Cyst Detection and Motion Artifact Elimination in Enface Optical Coherence Tomography Angiograms
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Image Acquisition
2.2. Cyst Detection and Motion Artifacts Elimination Algorithm
| Algorithm 1—Cysts detection and motion artifacts elimination. |
Input:Img—enface structural OCT-A grayscale image
|
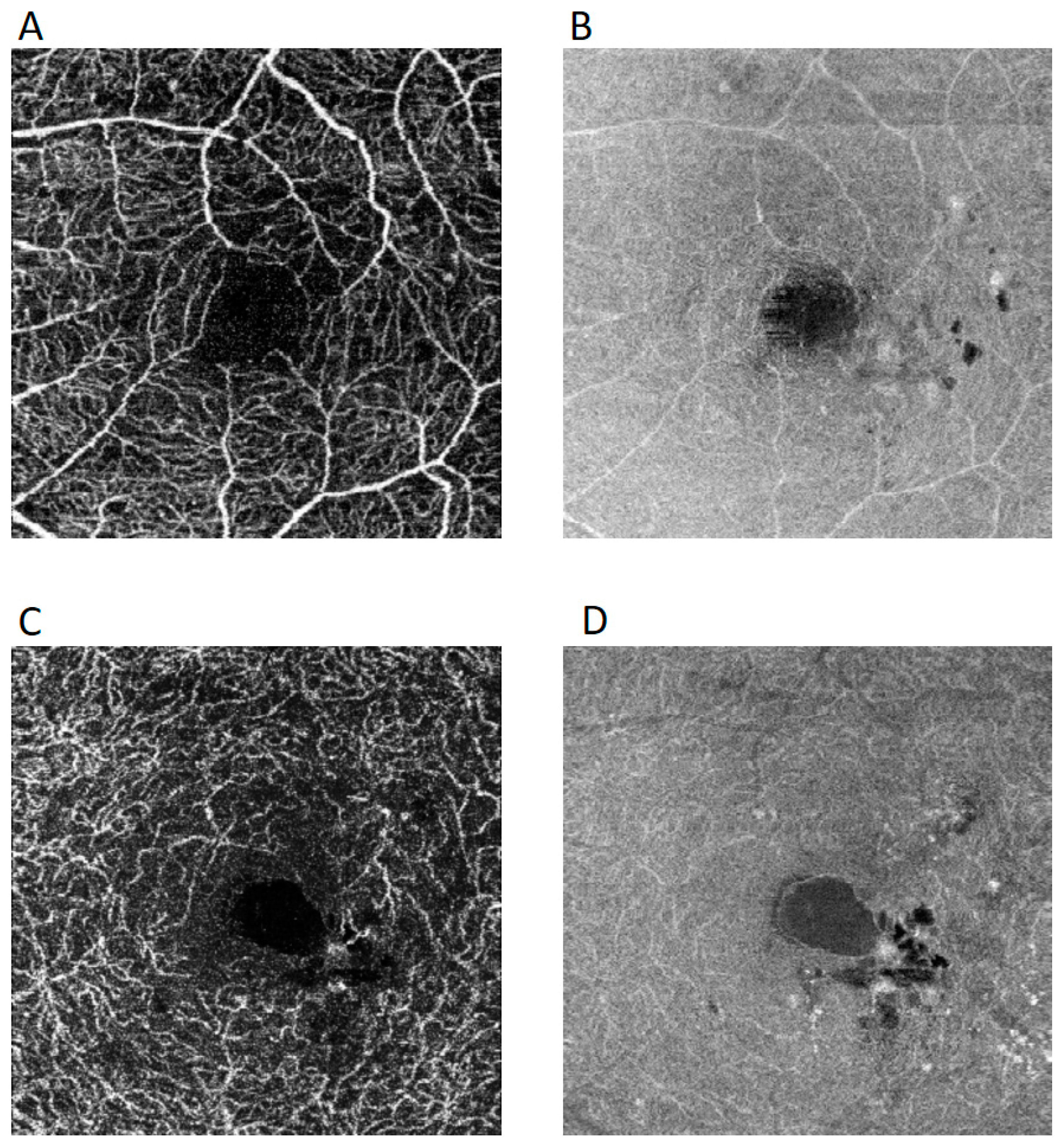
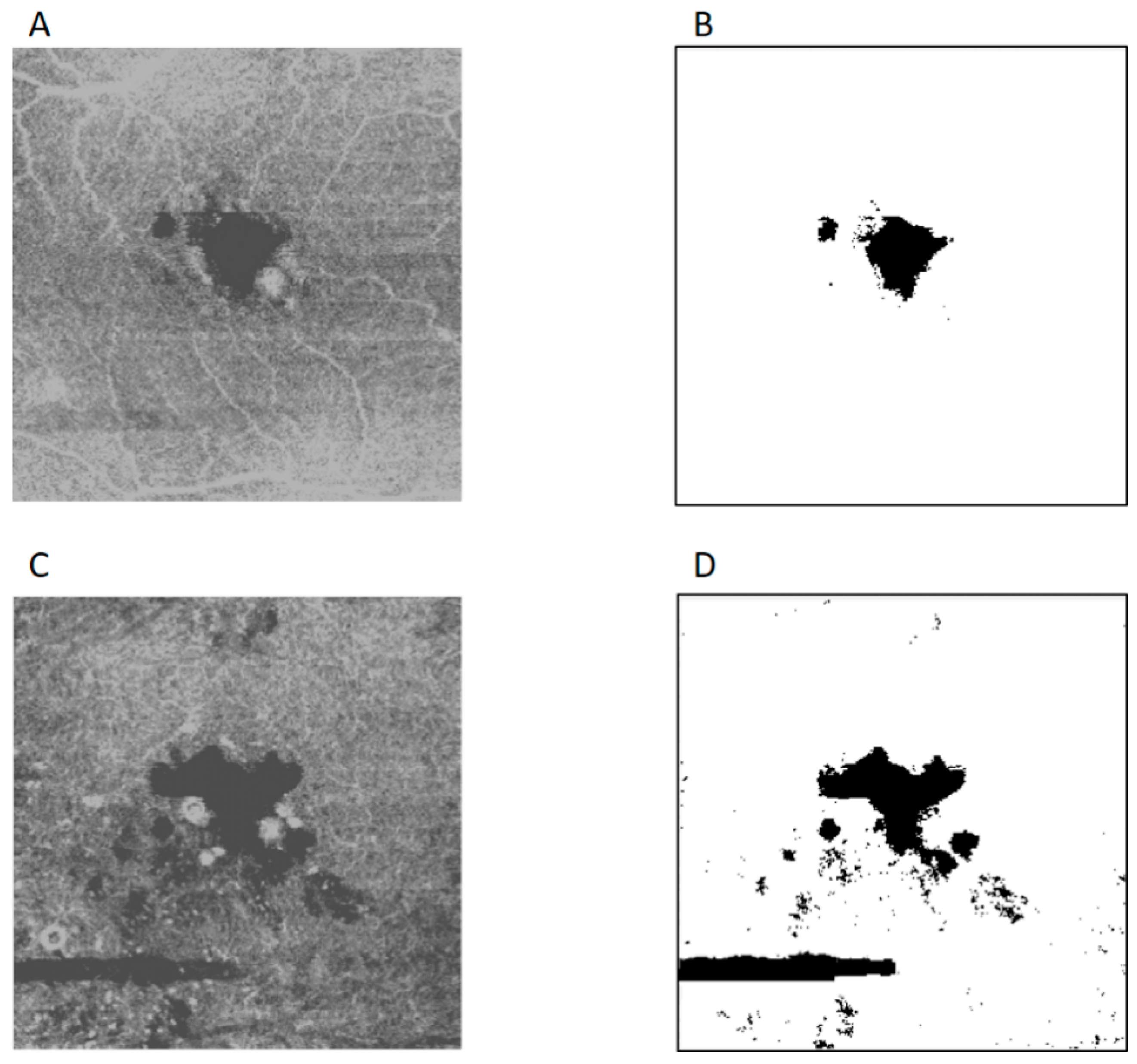
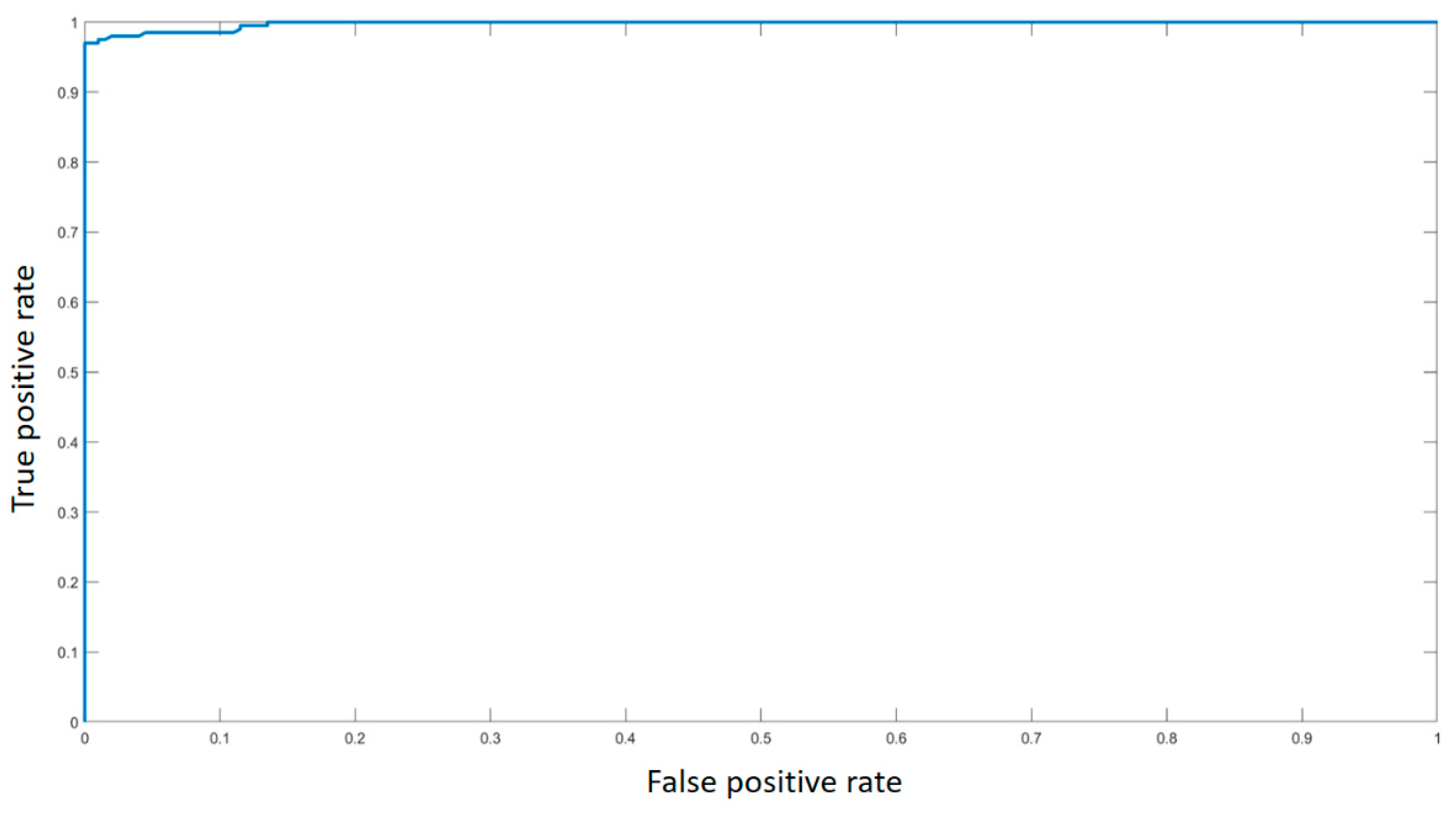
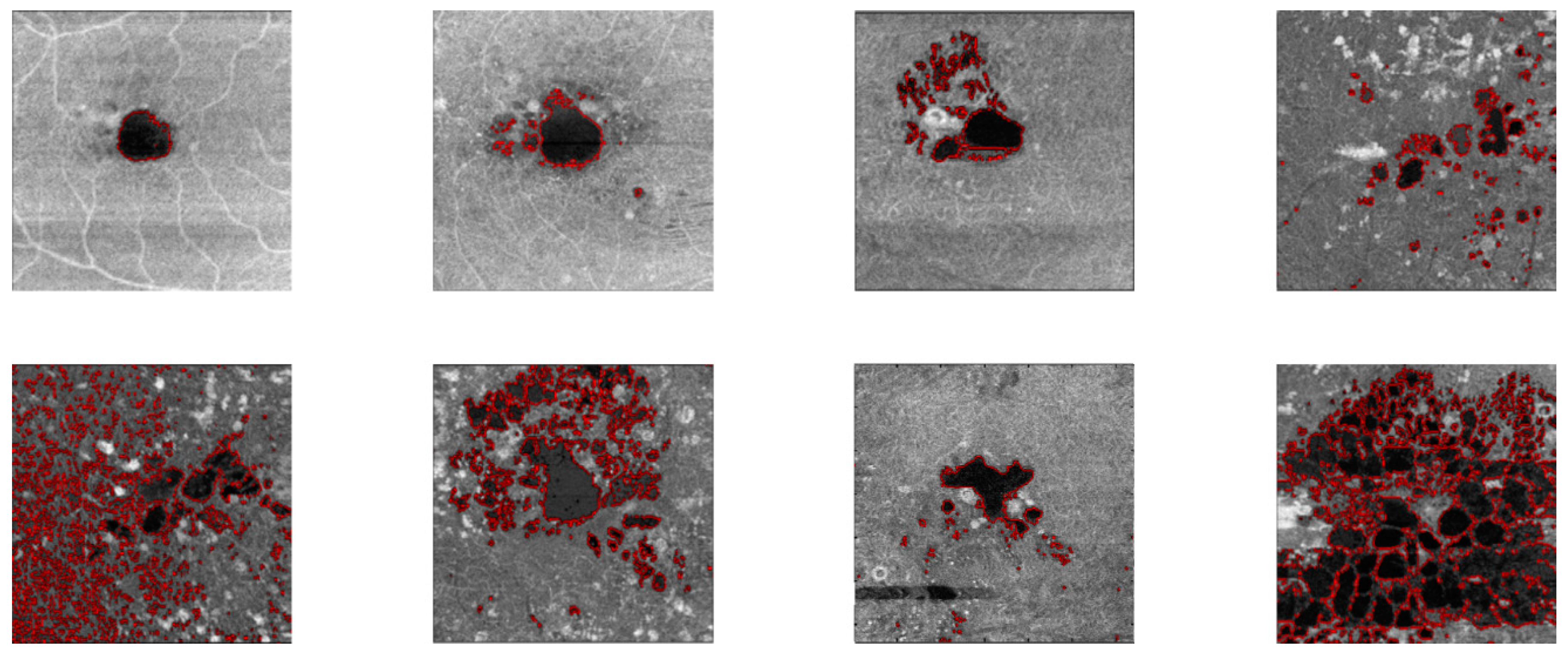
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A


References
- Bhanushali, D.; Anegondi, N.; Gadde, S.G.K.; Srinivasan, P.; Chidambara, L.; Yadav, N.K.; Roy, A.S. Linking retinal microvasculature features with severity of diabetic retinopathy using optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Muraca, A.; Gatti, V.; Masoero, L.; Brambilla, M.; Cannillo, B.; Villani, E.; Nucci, P.; De Cillà, S. Peripapillary microvascular and neural changes in diabetes mellitus: An oct-angiography study. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5074–5081. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, E.S.; Yu, S.Y. Optical coherence tomography angiography analysis of foveal microvascular changes and inner retinal layer thinning in patients with diabetes. Br. J. Ophthalmol. 