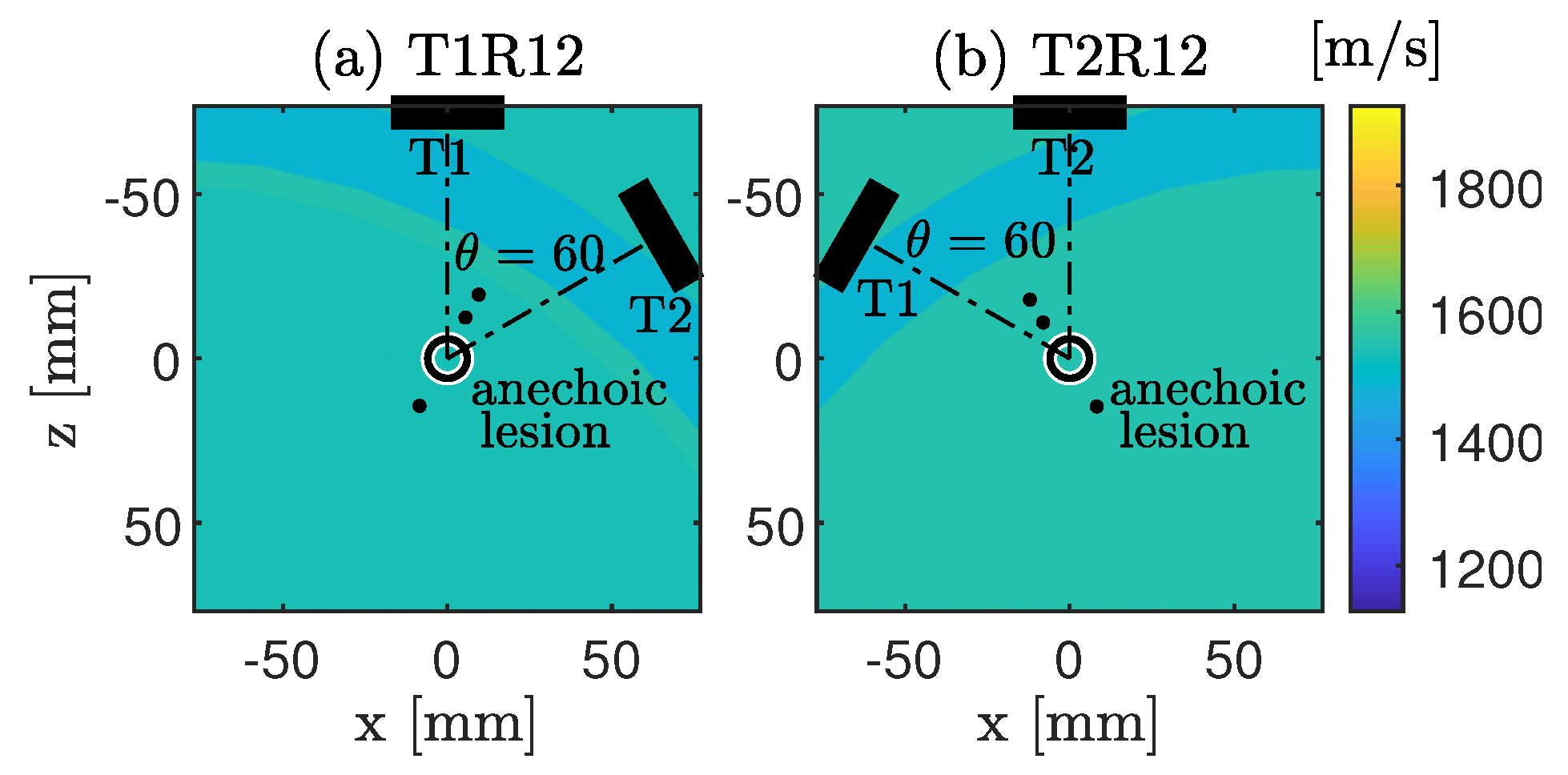
4.1.2. CoMTUS Discontinuous Effective Aperture
4.1.3. CoMTUS Imaging Depth
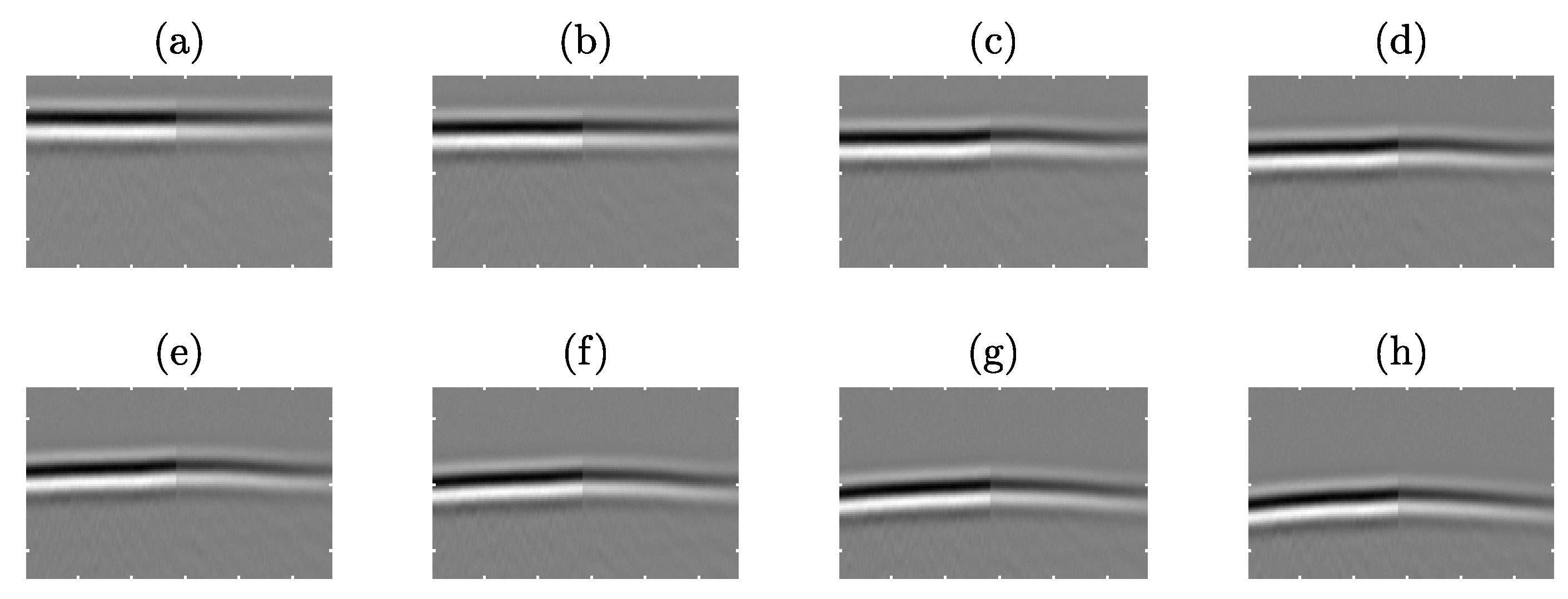
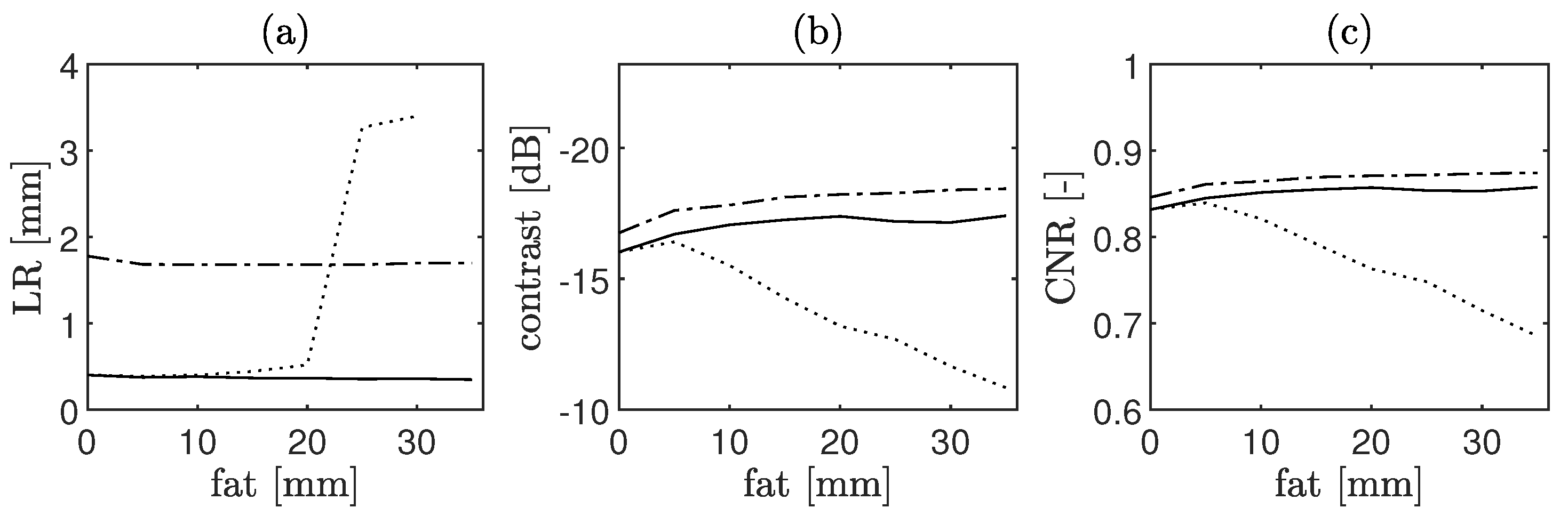
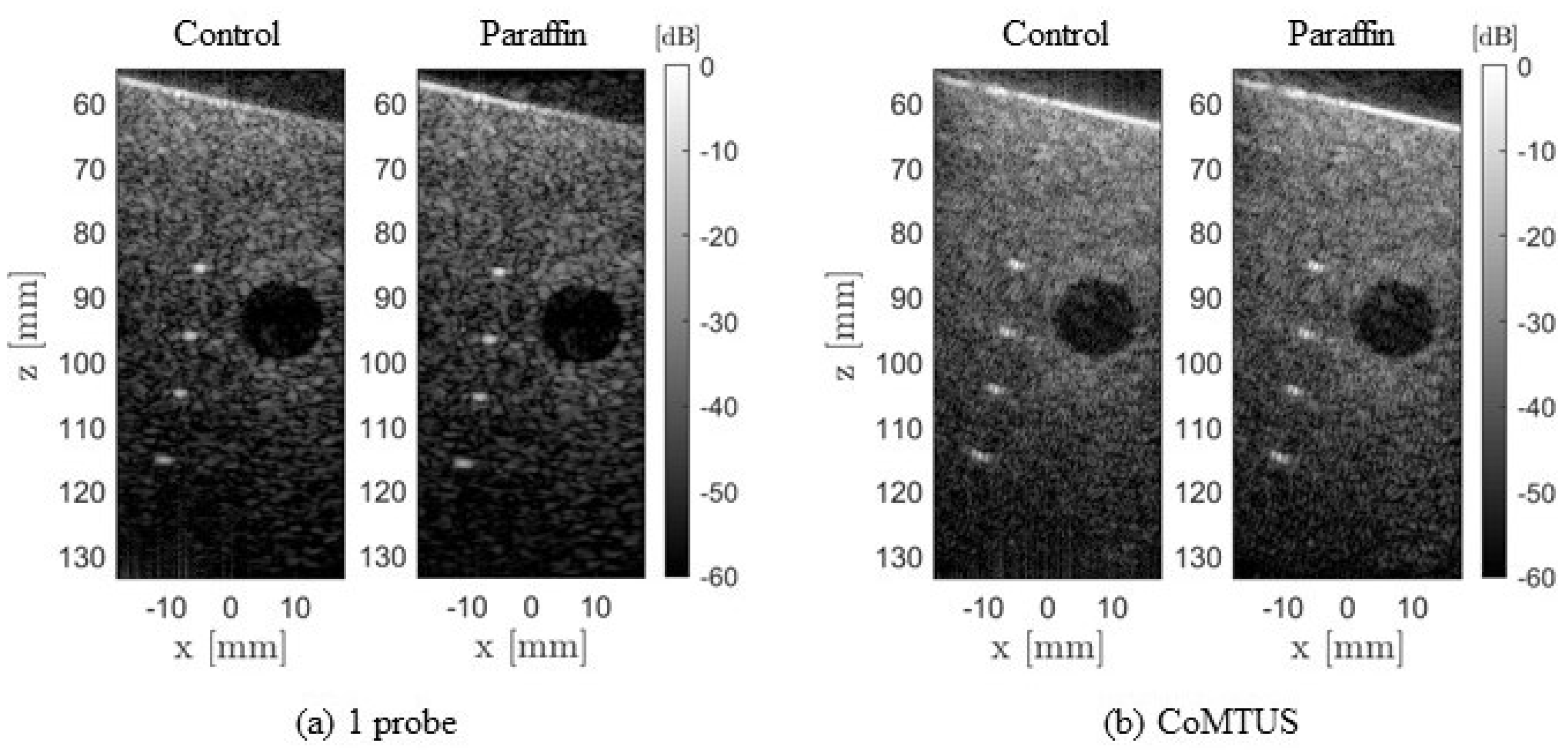
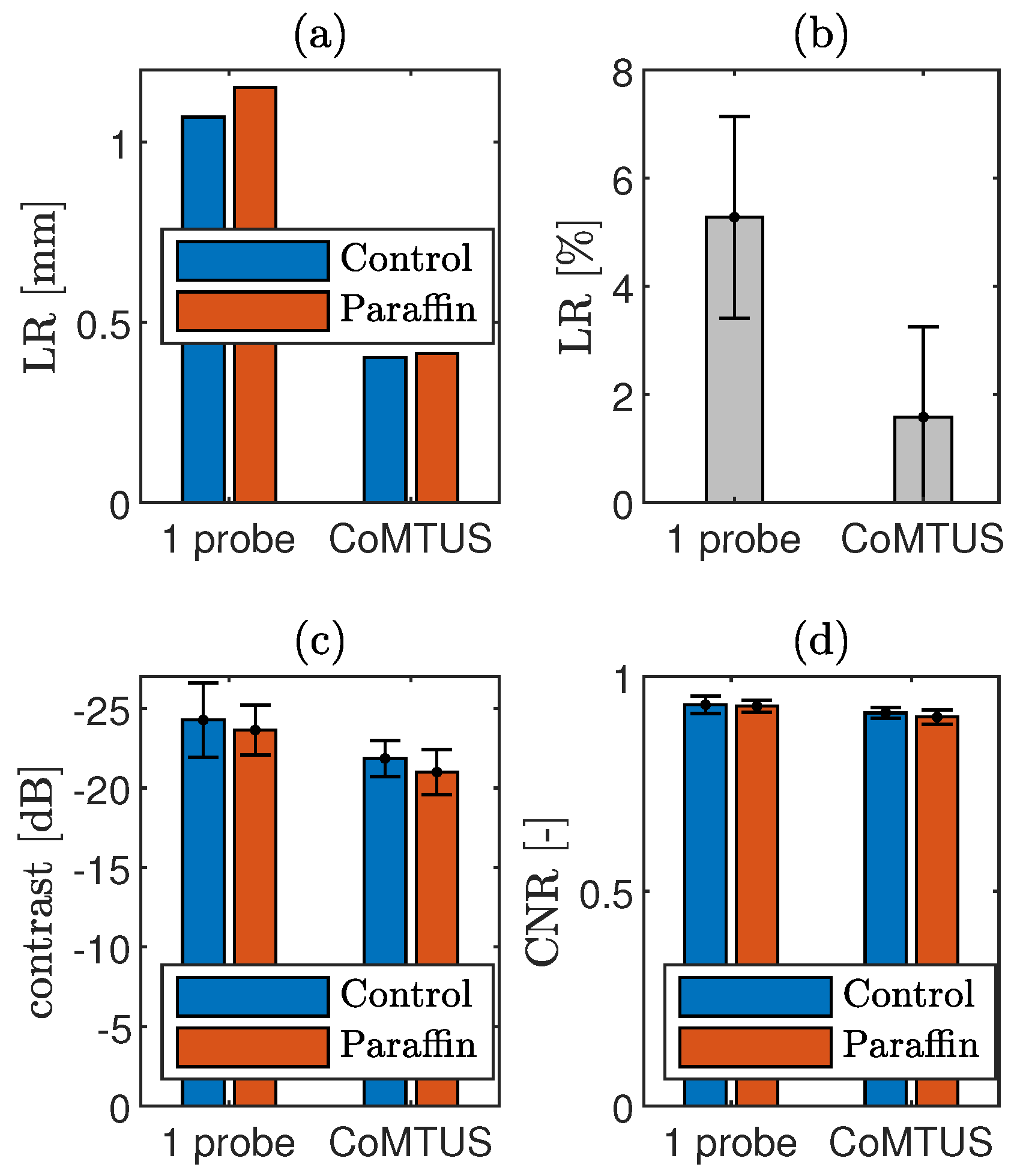
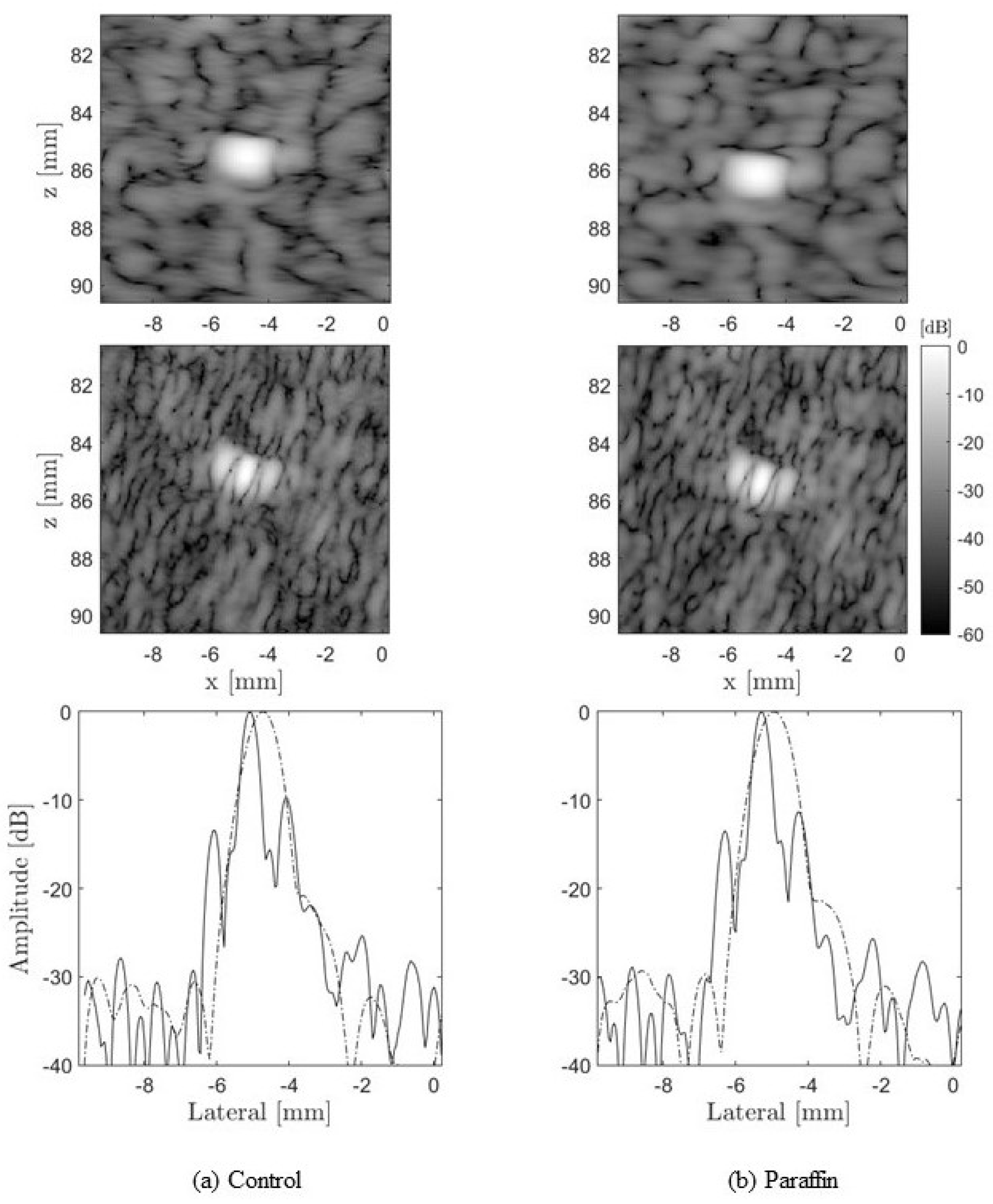
4.1.4. CoMTUS through Layered Media
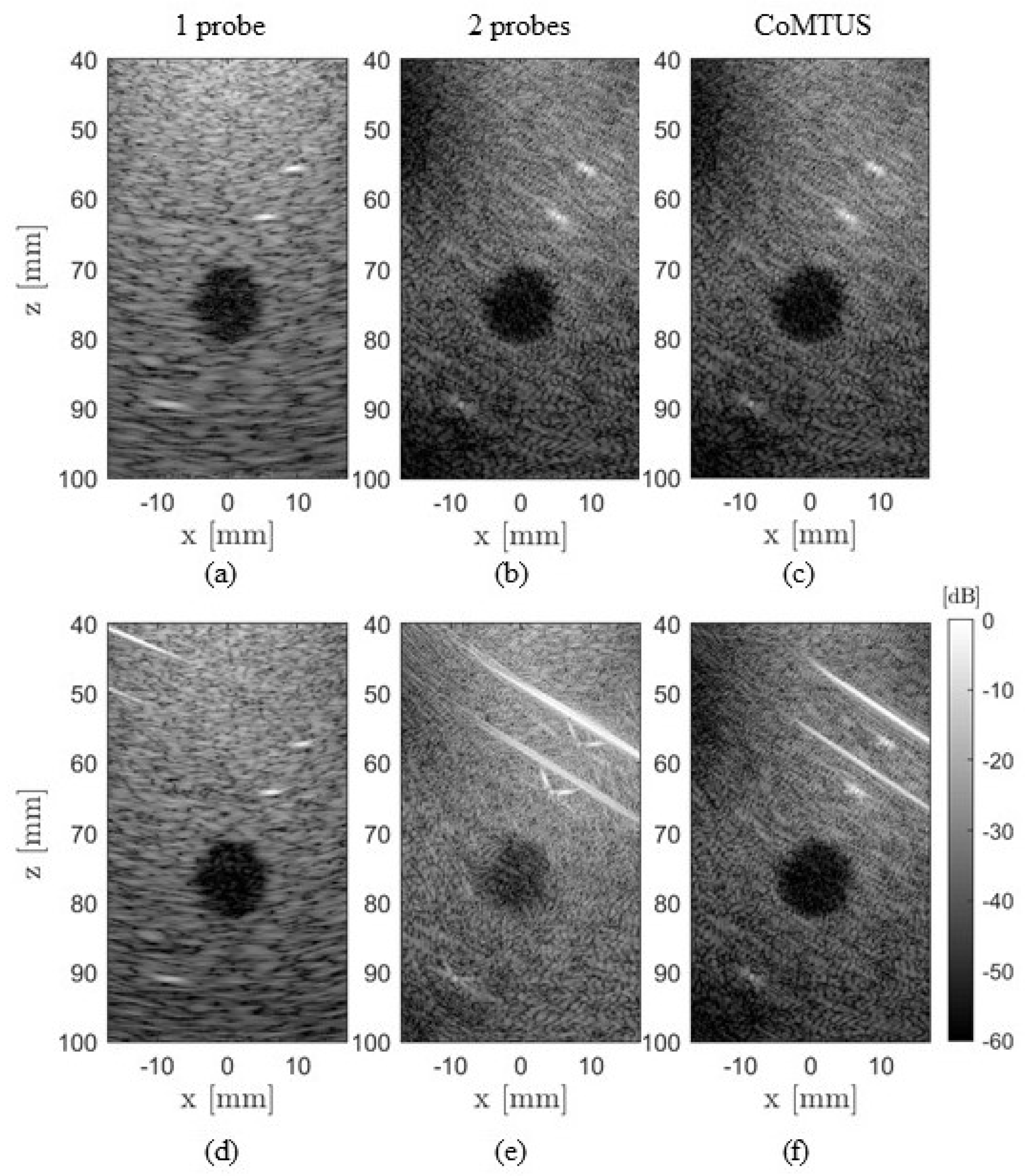
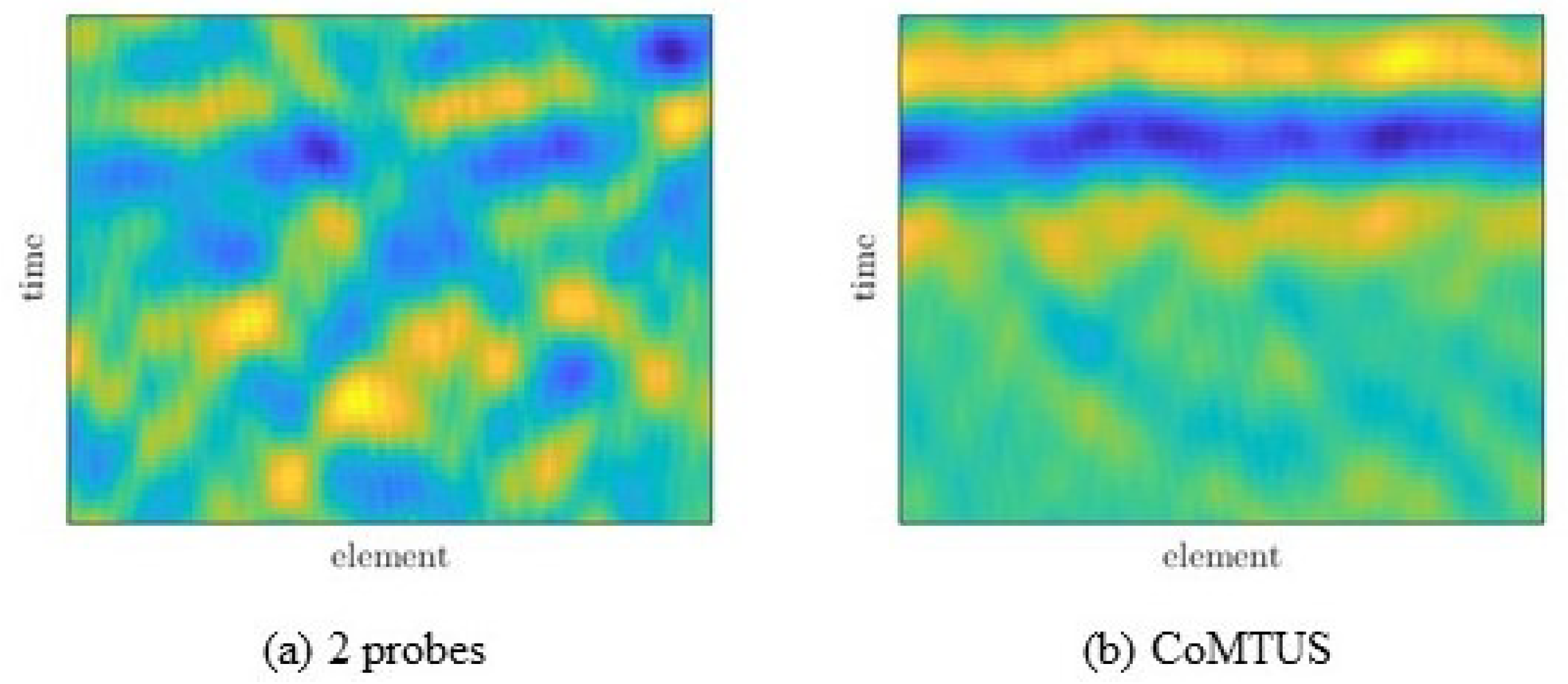
4.2. Experimental Results
5. Discussion
6. Conclusions
7. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cobbold, R.S. Foundations of Biomedical Ultrasound; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Harris, R.A.; Follett, D.; Halliwell, M.; Wells, P. Ultimate limits in ultrasonic imaging resolution. Ultrasound Med. Biol. 1991, 17, 547–558. [Google Scholar] [CrossRef]
- Moshfeghi, M.; Waag, R. In vivo and in vitro ultrasound beam distortion measurements of a large aperture and a conventional aperture focussed transducer. Ultrasound Med. Biol. 1988, 14, 415–428. [Google Scholar] [CrossRef]
- Tsai, P.J.S.; Loichinger, M.; Zalud, I. Obesity and the challenges of ultrasound fetal abnormality diagnosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Klysik, M.; Garg, S.; Pokharel, S.; Meier, J.; Patel, N.; Garg, K. Challenges of imaging for cancer in patients with diabetes and obesity. Diabetes Technol. Ther. 2014, 16, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Bottenus, N.; Long, W.; Zhang, H.K.; Jakovljevic, M.; Bradway, D.P.; Boctor, E.M.; Trahey, G.E. Feasibility of swept synthetic aperture ultrasound imaging. IEEE Trans. Med. Imaging 2016, 35, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Bottenus, N.; Long, W.; Morgan, M.; Trahey, G. Evaluation of Large-Aperture Imaging Through the ex Vivo Human Abdominal Wall. Ultrasound Med. Biol. 2018, 44, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, V.A.; Gomez, A.; Noh, Y.; Toussaint, N.; Khanal, B.; Wright, R.; Peralta, L.; van Poppel, M.; Skelton, E.; Matthew, J.; et al. Multi-view Image Reconstruction: Application to Fetal Ultrasound Compounding. In Data Driven Treatment Response Assessment and Preterm, Perinatal, and Paediatric Image Analysis; Melbourne, A., Licandro, R., DiFranco, M., Rota, P., Gau, M., Kampel, M., Aughwane, R., Moeskops, P., Schwartz, E., Robinson, E., et al., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 107–116. [Google Scholar]
- Peralta, L.; Gomez, A.; Hajnal, J.V.; Eckersley, R.J. Feasibility study of a coherent multi-transducer US imaging system. In Proceedings of the 2018 IEEE International Ultrasonics Symposium (IUS), Kobe, Japan, 22–25 October 2018; pp. 1–4. [Google Scholar]
- Peralta, L.; Gomez, A.; Hajnal, J.V.; Luan, Y.; Kim, B.; Eckersley, R.J. Coherent Multi-Transducer Ultrasound Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 66, 1316–1330. [Google Scholar] [CrossRef]
