A Novel Hybrid Machine Learning Classification for the Detection of Bruxism Patients Using Physiological Signals
Abstract
Featured Application
Abstract
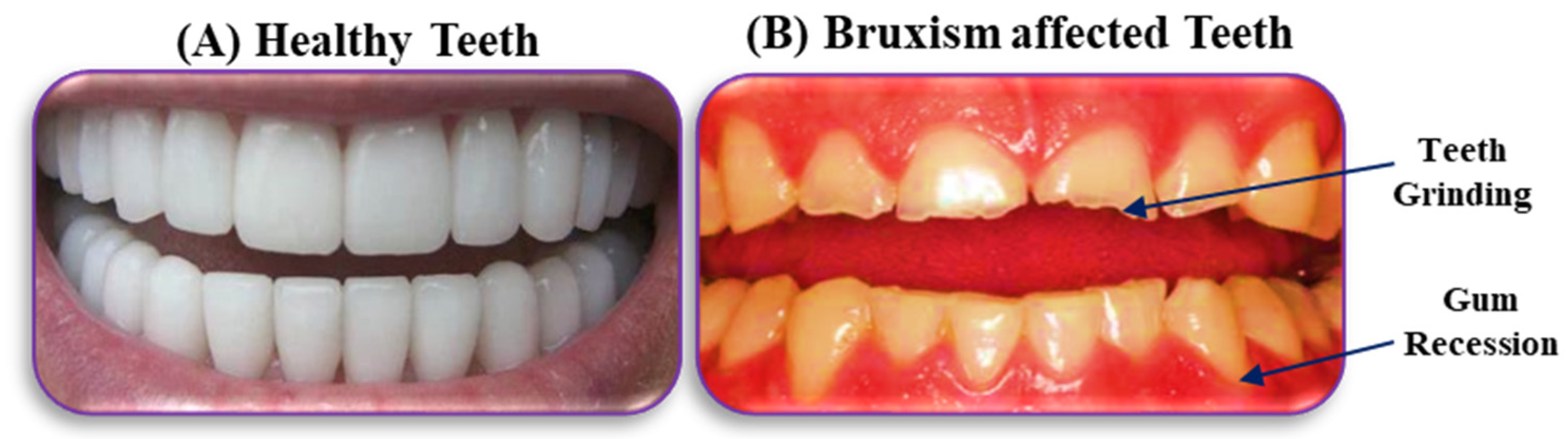
1. Introduction
2. Materials
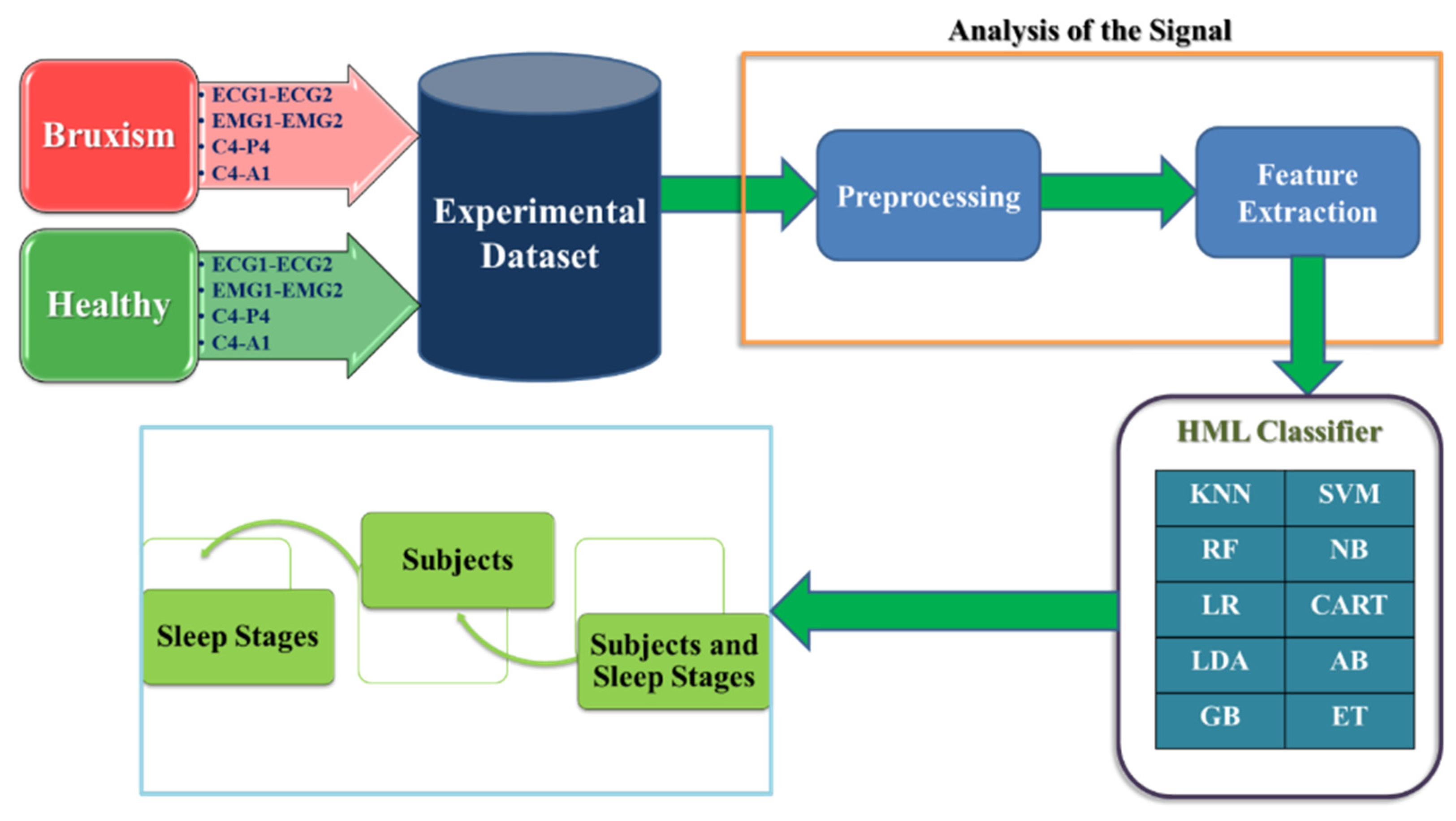
3. Methods
3.1. Power Spectral Density
3.2. Hybrid Machine Learning (HML) Classifier
3.3. Evaluation of the Proposed System
4. Results and Discussion
