High-Efficient Micro Reacting Pipe with 3D Internal Structure: Design, Flow Simulation, and Metal Additive Manufacturing
Abstract
1. Introduction
2. Research Preparation
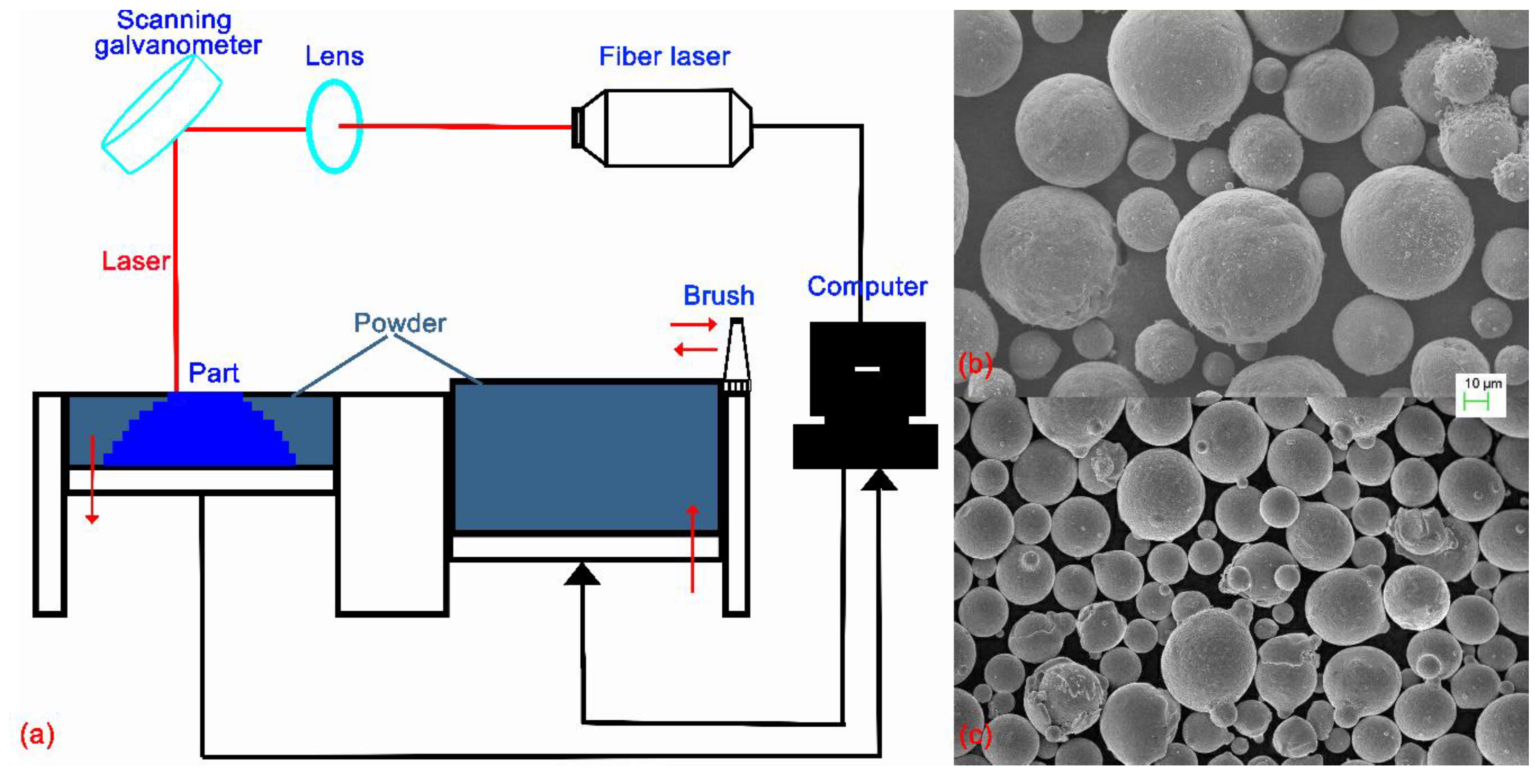
2.1. Equipment and Materials
2.2. Research Procedure
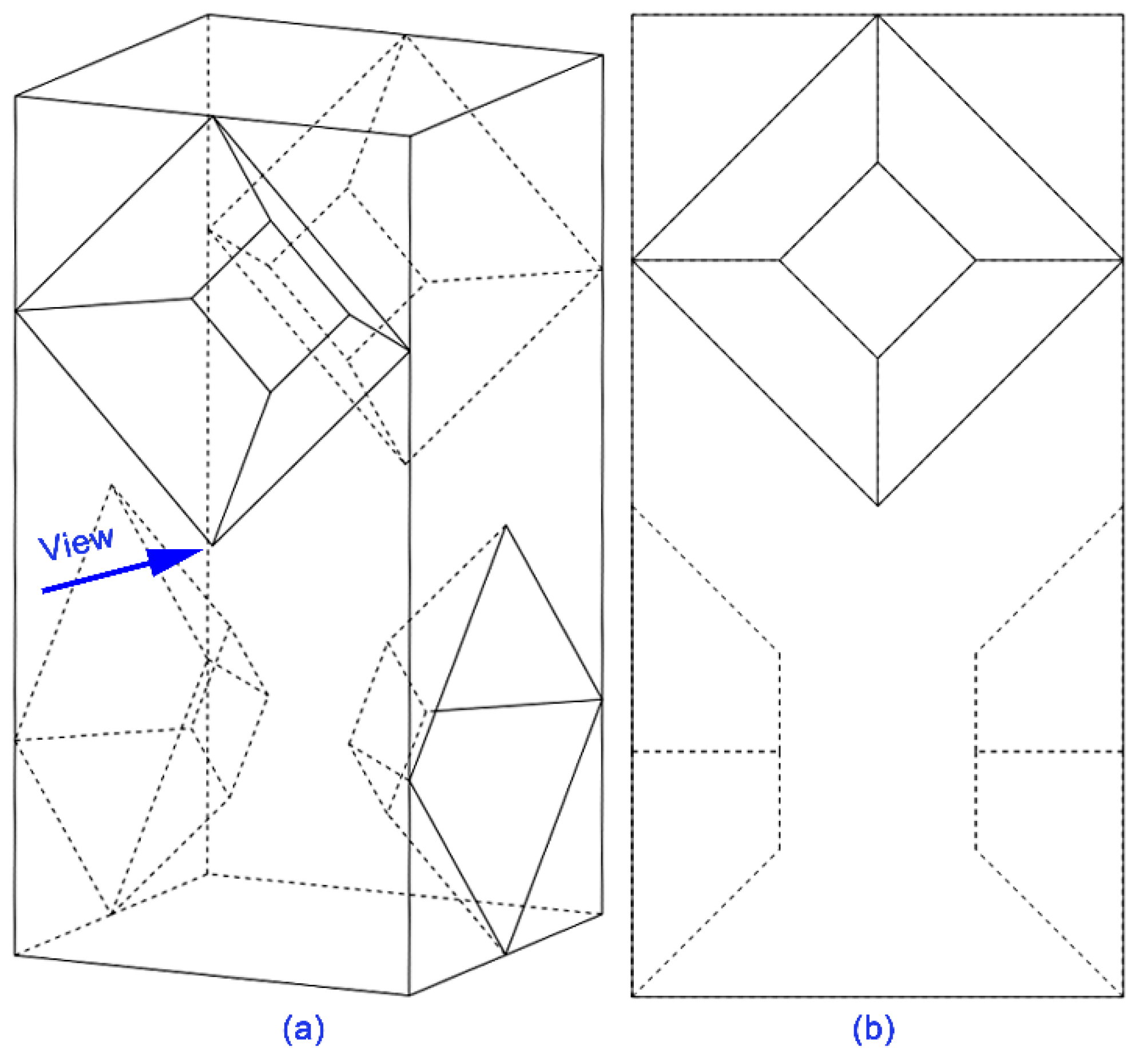
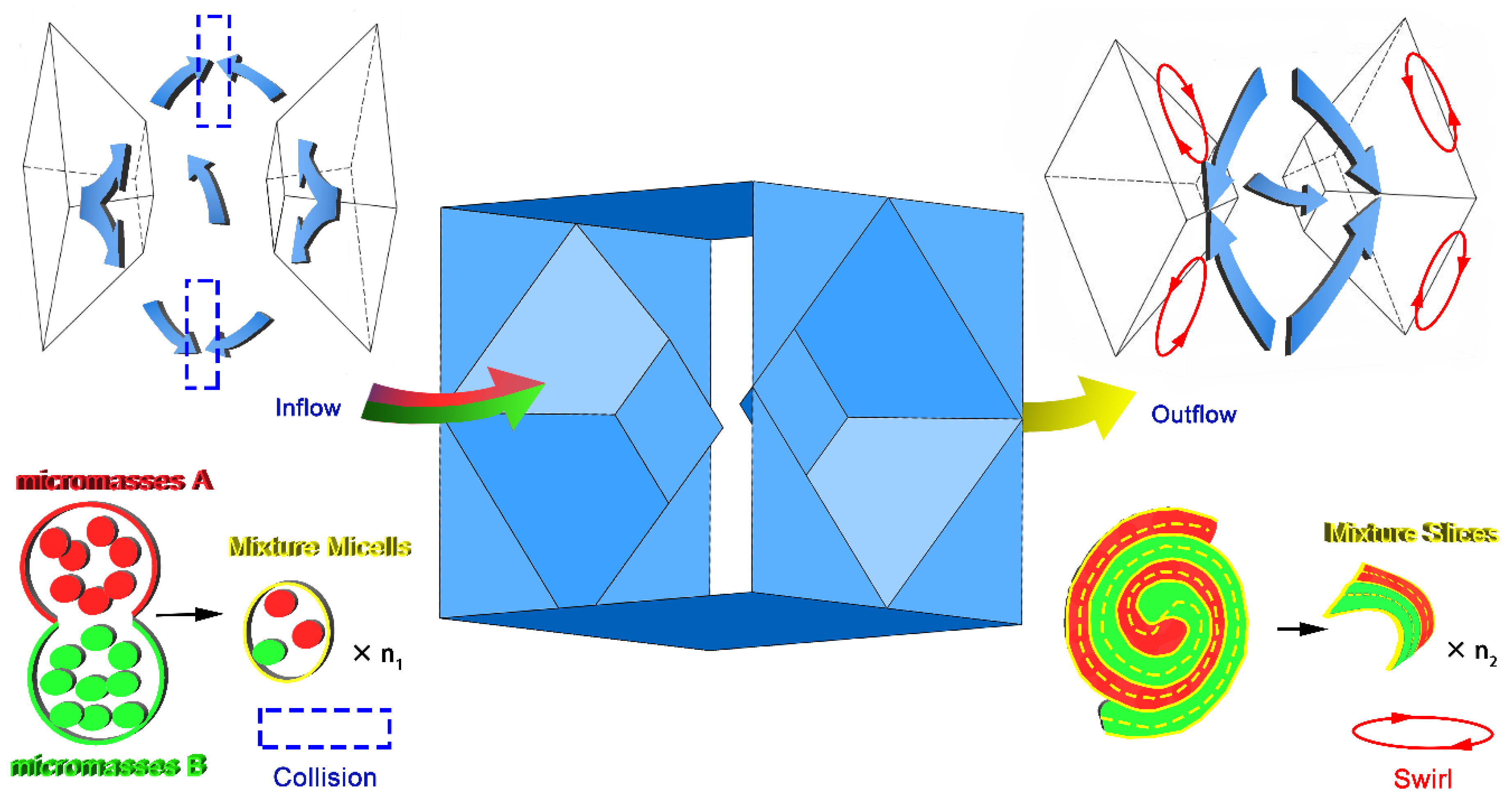
2.3. Design of Micro Reacting Pipe