- Peralta, L.; Gomez, A.; Hajnal, J.V.; Eckersley, R.J. Coherent multi-transducer ultrasound imaging in the presence of aberration. In Proceedings of the Medical Imaging 2019: Ultrasonic Imaging and Tomography, International Society for Optics and Photonics, San Diego, CA, USA, 17–18 February 2019; Volume 10955, p. 109550O. [Google Scholar]
- Peralta, L.; Reinwald, M.; Hajnal, J.V.; Eckersley, R.J. Coherent Multi-Transducer Ultrasound Imaging through aberrating media. In Proceedings of the 2019 IEEE International Ultrasonics Symposium (IUS), Glasgow, UK, 6–9 October 2019; pp. 324–327. [Google Scholar]
- Peralta, L.; Reinwald, M.; Ramalli, A.; Hajnal, J.V.; Eckersley, R.J. Extension of Coherent Multi-Transducer Ultrasound Imaging with Diverging Waves. In Proceedings of the 2019 IEEE International Ultrasonics Symposium (IUS), Glasgow, UK, 6–9 October 2019; pp. 2226–2229. [Google Scholar]
- Fitzgibbon, A.W. Robust registration of 2D and 3D point sets. Image Vis. Comput. 2003, 21, 1145–1153. [Google Scholar] [CrossRef]
- Montaldo, G.; Tanter, M.; Bercoff, J.; Benech, N.; Fink, M. Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 489–506. [Google Scholar] [CrossRef]
- Fang, B.T. Trilateration and extension to global positioning system navigation. J. Guid. Control. Dyn. 1986, 9, 715–717. [Google Scholar] [CrossRef]