2018, 102, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.W.; Tan, G.S.W.; Agrawal, R.; Yanagi, Y.; Sie, N.M.; Wong, C.W.; Yeo, I.Y.S.; Lee, S.Y.; Cheung, C.M.G.; Wong, T.Y. Optical coherence tomographic angiography in type 2 diabetes and diabetic retinopathy. JAMA Ophthalmol. 2017, 135, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Moon, B.G.; Cho, A.R.; Yoon, Y.H. Optical coherence tomography Aangiography of DME and its Aassociation with anti-VEGF treatment response. In Proceedings of the Ophthalmology; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 123, pp. 2368–2375. [Google Scholar]
- Toto, L.; D’Aloisio, R.; Di Nicola, M.; Di Martino, G.; Di Staso, S.; Ciancaglini, M.; Tognetto, D.; Mastropasqua, L. Qualitative and quantitative assessment of vascular changes in diabetic macular edema after dexamethasone implant using optical coherence tomography angiography. Int. J. Mol. Sci. 2017, 18, 1181. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, R.; D’Aloisio, R.; Di Nicola, M.; Di Martino, G.; Lamolinara, A.; Di Antonio, L.; Tognetto, D.; Toto, L. Relationship between aqueous humor cytokine level changes and retinal vascular changes after intravitreal aflibercept for diabetic macular edema. Sci. Rep. 2018, 8, 16548. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Gatti, V.; Muraca, A.; Brambilla, M.; Villani, E.; Nucci, P.; Rossetti, L.; De Cilla’, S. Optical coherence tomography angiography changes after subthreshold micropulse yellow laser in diabetic macular edema. Retina 2020, 1, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Toma, C.; Villani, E.; Muraca, A.; Torti, E.; Florimbi, G.; Leporati, F.; Brambilla, M.; Nucci, P.; De Cilla’, S. Diabetic macular edema with neuroretinal detachment: OCT and OCT-angiography biomarkers of treatment response to anti-VEGF and steroids. Acta Diabetol. 2020, 57, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Munk, M.; Sacu, S.; Huf, W.; Sulzbacher, F.; Mittermüller, T.; Eibenberger, K.; Rezar, S.; Bolz, M.; Kiss, C.; Simader, C.; et al. Differential diagnosis of macular edema of different pathophysiologic origins by spectral domain optical coherence tomography. Retina 2014, 34, 2218–2232. [Google Scholar] [PubMed]
- Otani, T.; Kishi, S.; Maruyama, Y. Patterns of diabetic macular edema with optical coherence tomography. Am. J. Ophthalmol. 1999, 127, 688–693. [Google Scholar] [CrossRef]
- De Carlo, T.E.; Chin, A.T.; Joseph, T.; Baumal, C.R.; Witkin, A.J.; Duker, J.S.; Waheed, N.K. Distinguishing diabetic macular edema from capillary nonperfusion using optical coherence tomography angiography. Ophthalmic Surg. Lasers Imaging Retin. 2016, 47, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K. Image artifacts in Optical coherence tomography angiography. Retina 2015, 35, 2163–2180. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Cahill, L.W. Adaptive Gaussian filter for noise reduction and edge detection. In Proceedings of the IEEE Nuclear Science Symposium & Medical Imaging Conference, San Francisco, CA, USA, 31 October–6 November 1993; IEEE: Piscataway, NJ, USA, 1994; pp. 1615–1619. [Google Scholar]
