Effect of Nano-Silica on the Autogenous Shrinkage, Strength, and Hydration Heat of Ultra-High Strength Concrete
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Specimen Preparation
2.2. Experimental Program
2.2.1. Isothermal Calorimetry
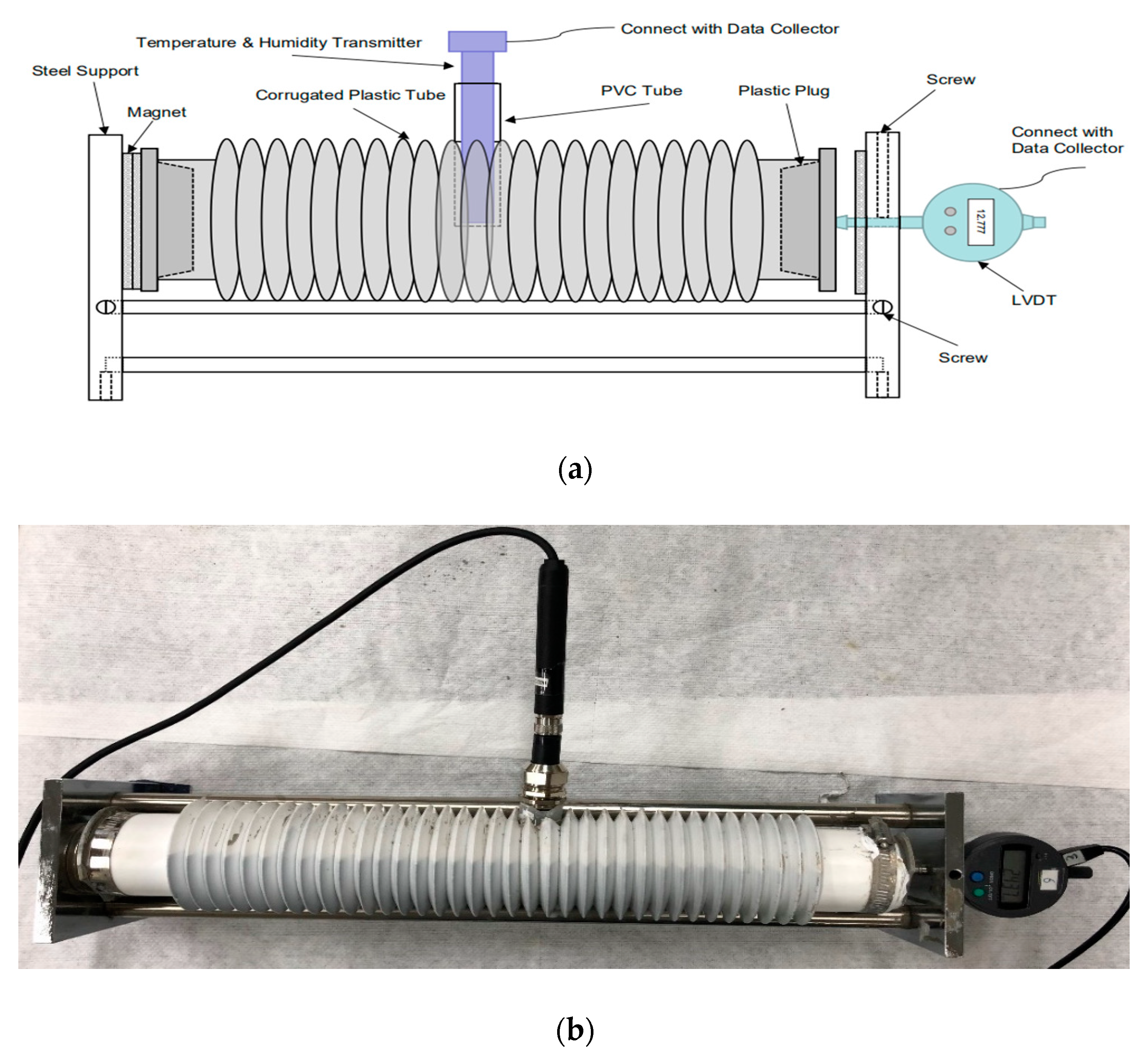
2.2.2. Autogenous Shrinkage, Internal Relative Humidity, and Internal Temperature