- Christensen-Jeffries, K.; Browning, R.J.; Tang, M.X.; Dunsby, C.; Eckersley, R.J. In vivo acoustic super-resolution and super-resolved velocity mapping using microbubbles. IEEE Trans. Med. Imaging 2014, 34, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Christensen-Jeffries, K.; Peralta, L.; Reinwald, M.; Hajnal, J.V.; Eckersley, R.J. Coherent Multi-Transducer Ultrasound Imaging with Micro-Bubble Contrast Agents. In Proceedings of the 2019 IEEE International Ultrasonics Symposium (IUS), Glasgow, UK, 6–9 October 2019; pp. 2310–2312. [Google Scholar]
- Treeby, B.E.; Jaros, J.; Rendell, A.P.; Cox, B. Modeling nonlinear ultrasound propagation in heterogeneous media with power law absorption using a k-space pseudospectral method. J. Acoust. Soc. Am. 2012, 131, 4324–4336. [Google Scholar] [CrossRef]
- Treeby, B.E.; Cox, B.T. k-Wave: MATLAB toolbox for the simulation and reconstruction of photoacoustic wave fields. J. Biomed. Opt. 2010, 15, 021314. [Google Scholar] [CrossRef] [PubMed]
- Denarie, B.; Tangen, T.A.; Ekroll, I.K.; Rolim, N.; Torp, H.; Bjåstad, T.; Lovstakken, L. Coherent plane wave compounding for very high frame rate ultrasonography of rapidly moving targets. IEEE Trans. Med. Imaging 2013, 32, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Christensen-Jeffries, K.; Harput, S.; Zhang, G.; Zhu, J.; Dunsby, C.; Tang, M.; Eckersley, R. Investigation of Microbubble Detection Methods for Super-resolution Imaging of Microvasculature. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2019. [Google Scholar] [CrossRef]