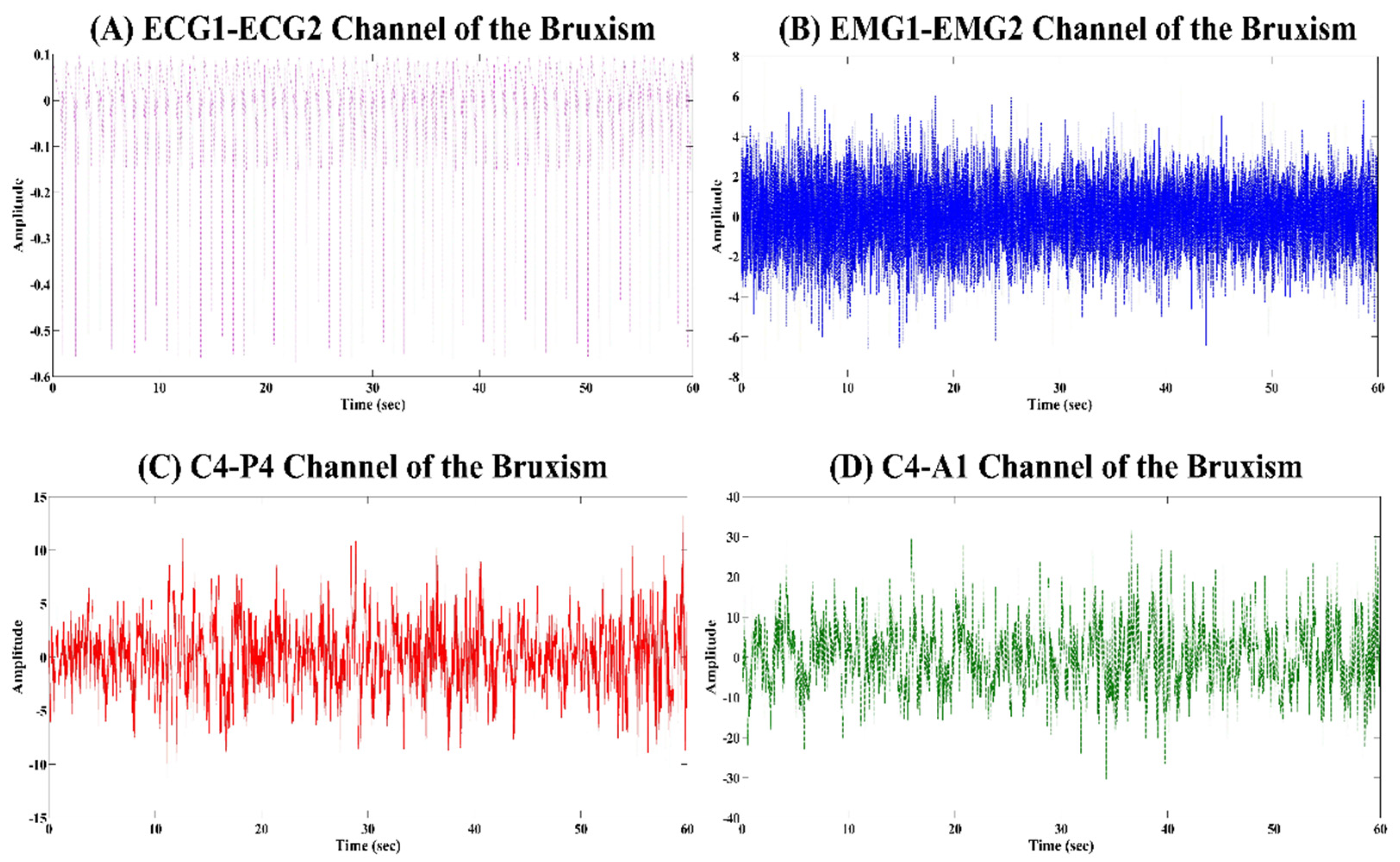
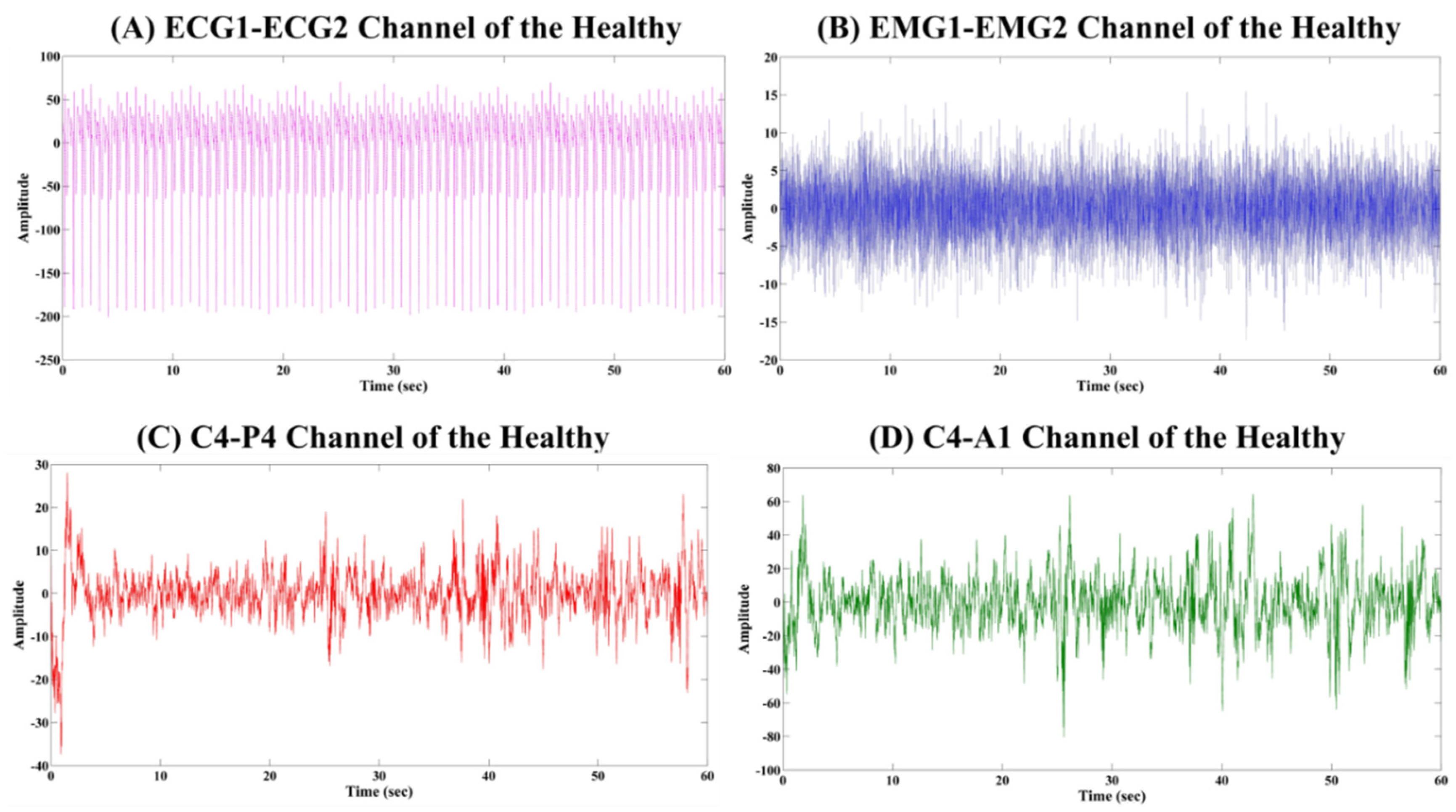
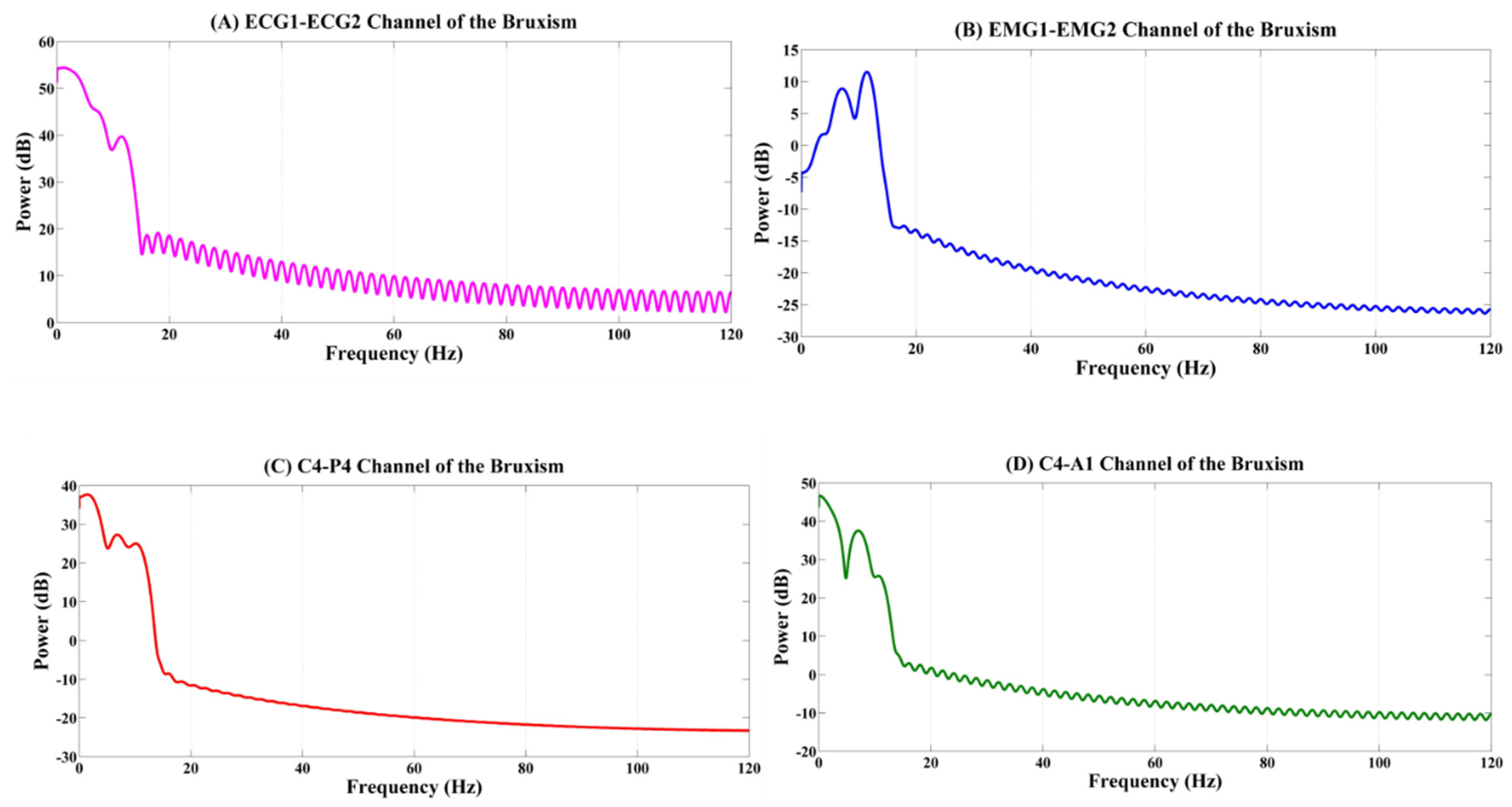
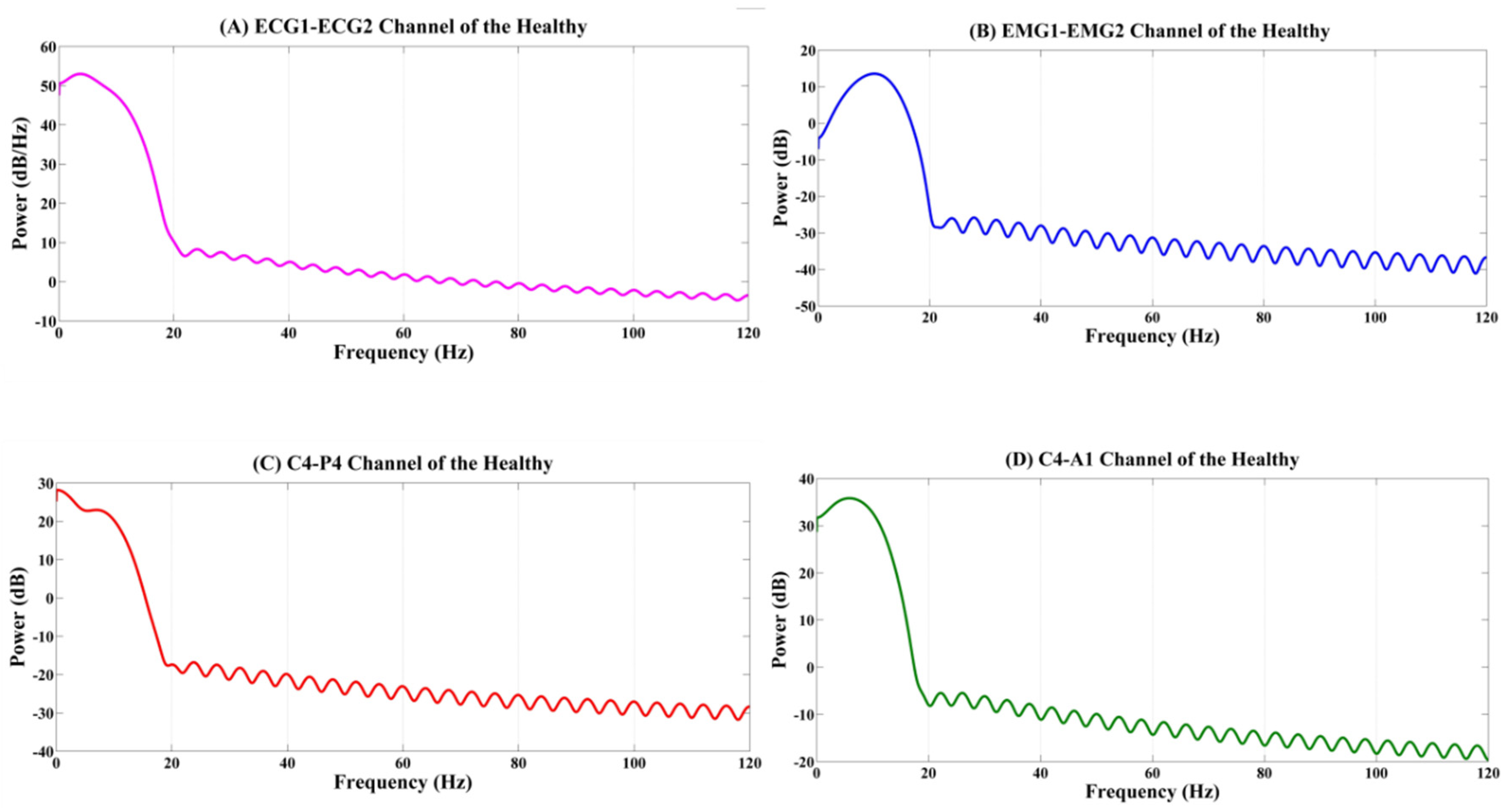
4.1. Analysis of the Physiological Signals
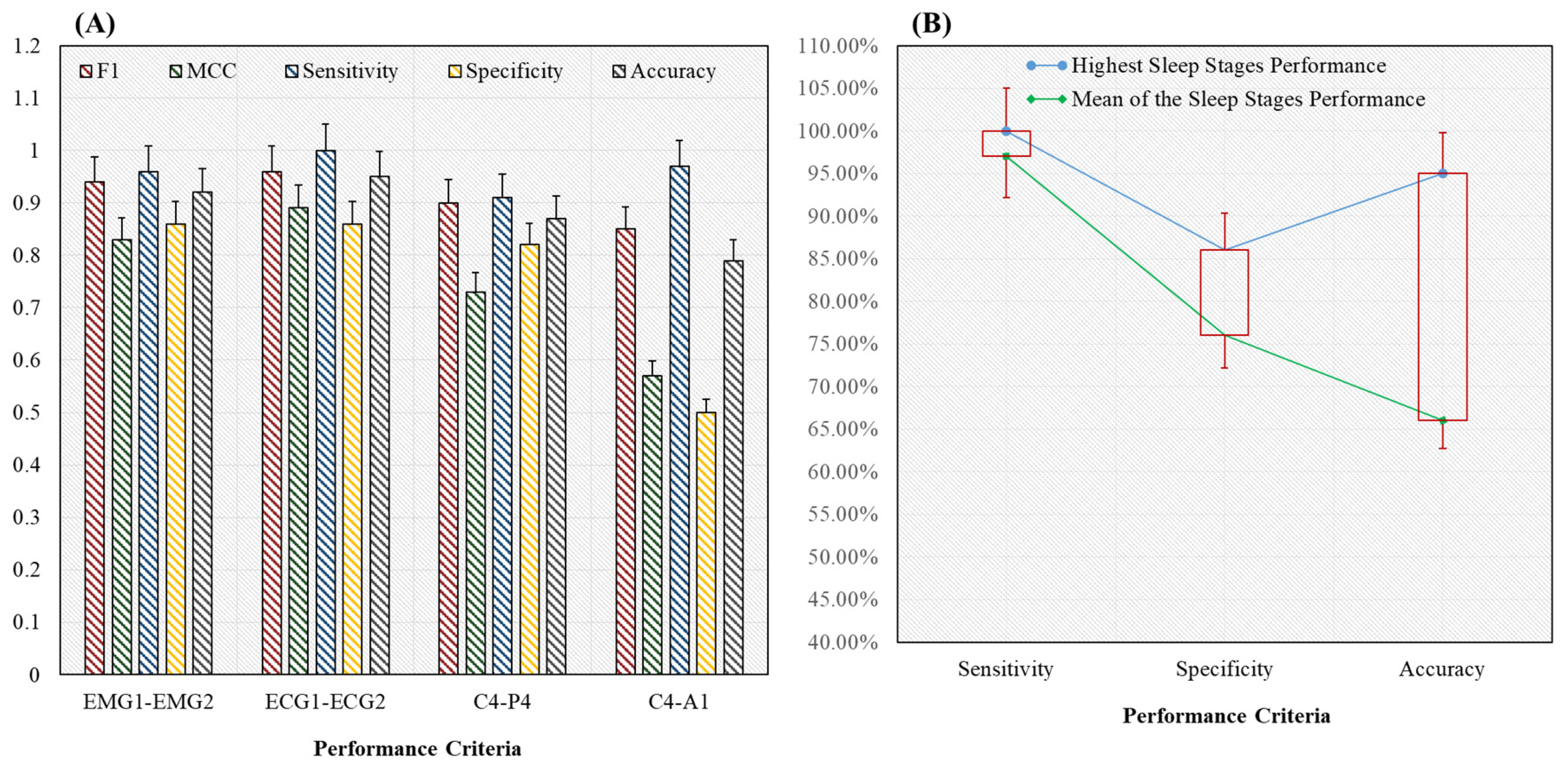
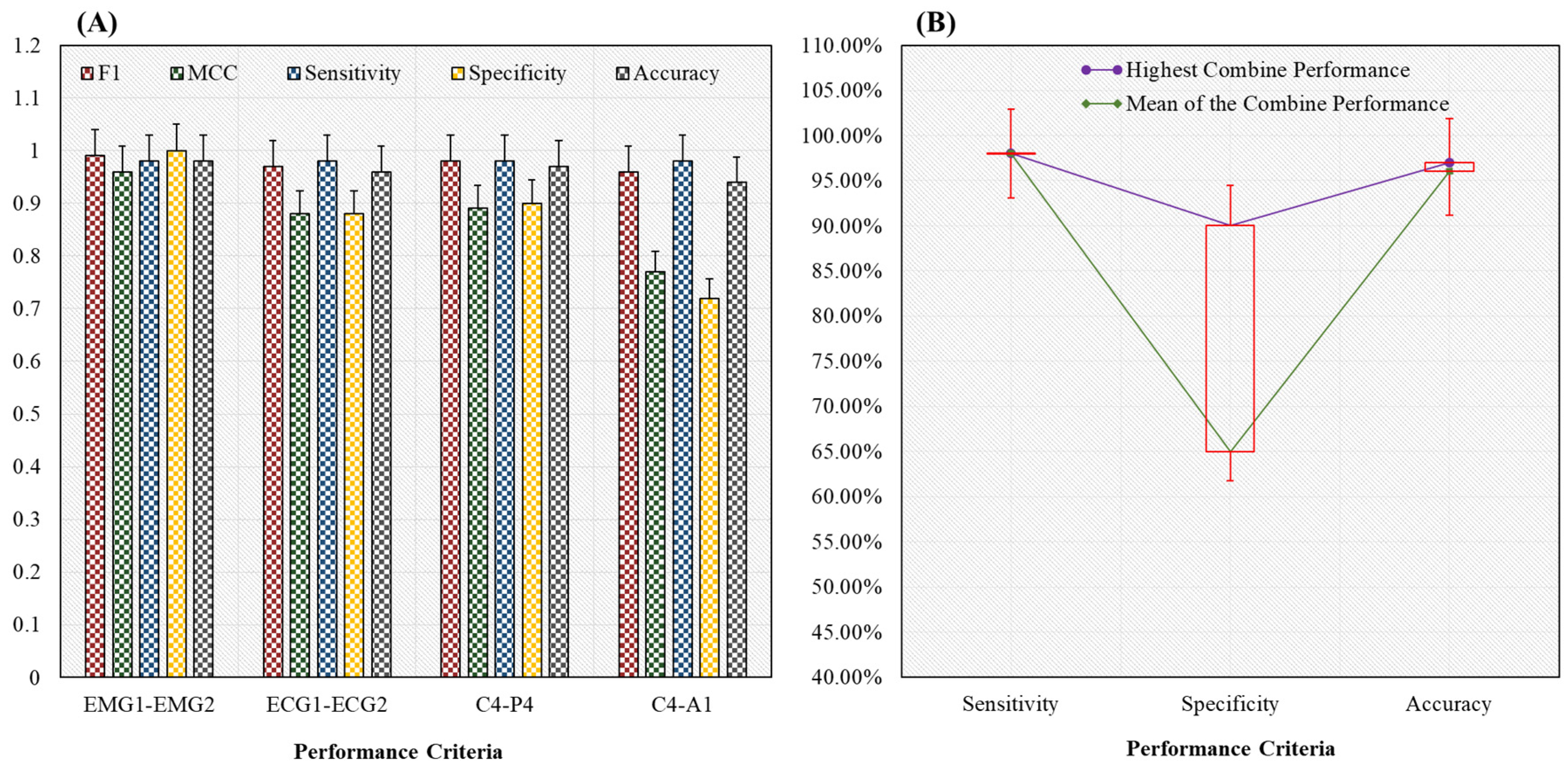
4.2. Classification Results Using Proposed Novel HML Classifier
4.3. Comparison with the Previous and Proposed Sleep Disorders and Sleep Stage Detection Methods
4.4. Application and Limitation of the Proposed System
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Crowley, K. Sleep and sleep disorders in older adults. Neuropsychol. Rev. 2011, 21, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Alschuler, V.; Claudatos, S.; Goldstone, A.; Baker, F.; Cellini, N.; Colrain, I.M.; de Zambotti, M. 1193 Accuracy of a Commercial Wearable in Detecting Sleep Stages Compared to Polysomnography in Adults: Considering Sleep Classification Methods and Effects of Evening Alcohol Consumption. Sleep 2020, 43, A456–A457. [Google Scholar] [CrossRef]
- Rechtschaffen, A.; Kales, A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects (Part 2); National Institutes of Health (U.S.): Bethesda, MD, USA, 1968.