2.2.3. X-ray Diffraction
2.2.4. Compressive Strength Test
3. Results and Discussion
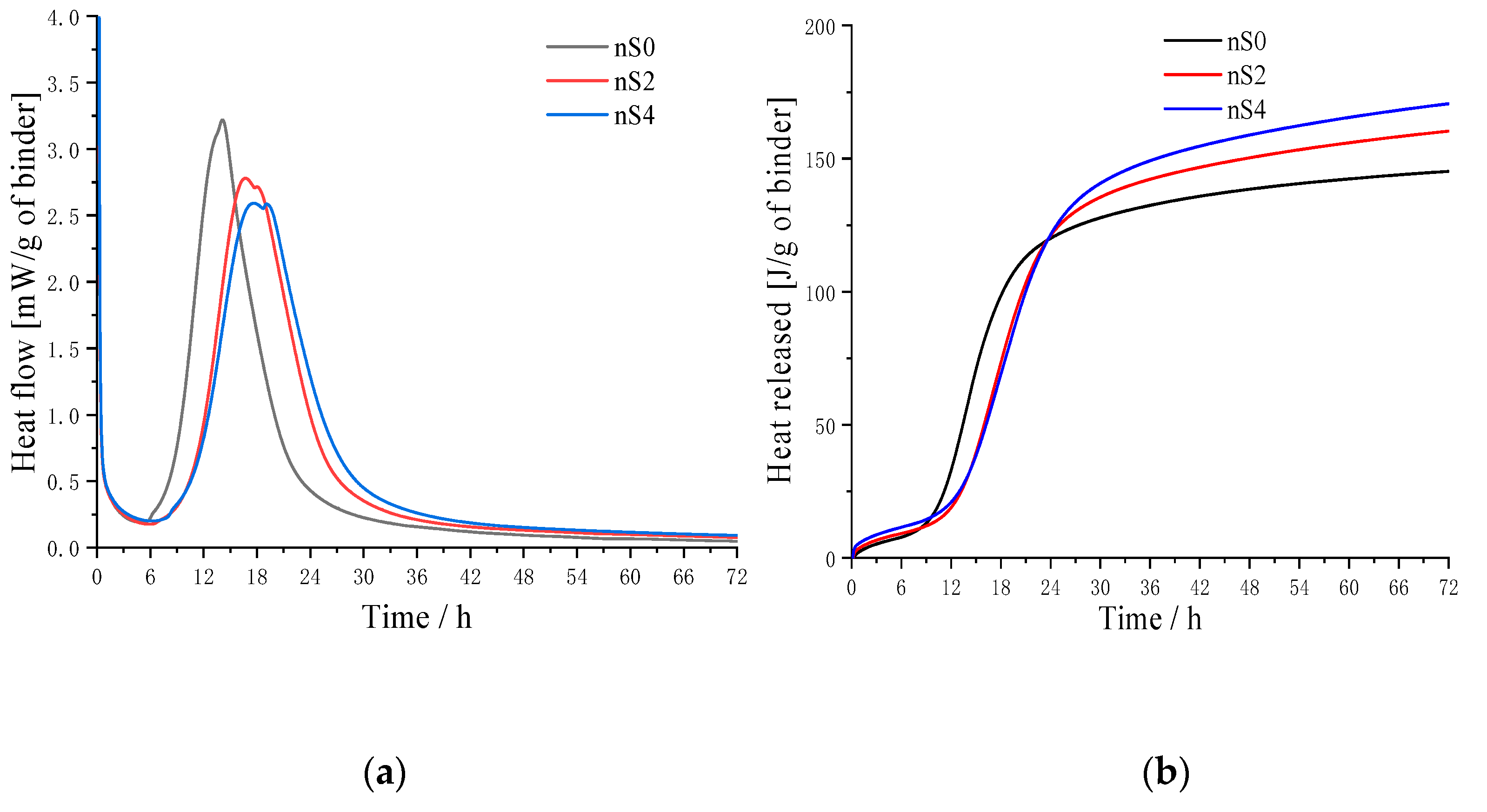
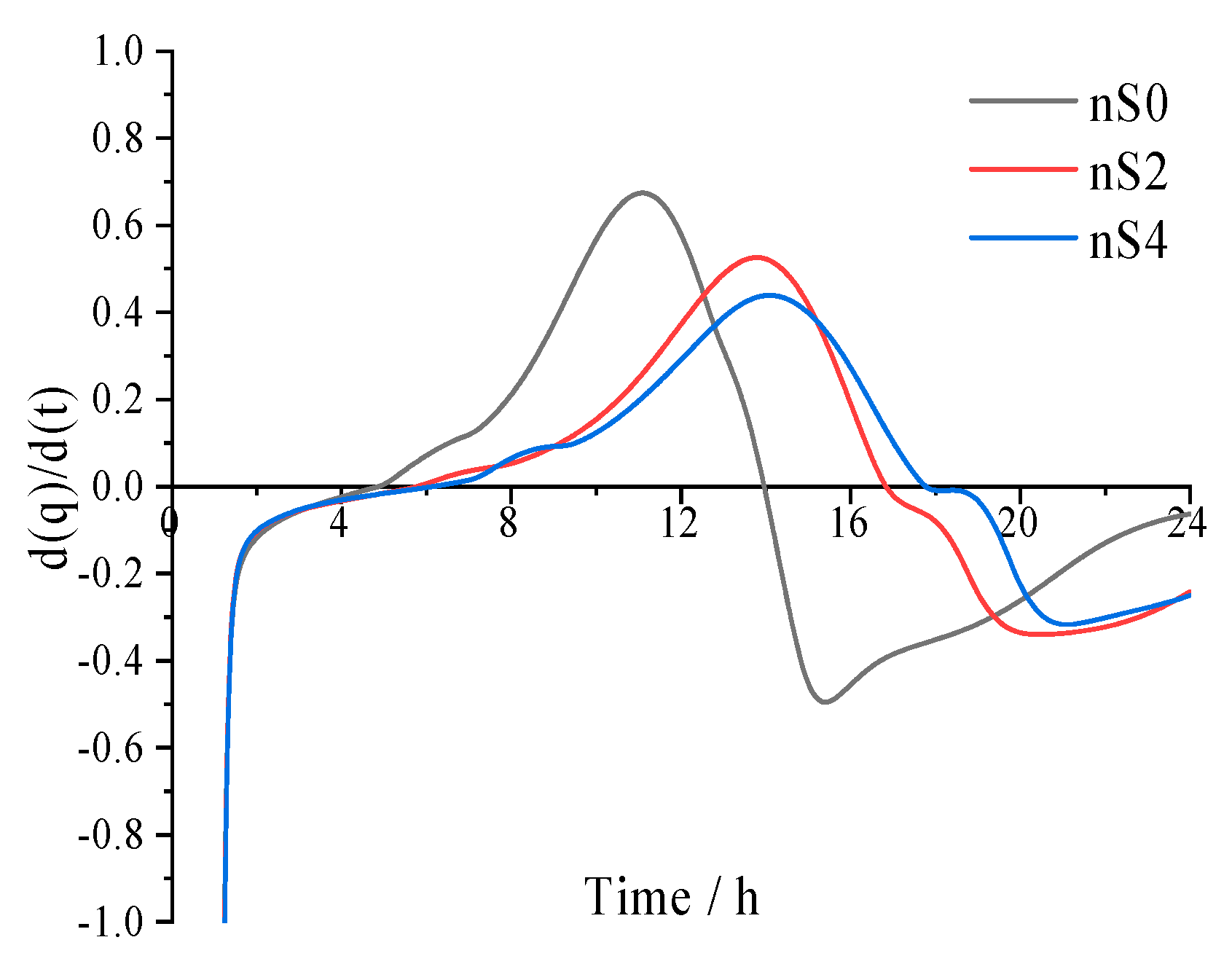
3.1. Isothermal Calorimetry
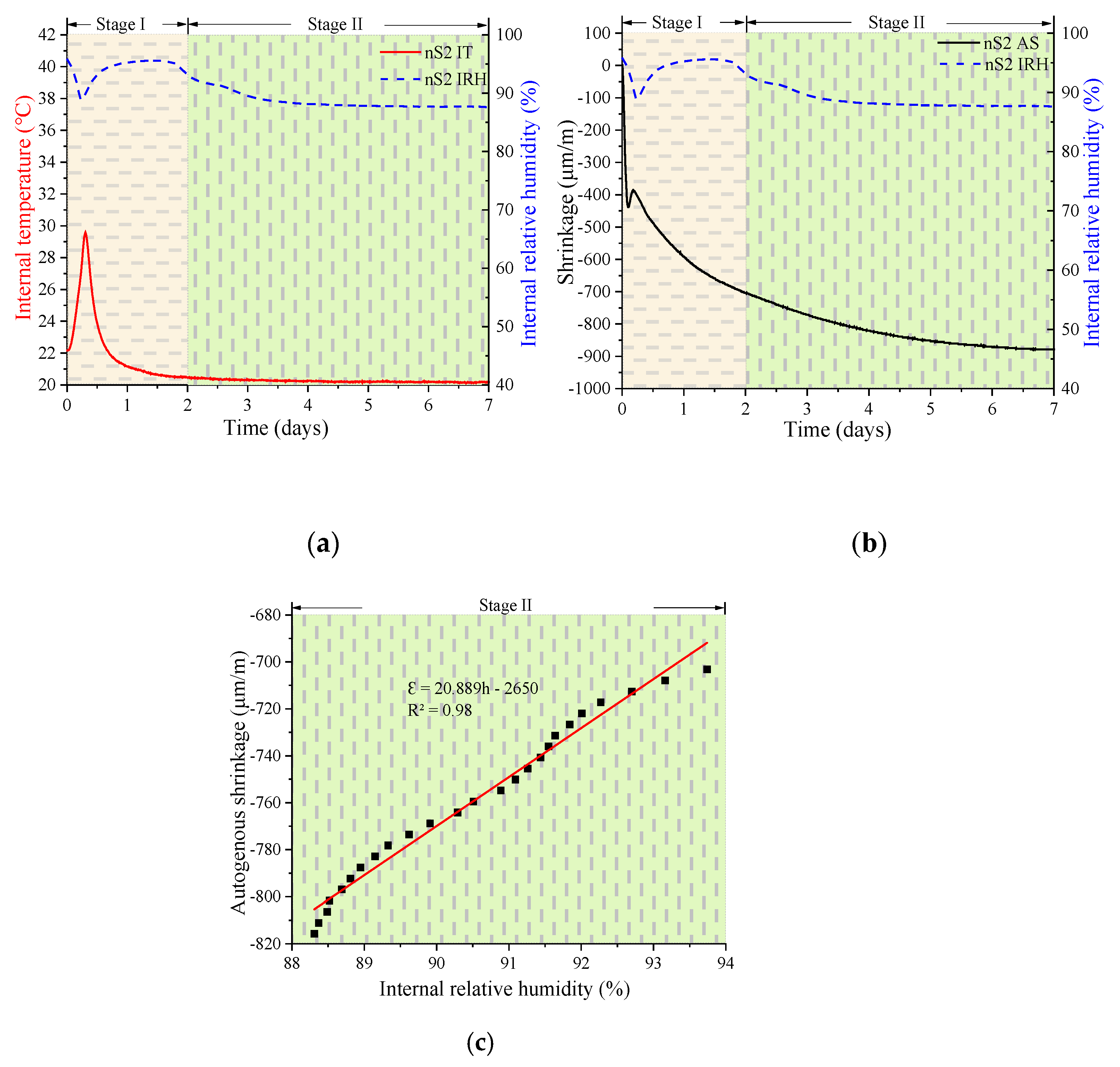
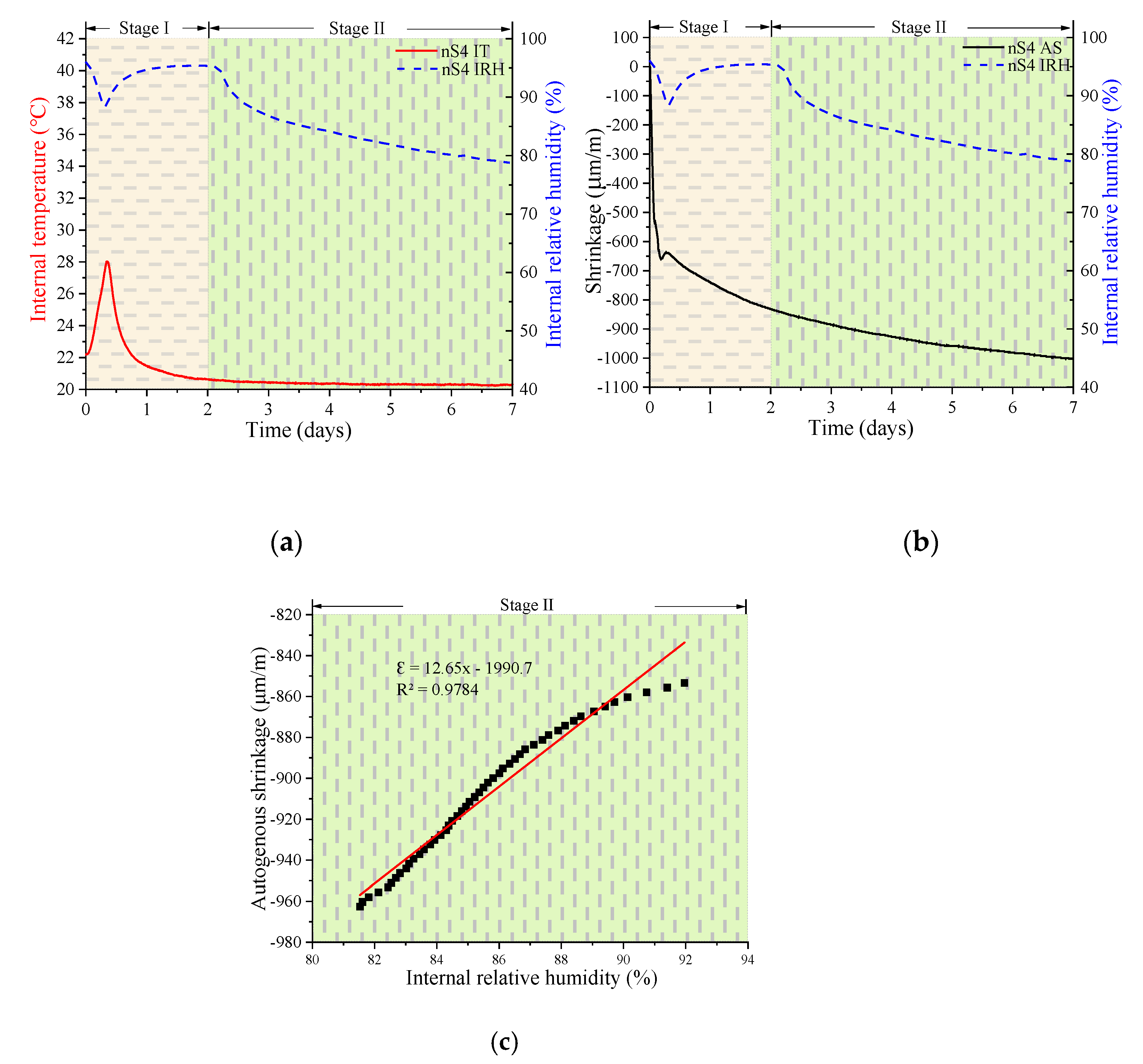
3.2. Development of Autogenous Shrinkage, Internal Relative Humidity, and Internal Temperature