- Treeby, B.E.; Cox, B. Modeling power law absorption and dispersion for acoustic propagation using the fractional Laplacian. J. Acoust. Soc. Am. 2010, 127, 2741–2748. [Google Scholar] [CrossRef] [PubMed]
- Pinton, G.F.; Dahl, J.; Rosenzweig, S.; Trahey, G.E. A heterogeneous nonlinear attenuating full-wave model of ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 474–488. [Google Scholar] [CrossRef]
- Robertson, J.L.; Cox, B.T.; Jaros, J.; Treeby, B.E. Accurate simulation of transcranial ultrasound propagation for ultrasonic neuromodulation and stimulation. J. Acoust. Soc. Am. 2017, 141, 1726–1738. [Google Scholar] [CrossRef]
- Wise, E.S.; Robertson, J.L.; Cox, B.T.; Treeby, B.E. Staircase-free acoustic sources for grid-based models of wave propagation. In Proceedings of the 2017 IEEE International Ultrasonics Symposium (IUS), Washington, DC, USA, 6–9 September 2017; pp. 1–4. [Google Scholar]
- Goss, S.; Johnston, R.; Dunn, F. Comprehensive compilation of empirical ultrasonic properties of mammalian tissues. J. Acoust. Soc. Am. 1978, 64, 423–457. [Google Scholar] [CrossRef]
- Liu, D.L.; Waag, R.C. Correction of ultrasonic wavefront distortion using backpropagation and a reference waveform method for time-shift compensation. J. Acoust. Soc. Am. 1994, 96, 649–660. [Google Scholar] [CrossRef]