2.4. Parameter Settings and Characterization Method in CFD
2.5. Experimental Method to Characterize Mixing Efficiency
2.5.1. The Process of Villermaux–Dushman Reaction System
2.5.2. Determination of
3. Results and Discussions
3.1. Practicality Experiment Results of Micro Reacting Pipe
3.1.1. Metal AM Printing and Compression Resistance Test of Micro Reacting Pipe
3.1.2. Comparison of Corrosion Resistance between Two Materials
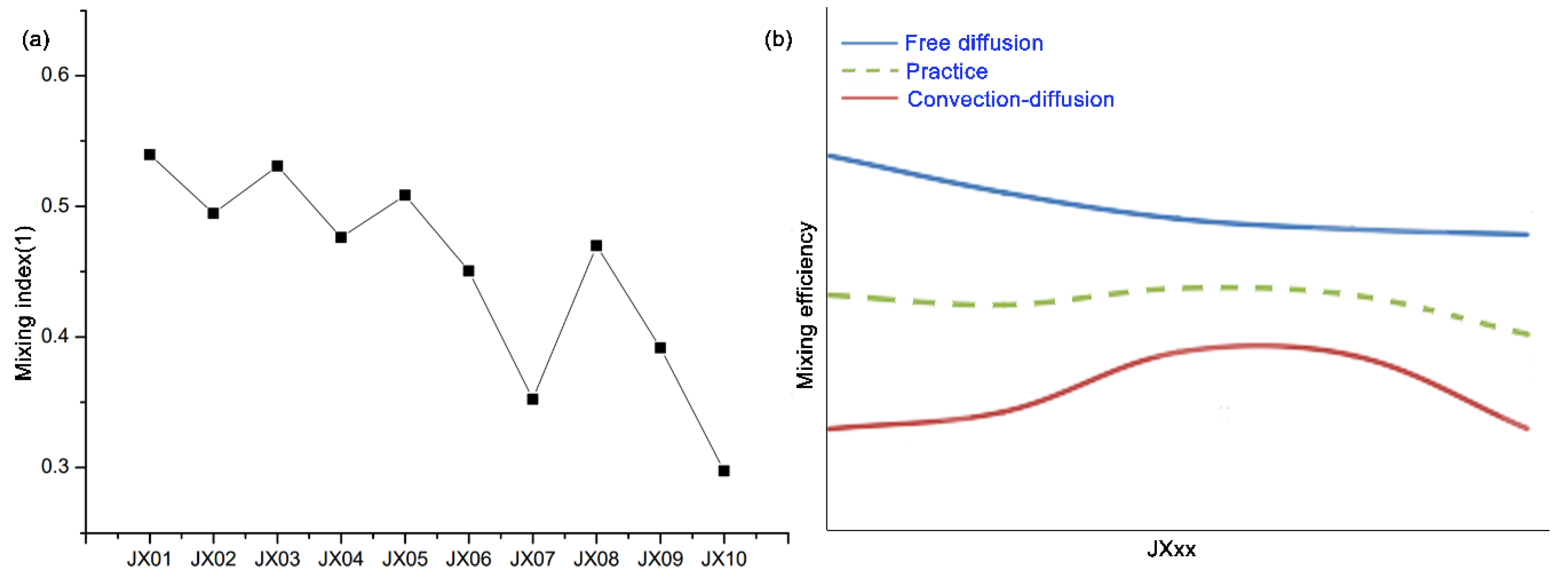
3.1.3. Test Results of Physical Mixing Efficiency of Micro Reacting Pipe
3.2. Simulation Results of Micro Reacting Pipe and Selection of Model Parameters
3.2.1. Selection of Model Parameters and the Three Design Principles
- The tilt angle of the internal structure is greater than or equal to 45° to avoid warping during laser melting;