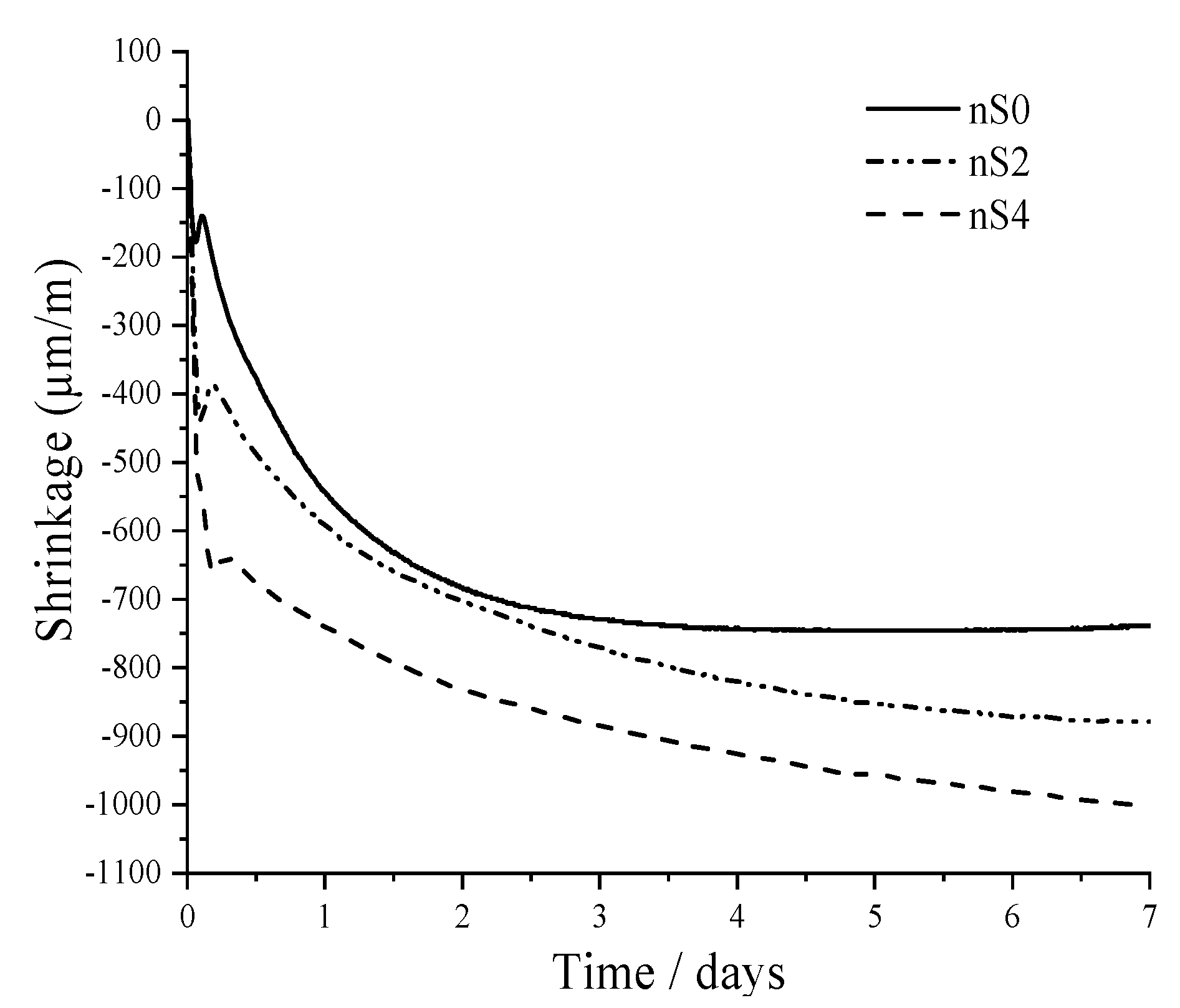
3.2.1. Autogenous Shrinkage
3.2.2. Development of the Internal Relative Humidity, Shrinkage, and Internal Temperature
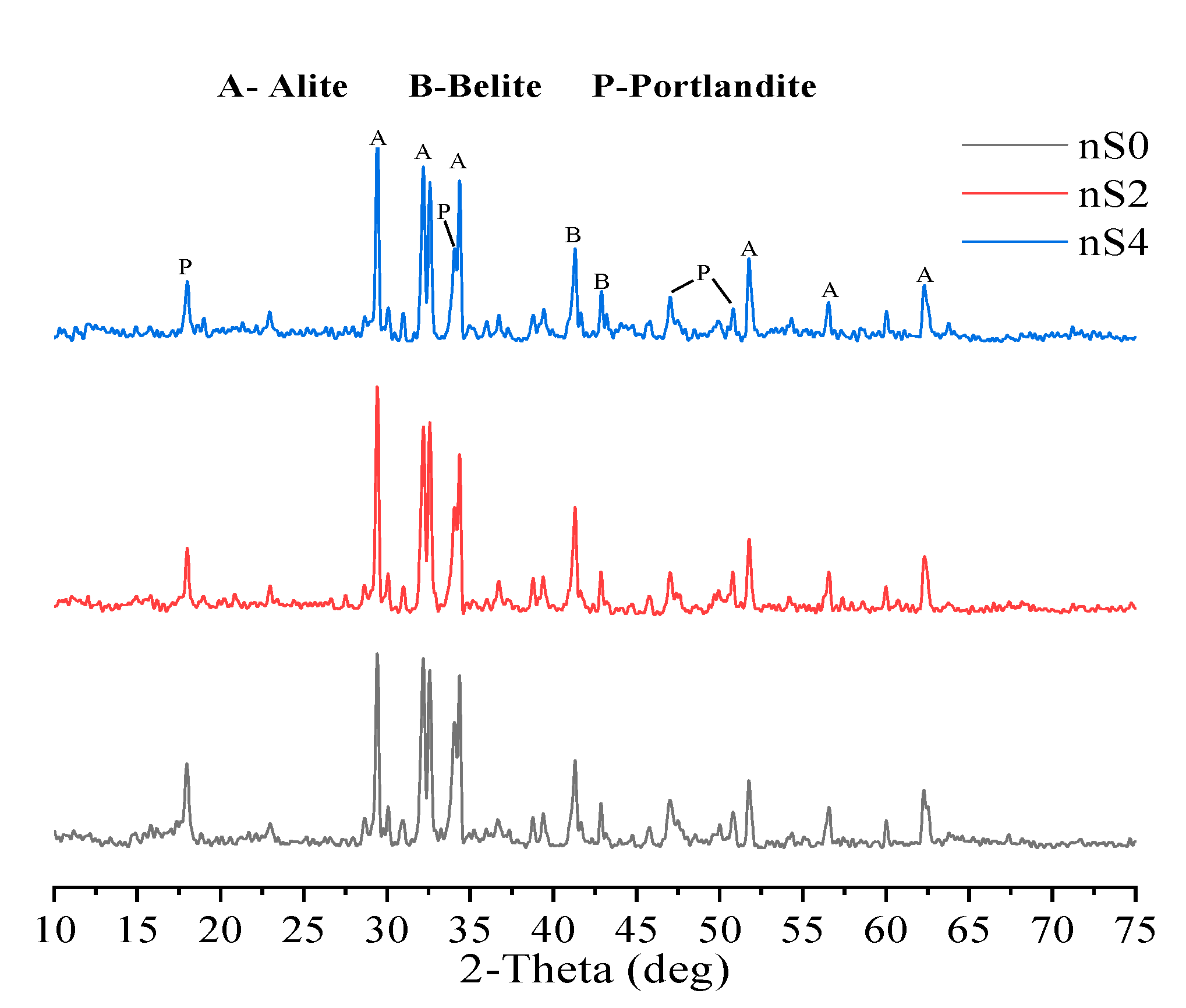
3.3. X-ray Diffraction
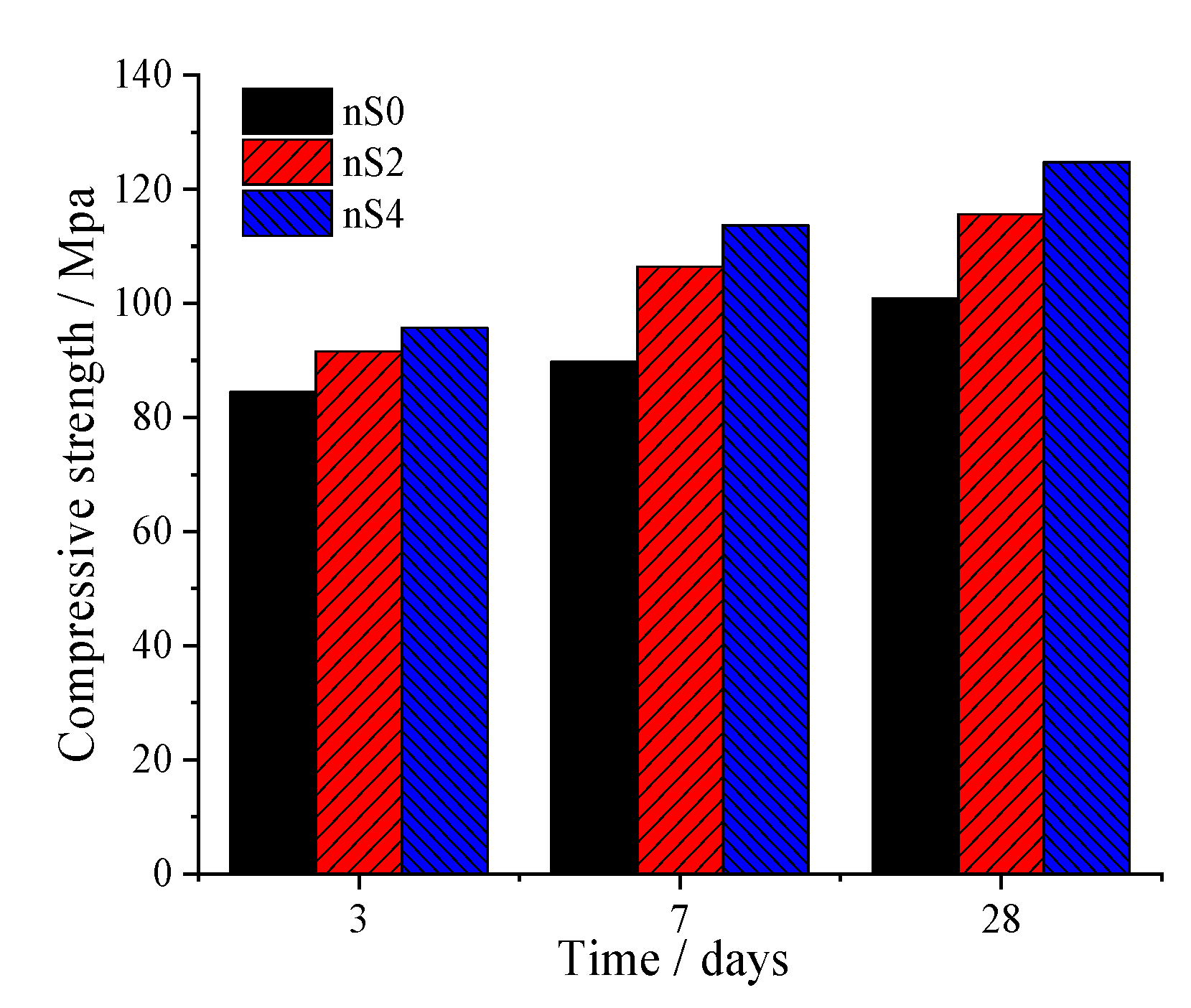
3.4. Compressive Strength
4. Conclusions
- The isothermal calorimetric data showed that due to the significantly delayed effect of polycarboxylate superplasticizers, the heat flow peaks of each hydration heat appeared in the order of nS0 > nS2 > nS4. Three peaks (dissolved peak of tricalcium aluminate, the exothermic peak of C3S and other topochemical reactions, and sulphate-type-AFm and C3A rehydration) were found in the heat flow curves in all UHSCs. Isothermal calorimetry is used to determine the setting time approximately. The setting time of UHSC specimens was delayed due to the addition of polycarboxylate superplasticizers. A greater amount of polycarboxylate superplasticizers led to more noticeable delayed effects.