- Mast, T.D.; Hinkelman, L.M.; Orr, M.J.; Sparrow, V.W.; Waag, R.C. Simulation of ultrasonic pulse propagation through the abdominal wall. J. Acoust. Soc. Am. 1997, 102, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Boni, E.; Bassi, L.; Dallai, A.; Guidi, F.; Meacci, V.; Ramalli, A.; Ricci, S.; Tortoli, P. ULA-OP 256: A 256-channel open scanner for development and real-time implementation of new ultrasound methods. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Boni, E.; Bassi, L.; Dallai, A.; Meacci, V.; Ramalli, A.; Scaringella, M.; Guidi, F.; Ricci, S.; Tortoli, P. Architecture of an ultrasound system for continuous real-time high frame rate imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Lopez, H.; Bodine, W., Jr. Frequency independent ultrasound contrast-detail analysis. Ultrasound Med. Biol. 1985, 11, 467–477. [Google Scholar] [CrossRef]
- Lacefield, J.C.; Pilkington, W.C.; Waag, R.C. Distributed aberrators for emulation of ultrasonic pulse distortion by abdominal wall. Acoust. Res. Lett. Online 2002, 3, 47–52. [Google Scholar] [CrossRef]
- Lee, S.; Lo, Y. Antenna Handbook: Theory, Applications, and Design; Van Nostrand Reinhold: New York, NY, USA, 1988. [Google Scholar]