- Grigg-Damberger, M.M. The AASM scoring manual four years later. J. Clin. Sleep Med. 2012, 8, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Anderer, P.; Moreau, A.; Woertz, M.; Ross, M.; Gruber, G.; Parapatics, S.; Loretz, E.; Heller, E.; Schmidt, A.; Boeck, M.; et al. Computer-assisted sleep classification according to the standard of the American Academy of sleep medicine: Validation study of the AASM version of the Somnolyzer 24 × 7. Neuropsychobiology 2010, 62, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.E.; Vaughn, B.V. Poor sleep challenging the health of a nation. Neurodiagn. J. 2012, 52, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Abdulghani, H.M.; Alrowais, N.A.; Bin-Saad, N.S.; Al-Subaie, N.M.; Haji, A.M.; Alhaqwi, A.I. Sleep disorder among medical students: Relationship to their academic performance. Med. Teach. 2012, 34, S37–S41. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Y.M.; Bin Heyat, M.B.; Siddiqui, M.M.; Azad, S.; Akhtar, F. An Overview of Sleep and Stages of Sleep. Int. J. Adv. Res. Comput. Commun. Eng. 2015, 4, 505–507. [Google Scholar]
- Asif, N.; Iqbal, R.; Nazir, C.F. Human immune system during sleep. Am. J. Clin. Exp. Immunol. 2017, 6, 92. [Google Scholar]
- Barnes, C.M.; Schaubroeck, J.; Huth, M.; Ghumman, S. Lack of sleep and unethical conduct. Organ. Behav. Hum. Decis. Process. 2011, 115, 169–180. [Google Scholar] [CrossRef]
- Heyat, M.B.B.; Lai, D.; Akhtar, F.; Hayat, M.A.B.; Azad, S.; Azad, S.; Azad, S. Bruxism Detection Using Single-Channel C4-A1 on Human Sleep S2 Stage Recording. In Intelligent Data Analysis: From Data Gathering to Data Comprehension, 1st ed.; Gupta, D., Bhattacharyya, S., Khanna, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 347–367. [Google Scholar]
- Hillman, D.R.; Lack, L.C. Public health implications of sleep loss: The community burden. Med. J. Aust. 2013, 199, S7–S10. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Y.; Wang, Y.; Jiang, Q.; Rao, X.; Lu, X.; Teng, H. Brief report: An epidemiologic survey of the prevalence of sleep disorders among children 2 to 12 years old in Beijing, China. Pediatrics 2005, 115, 266–268. [Google Scholar] [CrossRef]
- Casson, A.J.; Smith, S.; Duncan, J.S.; Rodriguez-Villegas, E. Wearable EEG: What is it, why is it needed and what does it entail? In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–24 August 2008. [Google Scholar] [CrossRef]
- Panda, S.; Taly, A.B.; Sinha, S.; Gururaj, G.; Girish, N.; Nagaraja, D. Sleep-related disorders among a healthy population in South India. Neurol. India 2012, 60, 68. [Google Scholar] [CrossRef]
- Monma, T.; Ando, A.; Asanuma, T.; Yoshitake, Y.; Yoshida, G.; Miyazawa, T.; Ebine, N.; Takeda, S.; Omi, N.; Satoh, M.; et al. Sleep disorder risk factors among student athletes. Sleep Med. 2018, 44, 76–81. [Google Scholar] [CrossRef]
- Melo, G.; Duarte, J.; Pauletto, P.; Porporatti, A.L.; Stuginski-Barbosa, J.; Winocur, E.; Flores-Mir, C.; De Luca Canto, G. Bruxism: An umbrella review of systematic reviews. J. Oral Rehabil. 2019, 46, 666–690. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.J.; Weber, K.K. Bruxism Management. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Heyat, M.B.B.; Lai, D.; Khan, F.I.; Zhang, Y. Sleep Bruxism Detection Using Decision Tree Method by the Combination of C4-P4 and C4-A1 Channels of Scalp EEG. IEEE Access 2019, 7, 102542–102553. [Google Scholar] [CrossRef]
- Lai, D.; Zhang, X.; Zhang, Y.; Heyat, M.B.B. Convolutional Neural Network Based Detection of Atrial Fibrillation Combing R-R intervals and F-wave Frequency Spectrum. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019. [Google Scholar]
- Lai, D.; Zhang, Y.; Zhang, X.; Su, Y.; Heyat, M.B.B. An Automated Strategy for Early Risk Identification of Sudden Cardiac Death by Using Machine Learning Approach on Measurable Arrhythmic Risk Markers. IEEE Access 2019, 7, 94701–94716. [Google Scholar] [CrossRef]
- Heyat, M.B.B.; Hasan, Y.M.; Siddiqui, M.M. EEG signals and wireless transfer of EEG Signals. Int. J. Adv. Res. Comput. Commun. Eng. 2015, 4, 10–12. [Google Scholar]
- Heyat, M.B.B.; Siddiqui, M.M. Recording of EEG, ECG, EMG Signal. Int. J. Adv Res. Comput. Sci. Softw. Eng. 2015, 5, 813–815. [Google Scholar]
- Imtiaz, S.A.; Rodriguez-Villegas, E. A Low Computational Cost Algorithm for REM Sleep Detection Using Single Channel EEG. Ann. Biomed. Eng. 2014, 42, 2344–2359. [Google Scholar] [CrossRef] [PubMed]
- Guillot, M.; Jungo, S.; Maniere, A.; Laplanche, O.; Tillier, Y.; Ehrmann, E. Diagnosis and management of bruxism: Evaluation of clinical practices in France. Cranio 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Saczuk, K.; Lapinska, B.; Wilmont, P.; Pawlak, L.; Lukomska-Szymanska, M. The Bruxoff Device as a Screening Method for Sleep Bruxism in Dental Practice. J. Clin. Med. 2019, 8, 930. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Yamaguchi, T.; Mikami, S.; Yachida, W.; Saito, T.; Sakuma, T.; Nakamura, H.; Saito, M.; Mizuno, M.; Yamada, K.; et al. Quantitative analyses of jaw-opening muscle activity during the active phase of jaw-closing muscles in sleep bruxism. J. Sleep Res. 2019, e12922. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, T.; Myllymaa, K.; Muraja-Murro, A.; Westeren-Punnonen, S.; Hukkanen, T.; Töyräs, J.; Lappalainen, R.; Mervaala, E.; Sipilä, K.; Myllymaa, S. Screen-printed ambulatory electrode set enables accurate diagnostics of sleep bruxism. J. Sleep Res. 2018, 27, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ruhland, J.L.; Jeutter, D.C.; Ackmann, J.J.; Hoge, H.W.; Jodat, R.W. Acquisition and analysis of electromyograms of the human masseter muscle. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, New Orleans, LA, USA, 4–7 November 1988. [Google Scholar] [CrossRef]