- Total shrinkage increased with an increase in the nano-silica content. All specimens expanded at the beginning of the aging (1.2–6.7 h). The expansion of nS2 was more pronounced compared to that of nS0. However, the expansion of nS4 did not show up more noticeably as the amount of nS increased.
- The trends in the autogenous shrinking of UHSC were divided into two stages: the variable temperature stage (Stage I) and the room temperature stage (Stage II). The dividing point between the two stages occurred at approximately 2 days. In stage I, the hydration rate values decreased as the nS content increased. The maximum IT values decreased as the nS content increased. The IRH curve peaks of all specimens showed distinct downward peaks in a short period of time, and the timing at which these peaks appeared corresponded to the location of the highest peak of IT. However, in stage II, the IRH of all specimens continued to decrease. The final IRH value of UHSC after 7 days decreased as the nS content increased.
- At 28 days, XRD analysis showed that compared with nS0, the CH intensity of the nS2 and nS4 specimens decreased. This was due to the pozzolanic activities of the added nano-silica and CH, which consumed the CH produced by cement hydration.
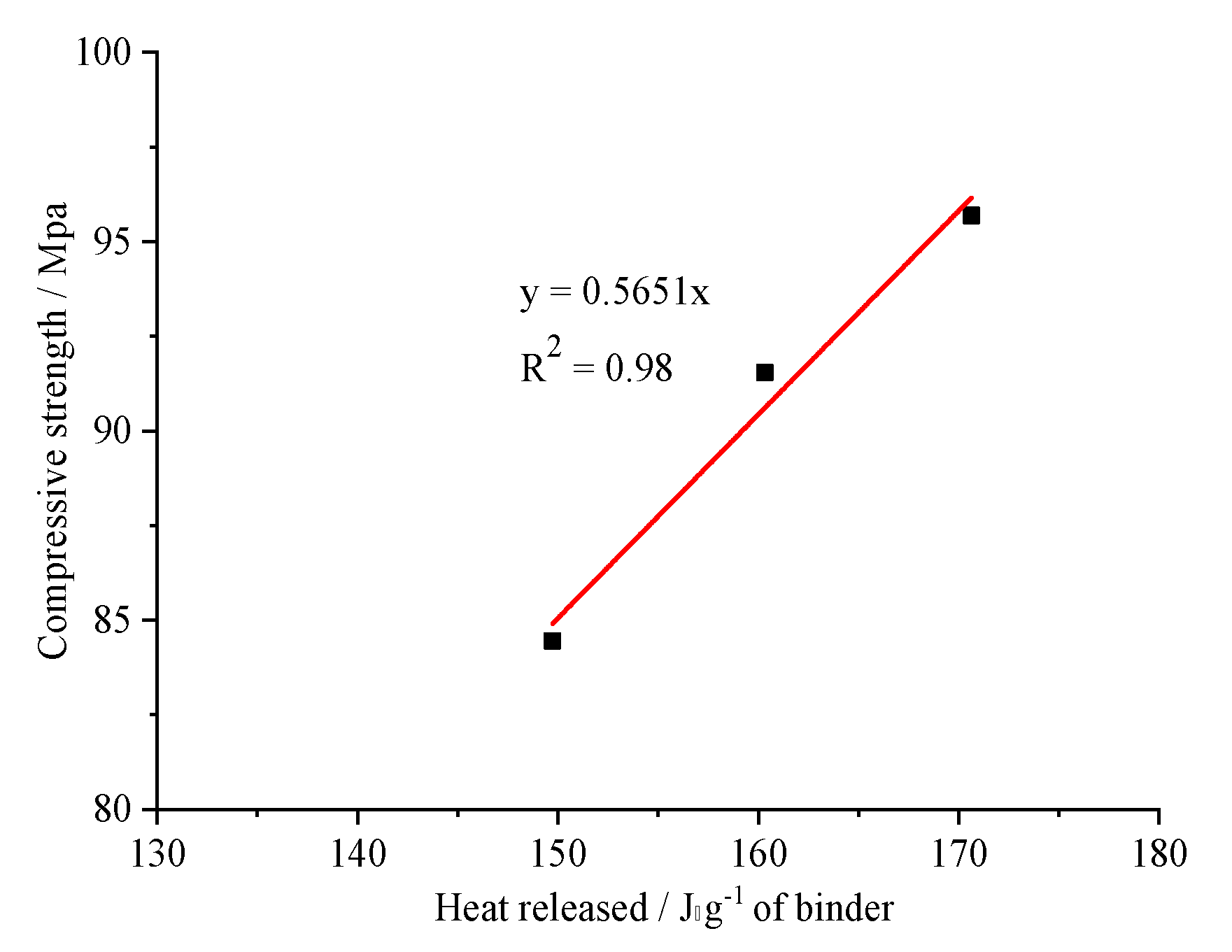
- Firstly, at 3 days, a linear relationship between the compressive strength and cumulative heat of hydration was clearly observed. Secondly, at 28 days, the addition of 2 wt.% and 4 wt.% nano-silica increased the strength of the specimen by 26.31% and 30.32%, respectively. As the amount of nS added increased, the ability of nS to enhance the compressive strength of UHSCs decreased significantly.