- Bottenus, N.; Pinton, G.; Trahey, G. Large coherent apertures: Improvements in deep abdominal imaging and fundamental limits imposed by clutter. In Proceedings of the 2016 IEEE International Ultrasonics Symposium (IUS), Tours, France, 18–21 September 2016; pp. 1–4. [Google Scholar]
- Karaman, M.; Li, P.C.; O’Donnell, M. Synthetic aperture imaging for small scale systems. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1995, 42, 429–442. [Google Scholar] [CrossRef]
- Trahey, G.; Freiburger, P.; Nock, L.; Sullivan, D. In vivo measurements of ultrasonic beam distortion in the breast. Ultrason. Imaging 1991, 13, 71–90. [Google Scholar] [CrossRef]
- Pinton, G.F.; Trahey, G.E.; Dahl, J.J. Spatial coherence in human tissue: Implications for imaging and measurement. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 1976–1987. [Google Scholar] [CrossRef][Green Version]
- Fatemi, A.; Berg, E.A.R.; Rodriguez-Molares, A. Studying the Origin of Reverberation Clutter in Echocardiography: In Vitro Experiments and In Vivo Demonstrations. Ultrasound Med. Biol. 2019. [Google Scholar] [CrossRef]
| Tissue | SOS | Density | Attenuation | Nonlinearity |
|---|---|---|---|---|
| Type | (m/s) | (kg/m) | (dB/MHz/cm) | B/A |
| Soft tissue | 1540 | 1000 | 0.75 | 6 |
| Fat | 1478 | 950 | 0.63 | 10 |
| Muscle | 1547 | 1050 | 0.15 | 7.4 |
| Fat + Muscle | rms ATF | ATF CL | rms ELF | ELF CL |
|---|---|---|---|---|
| Thickness (mm) | (ns) | (mm) | (dB) | (mm) |
| 5 + 8 | 0.94 | 0.35 | 2.48 | 4.86 |