- Hsiao, P.Y.; Chen, C.H.; Chou, S.S.; Li, L.T.; Chen, S.J. A parameterizable digital-approximated 2D Gaussion smoothing filter for edge detection in noisy image. In Proceedings of the IEEE International Symposium on Circuits and Systems, Island of Kos, Greece, 21–24 May 2006; pp. 3189–3192. [Google Scholar]
- Direkoglu, C.; Nixon, M.S. Image-based multiscale shape description using gaussian filter. In Proceedings of the 6th Indian Conference on Computer Vision, Graphics and Image Processing, ICVGIP 2008, Bhubaneswar, India, 16–19 December 2008; pp. 673–678. [Google Scholar]
- Ding, L.; Goshtasby, A. On the canny edge detector. Pattern Recognit. 2001, 34, 721–725. [Google Scholar] [CrossRef]
- Song, R.; Zhang, Z.; Liu, H. Edge connection based Canny edge detection algorithm. Pattern Recognit. Image Anal. 2017, 27, 740–747. [Google Scholar] [CrossRef]
- Vujosevic, S.; Toma, C.; Villani, E.; Gatti, V.; Brambilla, M.; Muraca, A.; Ponziani, M.C.; Aimaretti, G.; Nuzzo, A.; Nucci, P.; et al. Early detection of microvascular changes in patients with diabetes mellitus without and with diabetic retinopaty: Comparison between different swept-source OCT-A Instruments. J. Diabetes. Res. 2019, 2019, 2547216. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, G.R.; Houghton, O.M.; Oldenburg, A.L. Automated segmentation of intraretinal cystoid fluid in optical coherence tomography. IEEE Trans. Biomed. Eng. 2012, 59, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Remeseiro, B.; Ortega, M.; Penedo, M.G.; Charlon, P. Automatic cyst detection in OCT retinal images combining region flooding and texture analysis. In Proceedings of the CBMS 2013—26th IEEE International Symposium on Computer-Based Medical Systems, Porto, Portugal, 20–22 June 2013; pp. 397–400. [Google Scholar]
- Gopinath, K.; Sivaswamy, J. Segmentation of Retinal Cysts from Optical Coherence Tomography Volumes Via Selective Enhancement. IEEE J. Biomed. Heal. Informatics 2019, 23, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.H.; Green, K.M.; Kwon, J.; Chu, Z.; Zhang, Q.; Wang, R.K.; Garrity, S.; Sarraf, D.; Rebhun, C.B.; Waheed, N.K.; et al. Suspended Scattering Particles in Motion: A Novel Feature of OCT Angiography in Exudative Maculopathies. Ophthalmol. Retin. 2018, 2, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Parravano, M.; Costanzo, E.; Borrelli, E.; Sacconi, R.; Virgili, G.; Sadda, S.V.R.; Scarinci, F.; Varano, M.; Bandello, F.; Querques, G. Appearance of cysts and capillary non perfusion areas in diabetic macular edema using two different OCTA devices. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zheng, F.; Motulsky, E.H.; Gregori, G.; Chu, Z.; Chen, C.L.; Li, C.; De Sisternes, L.; Durbin, M.; Rosenfeld, P.J.; et al. A novel strategy for quantifying choriocapillaris flow voids using swept-source OCT angiography. Investig. Ophthalmol. Vis. Sci. 2018, 59, 203–211. [Google Scholar] [CrossRef] [PubMed]


| Index | Value [%] |
|---|---|
| 98.97 | |
| 99.49 | |
| 90.64 | |
| 99.62 | |
| 99.47 | |
| 99.48 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torti, E.; Toma, C.; Vujosevic, S.; Nucci, P.; De Cillà, S.; Leporati, F. Cyst Detection and Motion Artifact Elimination in Enface Optical Coherence Tomography Angiograms. Appl. Sci. 2020, 10, 3994. https://doi.org/10.3390/app10113994
Torti E, Toma C, Vujosevic S, Nucci P, De Cillà S, Leporati F. Cyst Detection and Motion Artifact Elimination in Enface Optical Coherence Tomography Angiograms. Applied Sciences. 2020; 10(11):3994. https://doi.org/10.3390/app10113994
Chicago/Turabian StyleTorti, Emanuele, Caterina Toma, Stela Vujosevic, Paolo Nucci, Stefano De Cillà, and Francesco Leporati. 2020. "Cyst Detection and Motion Artifact Elimination in Enface Optical Coherence Tomography Angiograms" Applied Sciences 10, no. 11: 3994. https://doi.org/10.3390/app10113994
APA StyleTorti, E., Toma, C., Vujosevic, S., Nucci, P., De Cillà, S., & Leporati, F. (2020). Cyst Detection and Motion Artifact Elimination in Enface Optical Coherence Tomography Angiograms. Applied Sciences, 10(11), 3994. https://doi.org/10.3390/app10113994