- The critical dimension of the micro reacting pipe’s channel should be reduced as much as possible to enhance the mixing efficiency dominated by free diffusion;
- To enhance the convection-diffusion dominated mixing efficiency, the fluid in the micro reacting pipe channel should be put in a turbulent state.
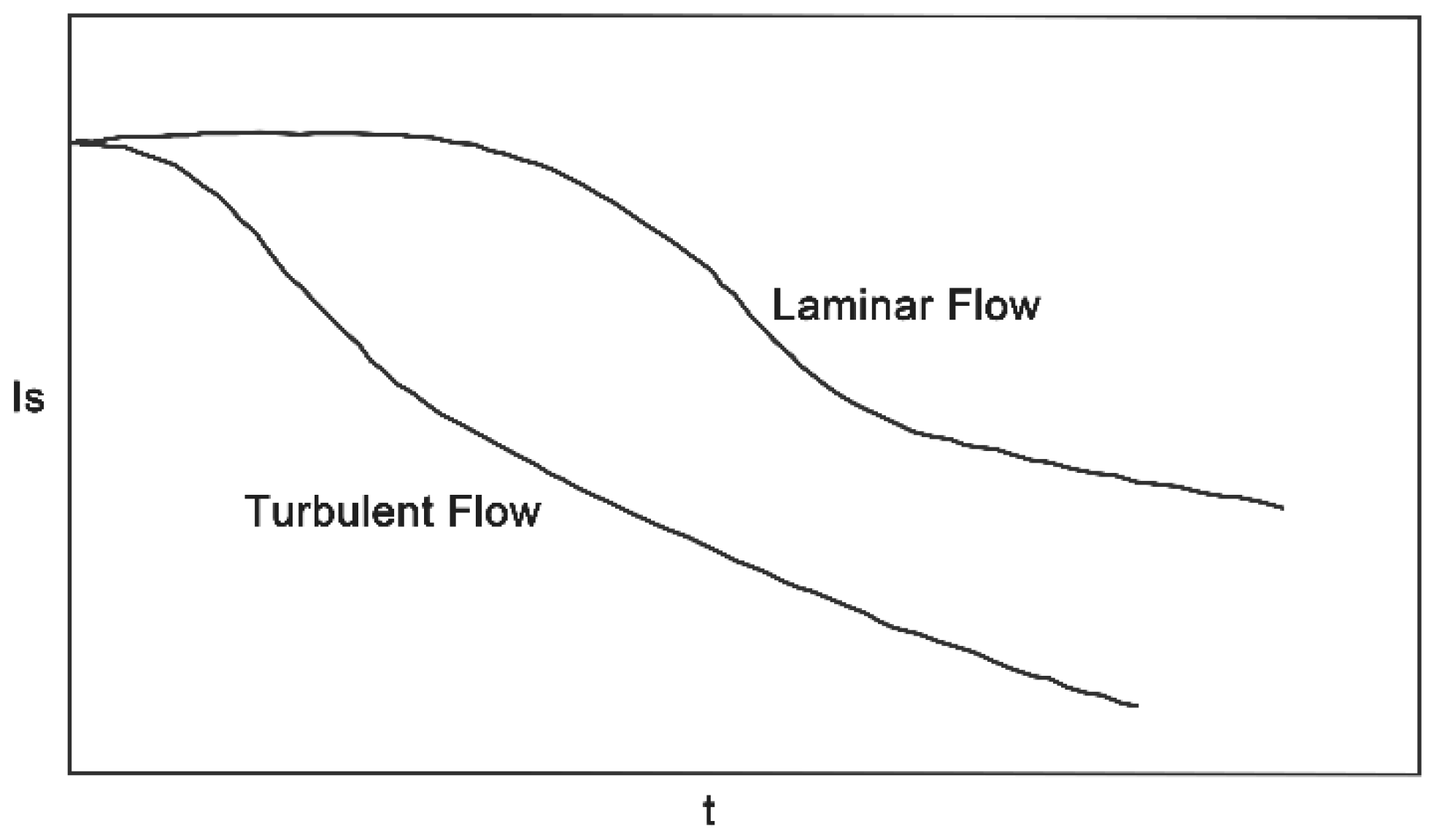
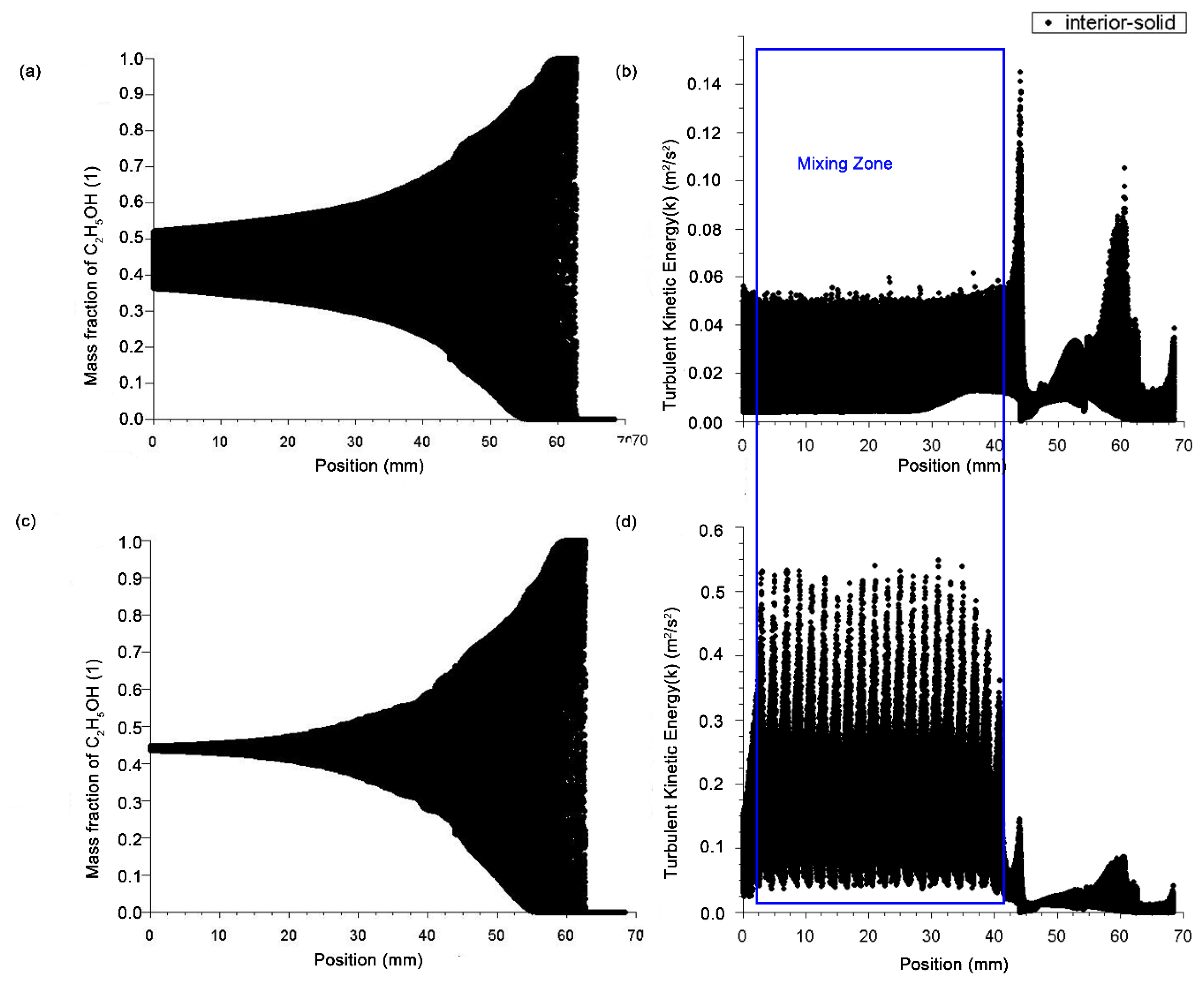
3.2.2. Simulation Results of Micro Reacting Pipe
- Reducing the micromixing distance and improving the mixing efficiency by means of sudden contraction and sudden enlargement of the cross-section of the micro reacting pipe;
- The fluid can be separated by means of a structure with a sharp edge;
- The fluid confluence can be guided by an inclined plane with a certain angle between the wall of the tube and the surfaces of the obstacles.