| 10 + 8 | 13.74 | 8.17 | 2.49 | 4.84 |
| 15 + 8 | 22.09 | 9.75 | 2.48 | 4.84 |
| 20 + 8 | 23.03 | 8.76 | 2.47 | 4.82 |
| 25 + 8 | 32.53 | 9.34 | 2.47 | 4.80 |
| 30 + 8 | 35.64 | 7.44 | 2.48 | 4.80 |
| 35 + 8 | 39.64 | 9.37 | 2.49 | 4.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peralta, L.; Ramalli, A.; Reinwald, M.; Eckersley, R.J.; Hajnal, J.V. Impact of Aperture, Depth, and Acoustic Clutter on the Performance of Coherent Multi-Transducer Ultrasound Imaging. Appl. Sci. 2020, 10, 7655. https://doi.org/10.3390/app10217655
Peralta L, Ramalli A, Reinwald M, Eckersley RJ, Hajnal JV. Impact of Aperture, Depth, and Acoustic Clutter on the Performance of Coherent Multi-Transducer Ultrasound Imaging. Applied Sciences. 2020; 10(21):7655. https://doi.org/10.3390/app10217655
Chicago/Turabian StylePeralta, Laura, Alessandro Ramalli, Michael Reinwald, Robert J. Eckersley, and Joseph V. Hajnal. 2020. "Impact of Aperture, Depth, and Acoustic Clutter on the Performance of Coherent Multi-Transducer Ultrasound Imaging" Applied Sciences 10, no. 21: 7655. https://doi.org/10.3390/app10217655
APA StylePeralta, L., Ramalli, A., Reinwald, M., Eckersley, R. J., & Hajnal, J. V. (2020). Impact of Aperture, Depth, and Acoustic Clutter on the Performance of Coherent Multi-Transducer Ultrasound Imaging. Applied Sciences, 10(21), 7655. https://doi.org/10.3390/app10217655