- Martínez, A.; Barrientos, A.; Díaz, A.; Lafont, P.; Colorado, J.; Castedo, P.L.; González, R. Polymeric piezoelectric sensors and remote communication for detection of Bruxism. In Proceedings of the 2010 IEEE International Conference on Industrial Technology, Vina del Mar, Chile, 14–17 March 2010. [Google Scholar] [CrossRef]
- Kostka, P.S.; Tkacz, E.J. Multi-sources data analysis with sympatho-vagal balance estimation toward early bruxism episodes detection. In Proceedings of the 2015 37th Annual international conference of the IEEE engineering in medicine and biology society (EMBC), Milan, Italy, 25–29 August 2015. [Google Scholar] [CrossRef]
- Jirakittayakorn, N.; Wongsawat, Y. An EMG instrument designed for bruxism detection on masseter muscle. In Proceedings of the 7th 2014 Biomedical Engineering International Conference, Fukuoka, Japan, 26–28 November 2014. [Google Scholar] [CrossRef]
- Bb, H.; Akhtar, F.; Mehdi, A.; Azad, S.; Azad, S.; Azad, S. Normalized Power are used in the Diagnosis of Insomnia Medical Sleep Syndrome through EMG1-EMG2 Channel. Austin J. Sleep Disord. 2017, 4, 2–4. [Google Scholar]
- Heyat, M.B.B.; Akhtar, F.; Ammar, M.; Hayat, B.; Azad, S. Power Spectral Density are used in the Investigation of insomnia neurological disorder. In Proceedings of the XL- Pre Congress Symposium, organized by Indian Academy of Social Sciences, State Takmeel-ut-Tib-College & Hospital, and King George Medical College & Hospital, Lucknow, UP, India, October 2016; pp. 45–50. [Google Scholar]
- Heyat, M.B.B.; Akhtar, F.; Sikandar, M.; Siddiqui, H.; Azad, S. An Overview of Dalk Therapy and Treatment of Insomnia in Dalk Therapy Abstract- Treatment of Insomnia in Dalk Therapy. In Proceedings of the National Seminar on Research Methodology in Ilaj-Bit-Tadbeer, organized by State Takmeel-ut-Tib-College & Hospital, Lucknow, India, 10–11 October 2015. [Google Scholar]
- Bin Heyat, B.; Akhtar, F.; Singh, S.K.; Siddiqui, M.M. Hamming Window are used in the Prognostic of Insomnia. In Proceedings of the International Seminar Present Scenario Future Prospectives Res. Eng. Sci. (ISPSFPRES), organized by Integral University, Lucknow, UP, India, January 2017; pp. 65–71. [Google Scholar]
- Heyat, M.B.B. Insomnia: Medical Sleep Disorder & Diagnosis, 1st ed.; Anchor Academic Publishing: Hamburg, Germany, 2016. [Google Scholar]
- Heyat, M.B.B.; Akhtar, F.; Azad, S. Comparative Analysis of Original Wave and Filtered Wave of EEG signal Used in the Prognostic of Bruxism medical Sleep syndrome. Int. J. Trend Sci. Res. Dev. 2016, 1, 7–9. [Google Scholar] [CrossRef]
- Heyat, M.B.B.; Lai, D.; Akhtar, F.; Hayat, M.A.B. Short Time Frequency Analysis of Theta Activity for the Diagnosis of Bruxism on EEG Sleep. In Advanced Computational Intelligence Techniques for Virtual Reality in Healthcare Studies in Computational Intelligence; Gupta, K.D., Hassanien, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 63–83. [Google Scholar]
- Lai, D.; Heyat, M.B.B.; Khan, F.I.; Zhang, Y. Prognosis of Sleep Bruxism Using Power Spectral Density Approach Applied on EEG Signal of Both EMG1-EMG2 and ECG1-ECG2 Channels. IEEE Access 2019, 7, 82553–82562. [Google Scholar] [CrossRef]
- Rahman, T.; Farook, O.; Bin Heyat, B.; Siddiqui, M.M. An Overview of Narcolepsy. Int. Adv. Res. J. Sci. Eng. Technol. 2016, 3, 2393–2395. [Google Scholar]
- Farooq, O.; Rahman, T.; Bin Heyat, B.; Siddiqui, M.M.; Akhtar, F. An Overview of NFLE. Int. J. Innov. Res. Electr. Electron. Instrum. Control. Eng. 2016, 4, 209–211. [Google Scholar]
- Shahin, M.; Ahmed, B.; Hamida, S.T.B.; Mulaffer, F.L.; Glos, M.; Penzel, T. Deep Learning and Insomnia: Assisting Clinicians with Their Diagnosis. IEEE J. Biomed. Heal. Inform. 2017, 21, 1546–1553. [Google Scholar] [CrossRef]
- Mulaffer, L.; Shahin, M.; Glos, M.; Penzel, T.; Ahmed, B. Comparing two insomnia detection models of clinical diagnosis techniques. In Proceedings of the 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju Island, Korea, 11–15 July 2017. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.-K.; Eugene Stanley, H.; et al. PhysioBank, PhysioToolkit, and PhysioNet. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef]
- Costa, M.; Moody, G.B.; Henry, I.; Goldberger, A.L. PhysioNet: An NIH Research Resource for Complex Signals. J. Electrocardiol. 2003, 36, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Welch, P.D. Welch_1967_Modified_Periodogram_Method. Trans. Audio Electroacoust. 1967, 15, 70–73. [Google Scholar] [CrossRef]
- Siddiqui, M.M.; Srivastava, G.; Saeed, S.H. Diagnosis of insomnia sleep disorder using short time frequency analysis of PSD approach applied on EEG signal using channel ROC-LOC. Sleep Sci. 2016, 9, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Barbé, K.; Pintelon, R.; Schoukens, J. Welch method revisited: Nonparametric power spectrum estimation via circular overlap. IEEE Trans. Signal. Process. 2010, 58, 553–565. [Google Scholar] [CrossRef]