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Luong, J.; Hua, Y.; Gras, R.; Yang, X.; Yang, P. Post-column reaction with a 3D-printed two-stage microreactor and flame ionization detection for carbon compound independent response in fast gas chromatography. J. Chromatogr. A 2020, 1609, 460. [Google Scholar] [CrossRef] [PubMed]
- Lin, W. Design and Application of Microreactor: Mixing of microfluidics, 1st ed.; Chemical Industry Press: Beijing, China, 2016; pp. 16–45. [Google Scholar]
- Liu, Z.; Zhang, P. Applications of microreactor in chemistry and chemical engineering. Chem. Ind. Eng. Prog. 2016, 35, 10–11. [Google Scholar]
- McPeak, K.; Opasanont, B.; Shibata, T.; Ko, D.; Becker, M.; Chattopadhyay, S.; Bui, H.; Beebe, T.; Bunker, B.; Murray, C.; et al. Microreactor Chemical Bath Deposition of Laterally Graded Cd1−xZnxS Thin Films: A Route to High-Throughput Optimization for Photovoltaic Buffer Layers. Chem. Mater. 2013, 25, 297–306. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P. Recent Advances in Micro Reaction Technology. Chem. Commun. 2011, 47, 6512–6535. [Google Scholar] [CrossRef]
- Zhang, F.; Marre, S.; Erriguible, A. Mixing intensification under turbulent conditions in a high pressure microreactor. Chem. Eng. J. 2020, 382, 94–101. [Google Scholar] [CrossRef]
- Jeon, W.J.; Shin, C.B. Design and simulation of passive mixing in microfluidic systems with geometric variations. Chem. Eng. J. 2009, 152, 575–582. [Google Scholar] [CrossRef]
- Shih, T.R.; Chung, C.K. A high-efficiency planar micromixer with convection and diffusion mixing over a wide Reynolds number range. Microfluid. Nanofluidics 2008, 5, 175–183. [Google Scholar] [CrossRef]
- Shi, H.; Nie, K.; Dong, B.; Chao, L.; Gao, F.; Ma, M.; Long, M.; Liu, Z. Mixing enhancement via a serpentine micromixer for real-time activation of carboxyl. Chem. Eng. J. 2020, 392. [Google Scholar] [CrossRef]
- Liu, Y.J.; Chen, P.Y.; Yang, J.Y.; Tsou, C.; Lee, Y.H.; Baldeck, P.L.; Lin, C.L. Three-Dimensional Passive Micromixer Fabricated by Two-Photon Polymerization. Sens. Mater. 2014, 26, 39–44. [Google Scholar]
- Schirmer, M.; Uhlemann, J.; Rebenklau, L.; Bauer, T.; Wolter, K.J. 3D-microfluidic reactor in LTCC. In Proceedings of the 2008 31st International Spring Seminar on Electronics Technology, Budapest, Hungary, 7–11 May 2008. [Google Scholar]
- Bhivgade, U.; Maralla, Y.; Bhanvase, B.A.; Sonawane, S. Hydrodynamic, Mass Transfer and RTD Studies of Fluid Flow in a Spiral Microreactor. J. Inst. Eng. (India) Ser. E 2019, 100, 139–146. [Google Scholar] [CrossRef]
- Naher, S.; Orpen, D.; Brabazon, D.; Poulsen, C.R.; Morshed, M.M. Effect of micro-channel geometry on fluid flow and mixing. Simul. Model. Pract. Theory 2011, 19, 1088–1095. [Google Scholar] [CrossRef]
- Ansari, M.A.; Kim, K.Y.; Anwar, K.; Kim, S.M. A novel passive micromixer based on unbalanced splits and collisions of fluid streams. J. Micromech. Microeng. 2010, 20, 1–10. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X. A novel design for 3D passive micromixer based on Cantor fractal structure. Microsyst. Technol. 2018, 5, 1–12. [Google Scholar] [CrossRef]
- Chen, X.; Li, T. A novel passive micromixer designed by applying an optimization algorithm to the zigzag microchannel. Chem. Eng. J. 2016, 313, 1406–1414. [Google Scholar] [CrossRef]