Author Contributions
Funding
Conflicts of Interest
References
- Sarma, K.C.; Adeli, H. Cost Optimization of Concrete Structures. J. Struct. Eng. 1998, 124, 570–578. [Google Scholar] [CrossRef]
- Chen, W.F. Concrete plasticity: Macro- and microapproaches. Int. J. Mech. Sci. 1993, 35, 1097–1109. [Google Scholar] [CrossRef]
- Aitcin, P.C. The durability characteristics of high performance concrete: A review. Cem. Concr. Compos. 2003, 25, 409–420. [Google Scholar] [CrossRef]
- Sato, T.; Beaudoin, J.J. Effect of nano-CaCO3 on hydration of cement containing supplementary cementitious materials. Adv. Cem. Res. 2011, 23, 33–43. [Google Scholar] [CrossRef]
- Meng, T.; Yu, Y.; Wang, Z. Effect of nano-CaCO3 slurry on the mechanical properties and micro-structure of concrete with and without fly ash. Compos. Part B Eng. 2017, 117, 124–129. [Google Scholar] [CrossRef]
- Mohseni, E.; Ranjbar, M.M.; Tsavdaridis, K.D. Durability Properties of High-Performance Concrete Incorporating Nano-TiO2 and Fly Ash. Am. J. Eng. Appl. Sci. 2015, 8, 519–526. [Google Scholar] [CrossRef]
- Mohseni, E.; Naseri, F.; Amjadi, R.; Khotbehsara, M.M.; Ranjbar, M.M. Microstructure and durability properties of cement mortars containing nano-TiO2 and rice husk ash. Constr. Build. Mater. 2016, 114, 656–664. [Google Scholar] [CrossRef]
- Chithra, S.; Kumar, S.S.; Chinnaraju, K. The effect of Colloidal Nano-silica on workability, mechanical and durability properties of High Performance Concrete with Copper slag as partial fine aggregate. Constr. Build. Mater. 2016, 113, 794–804. [Google Scholar] [CrossRef]
- Khaloo, A.; Mobini, M.H.; Hosseini, P. Influence of different types of nano-SiO2 particles on properties of high-performance concrete. Constr. Build. Mater. 2016, 113, 188–201. [Google Scholar] [CrossRef]
- Rupasinghe, M.; Nicolas, R.S.; Mendis, P.; Sofi, M.; Ngo, T. Investigation of strength and hydration characteristics in nano-silica incorporated cement paste. Cem. Concr. Compos. 2017, 80, 17–30. [Google Scholar] [CrossRef]
- Yu, R.; Spiesz, P.; Brouwers, H.J.H. Effect of nano-silica on the hydration and microstructure development of Ultra-High Performance Concrete (UHPC) with a low binder amount. Constr. Build. Mater. 2014, 65, 140–150. [Google Scholar] [CrossRef]
- Ghafari, E.; Arezoumandi, M.; Costa, H.; Julio, E. Influence of nano-silica addition on durability of UHPC. Constr. Build. Mater. 2015, 94, 181–188. [Google Scholar] [CrossRef]
- Ghafari, E.; Costa, H.; Julio, E.; Portugal, A.; Durães, L. The effect of nanosilica addition on flowability, strength and transport properties of ultra high performance concrete. Mater. Des. 2014, 59, 1–9. [Google Scholar] [CrossRef]
- Li, W.; Huang, Z.; Cao, F.; Sun, Z.; Shah, S.P. Effects of nano-silica and nano-limestone on flowability and mechanical properties of ultra-high-performance concrete matrix. Constr. Build. Mater. 2015, 95, 366–374. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, C.; Khayat, K.; Wan, S. Effects of different nanomaterials on hardening and performance of ultra-high strength concrete (UHSC). Cem. Concr. Compos. 2016, 70, 24–34. [Google Scholar] [CrossRef]
- Baloch, H.; Usman, M.; Rizwan, S.A.; Hanif, A. Properties enhancement of super absorbent polymer (SAP) incorporated self-compacting cement pastes modified by nano silica (NS) addition. Constr. Build. Mater. 2019, 203, 18–26. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, Y.-F.; Li, M.-Q. Influence of Nano-SiO2 on the Consistency, Setting Time, Early-Age Strength, and Shrinkage of Composite Cement Pastes. Adv. Mater. Sci. Eng. 2016, 2016, 1–8. [Google Scholar]
- Chen, H.; Xu, Y.; Zhang, D.; Huang, L.; Zhu, Y.; Huang, L. The Influence of Nano-SiO2 and Recycled Polypropylene Plastic Content on Physical, Mechanical, and Shrinkage Properties of Mortar. Adv. Civ. Eng. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Standard Test Method for Autogenous Strain of Cement Paste and Mortar; ASTM C1698-09(2014); ASTM International: West Conshohocken, PA, USA, 2014. [CrossRef]
- Zhang, J.; Wang, Q.; Zhang, J. Shrinkage of internal cured high strength engineered cementitious composite with pre-wetted sand-like zeolite. Constr. Build. Mater. 2017, 134, 664–672. [Google Scholar] [CrossRef]
- Stefanidou, M.; Papayianni, I. Influence of nano-SiO2 on the Portland cement pastes. Compos. Part B Eng. 2012, 43, 2706–2710. [Google Scholar] [CrossRef]
- Hu, J.; Ge, Z.; Wang, K. Influence of cement fineness and water-to-cement ratio on mortar early-age heat of hydration and set times. Constr. Build. Mater. 2014, 50, 657–663. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry; Thomas Telford Publishing: London, UK, 1997. [Google Scholar]
- Lin, R.-S.; Wang, X.-Y.; Lee, H.-S.; Cho, H.-K. Hydration and Microstructure of Cement Pastes with Calcined Hwangtoh Clay. Materials 2019, 12, 458. [Google Scholar] [CrossRef] [PubMed]
- Standard Test Method for Compressive Strength of HydraulicCement Mortars (Using Portions of Prisms Broken in Flexure); ASTM C349-14; ASTM International: West Conshohocken, PA, USA, 2014. [CrossRef]
- Yuan, B.; Yu, Q.; Brouwers, H. Reaction kinetics, reaction products and compressive strength of ternary activators activated slag designed by Taguchi method. Mater. Des. 2015, 86, 878–886. [Google Scholar] [CrossRef]
- Meng, W.; Kumar, A.; Khayat, K.H. Effect of silica fume and slump-retaining polycarboxylate-based dispersant on the development of properties of portland cement paste. Cem. Concr. Compos. 2019, 99, 181–190. [Google Scholar] [CrossRef]