- Liu, D.; Pang, Z.; Lloyd, S.R. A neural network method for detection of obstructive sleep apnea and narcolepsy based on pupil size and EEG. IEEE Trans. Neural Netw. 2008, 19, 308–318. [Google Scholar] [CrossRef]
- Rahi, P.K.; Mehra, R. Analysis of Power Spectrum Estimation Using Welch Method for Various Window Techniques. Int. J. Emerg. Technol. Eng. 2014, 2, 106–109. [Google Scholar]
- Xiang, C.; Yong, P.C.; Meng, L.S. Design of multiple-level hybrid classifier for intrusion detection system using Bayesian clustering and decision trees. Pattern Recognit. Lett. 2008, 29, 918–924. [Google Scholar] [CrossRef]
- Bezdek, J.C.; Reichherzer, T.R.; Lim, G.S.; Attikiouzel, Y. Multiple-prototype classifier design. IEEE Trans. Syst. Man Cybern. Part C Appl. Rev. 1998, 28, 67–79. [Google Scholar] [CrossRef]
- Chen, S.; Shen, B.; Wang, X.; Yoo, S.J. A strong machine learning classifier and decision stumps based hybrid adaboost classification algorithm for cognitive radios. Sensors 2019, 19, 5077. [Google Scholar] [CrossRef]
- Rawat, K.S.; Malhan, I.V. A Hybrid Classification Method Based on Machine Learning Classifiers to Predict Performance in Educational Data Mining. In Lecture Notes in Networks and Systems; Springer: Singapore, 2019. [Google Scholar]
- Miškovic, V. Machine Learning of Hybrid Classification Models for Decision Support. Sinteza 2014 Impact Internet Bus. Act. Serbia Worldw. 2014, 2014, 318–324. [Google Scholar] [CrossRef][Green Version]
- Chen, T.; Sun, J.; Lin, H.; Liu, Y. Hybrid. Machine Learning Models of Classifying Residential Requests for Smart Dispatching; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Längkvist, M.; Karlsson, L.; Loutfi, A. Sleep Stage Classification Using Unsupervised Feature Learning. Adv. Artif. Neural Syst. 2012, 2012, 107046. [Google Scholar] [CrossRef]
- Boe, A.J.; Koch, L.L.M.; O’Brien, M.K.; Shawen, N.; Rogers, J.A.; Lieber, R.L.; Reid, K.J.; Zee, P.C.; Jayaraman, A. Automating sleep stage classification using wireless, wearable sensors. NPJ Digit. Med. 2019, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, V.; Pachori, R.B. Automatic classification of sleep stages based on the time-frequency image of EEG signals. Comput. Methods Prog. Biomed. 2013, 112, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Mitsukura, Y.; Fukunaga, K.; Yasui, M.; Mimura, M. Sleep stage detection using only heart rate. Health Inform. J. 2019, 26, 376–387. [Google Scholar] [CrossRef]
- Pintado, M.R.; Anderson, G.C.; DeLong, R.; Douglas, W.H. Variation in tooth wear in young adults over a two-year period. J. Prosthet. Dent. 1997, 77, 313–320. [Google Scholar] [CrossRef]
- Ekfeldt, A.; Hugoson, A.; Bergendal, T.; Helkimo, M. An individual tooth wear index and an analysis of factors correlated to incisal and occlusal wear in an adult swedish population. Acta Odontol. Scand. 1990, 48, 343–349. [Google Scholar] [CrossRef]
- Takeuchi, H.; Ikeda, T.; Clark, G.T. A piezoelectric film-based intrasplint detection method for bruxism. J. Prosthet. Dent. 2001, 86, 195–202. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Saha, S.; Fattah, S.A.; Zhu, W.P.; Ahmad, M.O. Sleep apnea detection based on rician modeling of feature variation in multiband EEG signal. IEEE J. Biomed. Heal. Inform. 2018, 23, 1066–10174. [Google Scholar] [CrossRef]
- Zarei, A.; Asl, B.M. Automatic Detection of Obstructive Sleep Apnea Using Wavelet Transform and Entropy-Based Features from Single-Lead ECG Signal. IEEE J. Biomed. Heal. Inform. 2018, 23, 1011–1021. [Google Scholar] [CrossRef]
- Dong, Z.; Li, X.; Chen, W. Frequency Network Analysis of Heart Rate Variability for Obstructive Apnea Patient Detection. IEEE J. Biomed. Heal. Inform. 2017, 22, 1895–1905. [Google Scholar] [CrossRef]
- Kassiri, H.; Chemparathy, A.; Salam, M.T.; Boyce, R.; Adamantidis, A.; Genov, R. Electronic Sleep Stage Classifiers: A Survey and VLSI Design Methodology. IEEE Trans. Biomed. Circ. Syst. 2017, 11, 177–188. [Google Scholar] [CrossRef]
- Kohtoh, S.; Taguchi, Y.; Matsumoto, N.; Wada, M.; Huang, Z.L.; Urade, Y. Algorithm for sleep scoring in experimental animals based on fast Fourier transform power spectrum analysis of the electroencephalogram. Sleep Biol. Rhythms 2008, 6, 163–171. [Google Scholar] [CrossRef]
- Louis, R.P.; Lee, J.; Stephenson, R. Design and validation of a computer-based sleep-scoring algorithm. J. Neurosci. Methods 2004, 133, 71–80. [Google Scholar] [CrossRef]
- Asri, H.; Mousannif, H.; Al Moatassim, H. A Hybrid. Data Mining Classifier for Breast Cancer Prediction; Springer: Cham, Swizerland, 2020. [Google Scholar] [CrossRef]
- Mohan, S.; Thirumalai, C.; Srivastava, G. Effective heart disease prediction using hybrid machine learning techniques. IEEE Access 2019, 7, 81542–81554. [Google Scholar] [CrossRef]
- Reddy, G.T.M.; Reddy, P.K.; Lakshmanna, K.; Rajput, D.S.; Kaluri, R.; Srivastava, G. Hybrid genetic algorithm and a fuzzy logic classifier for heart disease diagnosis. Evol. Intell. 2020, 13, 185–196. [Google Scholar] [CrossRef]