- Frazier, W.E. Metal Additive Manufacturing: A Review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Wong, K.V.; Hernandez, A. A Review of Additive Manufacturing. ISRN Mech. Eng. 2012, 1–10. [Google Scholar] [CrossRef]
- Gu, D.D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser additive manufacturing of metallic components: Materials, processes and mechanisms. Int. Mater. Rev. 2012, 57, 133–164. [Google Scholar] [CrossRef]
- Okafor, O.; Weilhard, A.; Fernandes, J.A.; Karjalainen, E.; Goodridgea, R.; Sans, V. Advanced reactor engineering with 3D printing for the continuous-flow synthesis of silver nanoparticles. React. Chem. Eng. 2017, 2, 129–136. [Google Scholar] [CrossRef]
- Fornells, E.; Murray, E.; Waheed, S.; Morrin, A.; Diamond, D.; Paull, B.; Breadmore, M. Integrated 3D printed heaters for microfluidic applications: Ammonium analysis within environmental water. Anal. Chim. Acta 2020, 1098, 94–101. [Google Scholar] [CrossRef]
- Meng, J.; Loh, N.H.; Fu, G.; Tor, S.B.; Tay, B.Y. Replication and characterization of 316L stainless steel micro-mixer by micro powder injection molding. J. Alloys Compd. 2010, 496, 293–299. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Fan, Y.; Zhou, W. Fabrication of porous metal by selective laser melting as catalyst support for hydrogen production microreactor. Int. J. Hydrog. Energy 2020, 45, 10–22. [Google Scholar] [CrossRef]
- Kathryn, L.K.; Karen, A.T. Experimental Investigation of Numerically Optimized Wavy Microchannels Created Through Additive Manufacturing. J. Turbomach. 2018, 140, 021002. [Google Scholar]
- Santana, H.S.; da Silva, A.G.P.; Lopes, M.G.M.; Rodrigues, A.C.; Taranto, O.P.; Silva, J.L. Computational methodology for the development of microdevices and microreactors with ANSYS CFX. MethodsX 2020, 7, 82–103. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.S.; Curtis, K.S.; Karen, A.T.; Dominic, J.M. Build Direction Effects on Microchannel Tolerance and Surface Roughness. J. Mech. Des. 2015, 137, 111411. [Google Scholar]
- Investigation of crystal growth mechanism during selective laser melting and mechanical property characterization of 316L stainless steel parts. Mater. Des. 2016, 100, 291–299. [CrossRef]
- Wang, D.; Yang, Y.Q.; Liu, R.C.; Xiao, D.M.; Sun, J.F. Study on the designing rules and processability of porous structure based on selective laser melting (SLM). J. Mater. Process. Technol. 2013, 213, 1734–1742. [Google Scholar] [CrossRef]
- Launder, B.E.; Spalding, D.B. The numerical computation of turbulent flows. Comput. Methods Appl. Mech. Eng. 1974, 32, 269–289. [Google Scholar] [CrossRef]
- Fluent Inc. Fluent 6.0 Users Guide; Fluent Inc.: Lebanon, NH, USA, 2001. [Google Scholar]
- Ansari, M.A.; Kim, K. A numerical study of mixing in a microchannel with circular mixing chambers. AICHE J. 2009, 55, 2217–2225. [Google Scholar] [CrossRef]
- Guichardon, P.; Falk, L. Characterisation of micromixing efficiency by the iodide–iodate reaction system. Part, I. Experimental procedure. Chem. Eng. Sci. 2000, 55, 4233–4243. [Google Scholar] [CrossRef]
- Palmer, D.A.; Ramette, R.W.; Mesmer, R.E. Triodide ion formation equilibrium and activity coefficients in aqueous solution. J. Solut. Chem. 1984, 13, 673–683. [Google Scholar] [CrossRef]