- Budnikov, P.P.; Strelkov, M.I. Some Recent Concepts on Portland Cement Hydration and Hardening; Highway Research Board: Washington, DC, USA, 1966; pp. 447–464. [Google Scholar]
- Land, G.; Stephan, D. The influence of nano-silica on the hydration of ordinary Portland cement. J. Mater. Sci. 2011, 47, 1011–1017. [Google Scholar] [CrossRef]
- Jo, B.W.; Kim, C.H.; Lim, J.H. Investigations on the development of powder concrete with nano-SiO2 particles. KSCE J. Civ. Eng. 2007, 11, 37–42. [Google Scholar] [CrossRef]
- Sakai, E.; Kasuga, T.; Sugiyama, T.; Asaga, K.; Daimon, M. Influence of superplasticizers on the hydration of cement and the pore structure of hardened cement. Cem. Concr. Res. 2006, 36, 2049–2053. [Google Scholar] [CrossRef]
- Zhang, M.-H.; Sisomphon, K.; Ng, T.S.; Sun, D.J. Effect of superplasticizers on workability retention and initial setting time of cement pastes. Constr. Build. Mater. 2010, 24, 1700–1707. [Google Scholar] [CrossRef]
- Plank, J.; Sachsenhauser, B. Experimental determination of the effective anionic charge density of polycarboxylate superplasticizers in cement pore solution. Cem. Concr. Res. 2009, 39, 1–5. [Google Scholar] [CrossRef]
- Jansen, D.; Neubauer, J.; Goetz-Neunhoeffer, F.; Haerzschel, R.; Hergeth, W.-D. Change in reaction kinetics of a Portland cement caused by asuperplasticizer—Calculation of heatflow curves from XRD data. Cem. Concr. Res. 2012, 42, 327–332. [Google Scholar] [CrossRef]
- Quennoz, A.; Scrivener, K. Interactions between alite and C3A-gypsum hydrations in model cements. Cem. Concr. Res. 2013, 44, 46–54. [Google Scholar] [CrossRef]
- Mehta, P.; Wang, S. Expansion of ettringite by water adsorption. Cem. Concr. Res. 1982, 12, 121–122. [Google Scholar] [CrossRef]
- Tazawa, E.-I. Autogenous Shrinkage of Concrete, 1st ed.; Japan Concrete Institute: Tokio, Japan, 1999. [Google Scholar]
- Holt, E. Contribution of mixture design to chemical and autogenous shrinkage of concrete at early ages. Cem. Concr. Res. 2005, 35, 464–472. [Google Scholar] [CrossRef]
- Soliman, A.M.; Nehdi, M.L. Effect of drying conditions on autogenous shrinkage in ultra-high performance concrete at early-age. Mater. Struct. 2010, 44, 879–899. [Google Scholar] [CrossRef]
- Ishida, T.; Maekawa, K.; Kishi, T. Enhanced modeling of moisture equilibrium and transport in cementitiousmaterials under arbitrary temperature and relative humidity history. Cem. Concr. Res. 2006, 37, 565–578. [Google Scholar] [CrossRef]
- Bentz, D.P.; Geiker, M.R.; Hansen, K.K. Shrinkage-reducing admixtures and early-age desiccation in cement pastes and mortars. Cem. Concr. Res. 2001, 31, 11. [Google Scholar] [CrossRef]
- Morin, V.; Tenoudji, F.C.; Feylessoufi, A.; Richard, P. Superplasticizer effects on setting and structuration mechanisms of ultrahigh-performance concrete. Cem. Concr. Res. 2001, 31, 63–71. [Google Scholar] [CrossRef]
- Hakamy, A.; Shaikh, F.U.A.; Low, I.-M. Microstructures and mechanical properties of hemp fabric reinforced organoclay–cement nanocomposites. Constr. Build. Mater. 2013, 49, 298–307. [Google Scholar] [CrossRef]
- Rong, Z.; Sun, W.; Xiao, H.; Jiang, G. Effects of nano-SiO2 particles on the mechanical and microstructural properties of ultra-high performance cementitious composites. Cem. Concr. Compos. 2015, 56, 25–31. [Google Scholar] [CrossRef]
- Madani, H.; Bagheri, A.; Parhizkar, T. The pozzolanic reactivity of monodispersed nanosilica hydrosols and their influence on the hydration characteristics of Portland cement. Cem. Concr. Res. 2012, 42, 1563–1570. [Google Scholar] [CrossRef]
- Lee, H.-S.; Lim, S.-M.; Wang, X.-Y. Optimal Mixture Design of Low-CO2 High-Volume Slag Concrete Considering Climate Change and CO2 Uptake. Int. J. Concr. Struct. Mater. 2019, 13, 56. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Luan, Y. Modeling of Hydration, Strength Development, and Optimum Combinations of Cement-Slag-Limestone Ternary Concrete. Int. J. Concr. Struct. Mater. 2018, 12. [Google Scholar] [CrossRef]

| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | ZnO | K2O | P2O5 | Loss | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cement (%) | 21.95 | 6.07 | 2.45 | 61.38 | 2.80 | 2.54 | 0.11 | 1.58 | 0.10 | 0.46 |
| Nano-silica (%) | 99.80 | 0.05 | - | - | - | - | - | - | - | - |
| Number | Binder/% | w/b | Superplasticizer/% | |
|---|---|---|---|---|
| Cement | Nano-Silica | |||
| nS0 | 100 | 0 | 0.2 | 0.9 |
| nS2 | 98 | 2 | 0.2 | 2.4 |
| nS4 | 96 | 4 | 0.2 | 3.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.-Z.; Cho, H.-K.; Wang, X.-Y. Effect of Nano-Silica on the Autogenous Shrinkage, Strength, and Hydration Heat of Ultra-High Strength Concrete. Appl. Sci. 2020, 10, 5202. https://doi.org/10.3390/app10155202
Zhang G-Z, Cho H-K, Wang X-Y. Effect of Nano-Silica on the Autogenous Shrinkage, Strength, and Hydration Heat of Ultra-High Strength Concrete. Applied Sciences. 2020; 10(15):5202. https://doi.org/10.3390/app10155202
Chicago/Turabian StyleZhang, Guang-Zhu, Hyeong-Kyu Cho, and Xiao-Yong Wang. 2020. "Effect of Nano-Silica on the Autogenous Shrinkage, Strength, and Hydration Heat of Ultra-High Strength Concrete" Applied Sciences 10, no. 15: 5202. https://doi.org/10.3390/app10155202
APA StyleZhang, G.-Z., Cho, H.-K., & Wang, X.-Y. (2020). Effect of Nano-Silica on the Autogenous Shrinkage, Strength, and Hydration Heat of Ultra-High Strength Concrete. Applied Sciences, 10(15), 5202. https://doi.org/10.3390/app10155202