- Yang, H.; Liu, J.; Sui, J.; Pearlson, G.; Calhoun, V.D. A hybrid machine learning method for fusing fMRI and genetic data: Combining both improves classification of schizophrenia. Front. Hum. Neurosci. 2010, 4, 192. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Yang, M.Q.; Luo, Z.; Ma, Y.; Li, J.; Deng, Y.; Huang, X. A hybrid machine learning-based method for classifying the Cushing’s Syndrome with comorbid adrenocortical lesions. BMC Genom. 2008, 9, S23. [Google Scholar] [CrossRef]

| Name of the Physiological Signal | Channel of the Physiological Signal | Number of the Subjects (n) | Number of the Segment (n) | Duration of the Signal | ||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Total | Bruxism | Healthy | Total | (s) | ||
| ECG | ECG1-ECG2 | 6 | 3 | 9 | 149 | 95 | 244 | 14,640 |
| EMG | EMG1-EMG2 | 6 | 3 | 9 | 149 | 95 | 244 | 14,640 |
| EEG | C4-P4 | 4 | 4 | 8 | 140 | 84 | 224 | 13,440 |
| EEG | C4-A1 | 4 | 4 | 8 | 140 | 84 | 224 | 13,440 |
| Mean | 5 | 3.5 | 8.5 | 144.5 | 89.5 | 234 | 14,040 | |
| ±SD | 1.154 | 0.577 | 0.577 | 5.196 | 6.350 | 11.547 | 692.820 | |
| Name of the Channel | PT | NT | TP | TN | FP | FN | F1 | MCC | Sen | Spe | Acc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EMG1-EMG2 | 64 | 17 | 46 | 17 | 18 | 0 | 0.83 | 0.59 | 1 | 0.48 | 0.77 |
| ECG1-ECG2 | 78 | 3 | 46 | 3 | 32 | 0 | 0.74 | 0.22 | 1 | 0.08 | 0.60 |
| C4-P4 | 48 | 26 | 45 | 24 | 3 | 2 | 0.94 | 0.85 | 0.95 | 0.88 | 0.93 |
| C4-A1 | 47 | 27 | 45 | 25 | 2 | 2 | 0.95 | 0.88 | 0.95 | 0.92 | 0.94 |
| Mean | 59.2 | 18.2 | 45.5 | 17.2 | 13.7 | 1 | 0.86 | 0.63 | 0.97 | 0.59 | 0.81 |
| ±SD | 14.7 | 11.1 | 0.57 | 10.1 | 14.1 | 1.15 | 0.09 | 0.30 | 0.02 | 0.39 | 0.16 |
| Name of the Channel | PT | NT | TP | TN | FP | FN | F1 | MCC | Sen | Spe | Acc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EMG1-EMG2 | 54 | 27 | 50 | 25 | 4 | 2 | 0.94 | 0.83 | 0.96 | 0.86 | 0.92 |
| ECG1-ECG2 | 56 | 25 | 52 | 25 | 4 | 0 | 0.96 | 0.89 | 1 | 0.86 | 0.95 |
| C4-P4 | 47 | 27 | 42 | 23 | 5 | 4 | 0.9 | 0.73 | 0.91 | 0.82 | 0.87 |
| C4-A1 | 59 | 15 | 45 | 14 | 14 | 1 | 0.85 | 0.57 | 0.97 | 0.5 | 0.79 |
| Mean | 54 | 23.5 | 47.25 | 21.75 | 6.75 | 1.75 | 0.91 | 0.75 | 0.97 | 0.76 | 0.88 |
| ±SD | 5.09 | 5.74 | 4.57 | 5.25 | 4.85 | 1.70 | 0.04 | 0.13 | 0.03 | 0.17 | 0.06 |
| Name of the Channel | PT | NT | TP | TN | FP | FN | F1 | MCC | Sen | Spe | Acc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EMG1-EMG2 | 63 | 18 | 63 | 17 | 0 | 1 | 0.99 | 0.96 | 0.98 | 1.00 | 0.98 |
| ECG1-ECG2 | 65 | 16 | 63 | 15 | 2 | 1 | 0.97 | 0.88 | 0.98 | 0.88 | 0.96 |
| C4-P4 | 63 | 11 | 62 | 10 | 1 | 1 | 0.98 | 0.89 | 0.98 | 0.90 | 0.97 |
| C4-A1 | 65 | 9 | 62 | 8 | 3 | 1 | 0.96 | 0.77 | 0.98 | 0.72 | 0.94 |
| Mean | 64 | 13.5 | 62.5 | 12.5 | 1.5 | 1 | 0.97 | 0.87 | 0.98 | 0.65 | 0.96 |
| ±SD | 1.15 | 4.20 | 0.57 | 4.20 | 1.29 | 0 | 0.01 | 0.07 | 0 | 0.37 | 0.01 |
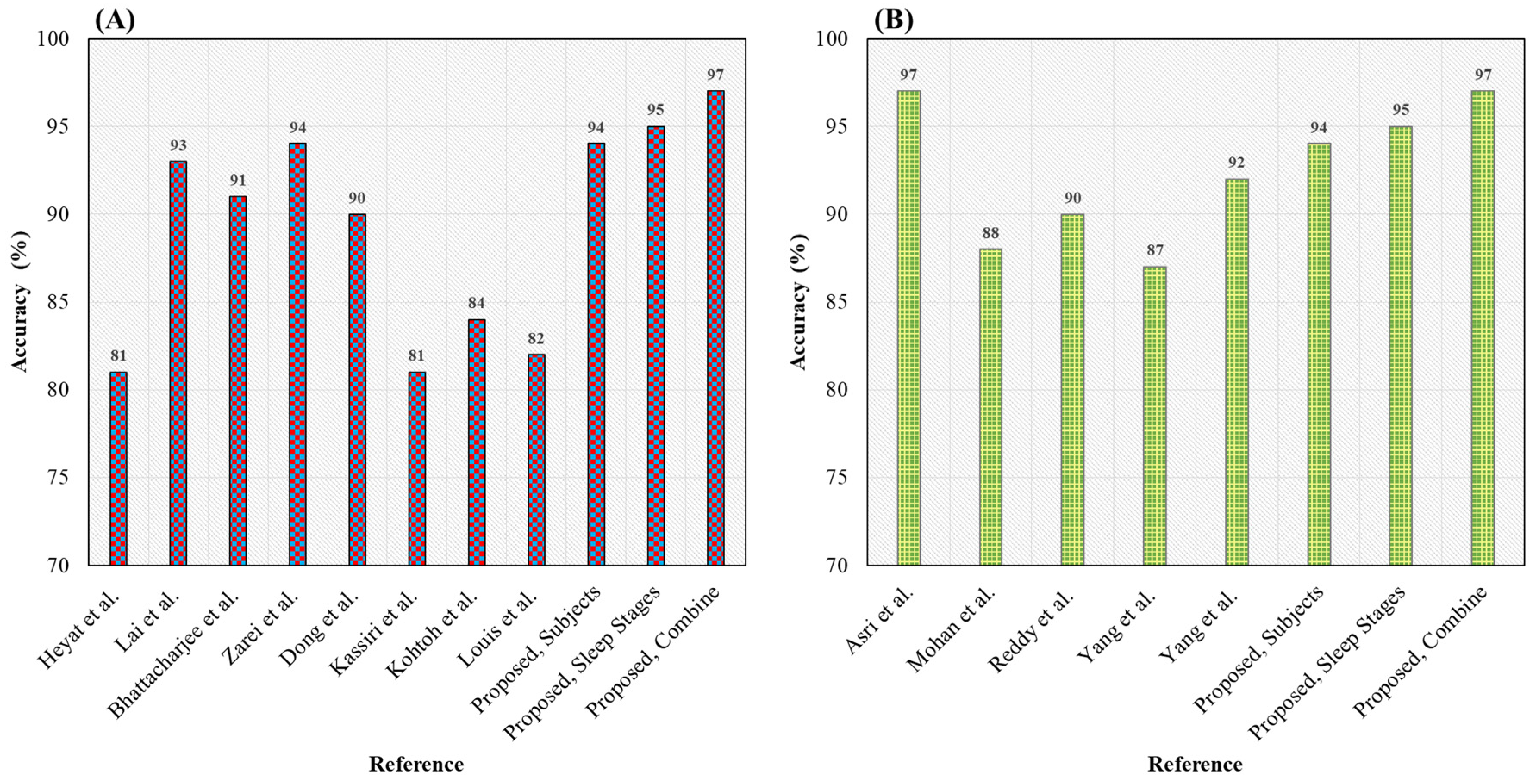
| Reference | Year | Disease | Signal | Classifier | Sen (%) | Spe (%) | Acc (%) |
|---|---|---|---|---|---|---|---|
| Heyat et al. [40] | 2019 | Bruxism | EEG | DT | 89 | 78 | 81 |
| Lai et al. [19] | 2019 | Bruxism | EMG | DT | 94 | 92 | 93 |
| Bhattacharjee et al. [65] | 2019 | SA | EEG | KNN | 98 | 83 | 91 |
| Zarei et al. [66] | 2019 | OSA | ECG | SVM RBF Kernel | 94 | 94 | 94 |
| Dong et al. [67] | 2018 | OSA | ECG | Threshold | 88 | 90 | 90 |
| Kassiri et al. [68] | 2017 | Sleep Stage | EEG, EMG | Threshold | 81 | 93 | 81 |
| Kohtoh et al. [69] | 2008 | Sleep Stage | EEG, EMG | Threshold | 71 | 96 | 84 |
| Louis et al. [70] | 2004 | Sleep Stage | EEG, EMG | Threshold | 66 | 84 | 82 |
| Subjects (Bruxism and Healthy) | EEG (C4-A1) | HML | 95 | 92 | 94 | ||
| Proposed | Sleep Stages (w and REM) | ECG (ECG1-ECG2) | 100 | 86 | 95 | ||
| Combine (Subjects and Sleep Stages) | EEG (C4-P4) | 98 | 90 | 97 | |||
| Reference | Year | Disease | Acc (%) |
|---|---|---|---|
| Asri et al. [71] | 2020 | Cancer | 97 |
| Mohan et al. [72] | 2019 | Heart | 88 |
| Reddy et al. [73] | 2019 | Heart | 90 |
| Yang et al. [74] | 2010 | Schizophrenia | 87 |
| Yang et al. [75] | 2007 | Cushing | 92 |
| 94 | |||
| Proposed | Bruxism | 95 | |
| 97 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin Heyat, M.B.; Akhtar, F.; Khan, A.; Noor, A.; Benjdira, B.; Qamar, Y.; Abbas, S.J.; Lai, D. A Novel Hybrid Machine Learning Classification for the Detection of Bruxism Patients Using Physiological Signals. Appl. Sci. 2020, 10, 7410. https://doi.org/10.3390/app10217410
Bin Heyat MB, Akhtar F, Khan A, Noor A, Benjdira B, Qamar Y, Abbas SJ, Lai D. A Novel Hybrid Machine Learning Classification for the Detection of Bruxism Patients Using Physiological Signals. Applied Sciences. 2020; 10(21):7410. https://doi.org/10.3390/app10217410
Chicago/Turabian StyleBin Heyat, Md Belal, Faijan Akhtar, Asif Khan, Alam Noor, Bilel Benjdira, Yumna Qamar, Syed Jafar Abbas, and Dakun Lai. 2020. "A Novel Hybrid Machine Learning Classification for the Detection of Bruxism Patients Using Physiological Signals" Applied Sciences 10, no. 21: 7410. https://doi.org/10.3390/app10217410
APA StyleBin Heyat, M. B., Akhtar, F., Khan, A., Noor, A., Benjdira, B., Qamar, Y., Abbas, S. J., & Lai, D. (2020). A Novel Hybrid Machine Learning Classification for the Detection of Bruxism Patients Using Physiological Signals. Applied Sciences, 10(21), 7410. https://doi.org/10.3390/app10217410