- Commoner, B.; Lipkin, D. The Application of the Beer-Lambert Law to Optically Anisotropic Systems. Science 1949, 110, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Foumier, M.C.; Falk, L.; Villermaux, J. A New Parallel Competing Reaction System for Assessing Micromixing Efficiency-Experimental Approach. Chem. Eng. Sci. 1996, 51, 5053–5064. [Google Scholar]
- Kjäldman, L.; Brink, A.; Hupa, M. Micro Mixing Time in the Eddy Dissipation Concept. Combust. Sci. Technol. 2000, 154, 207–227. [Google Scholar] [CrossRef]
- Hessel, V.; Löwe, H.; Schönfeld, F. Micromixers-a review on passive and active mixing principles. Chem. Eng. Sci. 2005, 60, 2479–2501. [Google Scholar] [CrossRef]
- Jiang, F.; Drese, K.S.; Hardt, S.; Küpper, M.; Schönfeld, F. Helical flows and chaotic mixing in curved micro channels. AICHE J. 2004, 50, 2297–2305. [Google Scholar] [CrossRef]
- Commenge, J.M.; Laurent, F. Villermaux-Dushman protocol for experimental characterization of micromixers. Chem. Eng. Process. Process. Intensif. 2011, 50, 979–990. [Google Scholar] [CrossRef]







| Material | Cr | Fe | Mo | Si | Mn | Ni | Ti | Al | P | Nb | N | Cu | O | C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IN718 | 19.20 | 17.32 | 3.17 | 0.33 | 0.23 | 52.91 | 0.65 | 0.54 | 0.10 | 5.16 | 0.14 | 0.13 | - | - |
| 316LSS | 17.5 | Bal | 2.06 | 0.86 | 0.3 | 12.06 | - | - | - | - | - | - | 0.09 | 0.03 |
| ID | Mass before the Experiment (g) | Mass after the Experiment (g) | Mass Loss (%) | Duration (h) | Medium |
|---|---|---|---|---|---|
| Sample 1 | 9.5814 | 9.5810 | 0.0042 | 24 | PBr3 |
| Sample 2 | 9.5810 | 9.5810 | 0.0000 | 24 | PBr3 |
| Sample 3 | 9.5810 | 9.5753 | 0.0595 | 24 | 3% HCl |
| Sample 4 | 9.5753 | 9.5737 | 0.0167 | 24 | 3% HCl |
| ID | Mass before the Experiment (g) | Mass after the Experiment (g) | Mass Loss (%) | Duration (h) | Medium |
|---|---|---|---|---|---|
| Sample 1 | 5.0909 | 5.0881 | 0.055 | 24 | PBr3 |
| Sample 2 | 5.0881 | 5.0881 | 0 | 24 | PBr3 |
| Sample 3 | 5.0881 | 5.0727 | 0.3027 | 24 | 3% HCl |
| Sample 4 | 5.0727 | 5.0573 | 0.3036 | 24 | 3% HCl |
| Chemical Constituents | Initial Concentration (mol/L) |
|---|---|
| H+ | 0.05/0.06 |
| IO3− | 0.00233 |
| H3BO3 | 0.1818 |
| I− | 0.01167 |
| NaOH | 0.0909 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wang, D.; Mai, J.; Chen, X.; Dou, W. High-Efficient Micro Reacting Pipe with 3D Internal Structure: Design, Flow Simulation, and Metal Additive Manufacturing. Appl. Sci. 2020, 10, 3779. https://doi.org/10.3390/app10113779
Chen X, Wang D, Mai J, Chen X, Dou W. High-Efficient Micro Reacting Pipe with 3D Internal Structure: Design, Flow Simulation, and Metal Additive Manufacturing. Applied Sciences. 2020; 10(11):3779. https://doi.org/10.3390/app10113779
Chicago/Turabian StyleChen, Xiaomin, Di Wang, Jingming Mai, Xiaojun Chen, and Wenhao Dou. 2020. "High-Efficient Micro Reacting Pipe with 3D Internal Structure: Design, Flow Simulation, and Metal Additive Manufacturing" Applied Sciences 10, no. 11: 3779. https://doi.org/10.3390/app10113779
APA StyleChen, X., Wang, D., Mai, J., Chen, X., & Dou, W. (2020). High-Efficient Micro Reacting Pipe with 3D Internal Structure: Design, Flow Simulation, and Metal Additive Manufacturing. Applied Sciences, 10(11), 3779. https://doi.org/10.3390/app10113779






