The Cytotoxic Effect of Newly Synthesized Ferrocenes against Cervical Carcinoma Cells Alone and in Combination with Radiotherapy
Abstract
1. Introduction
2. Materials and Methods
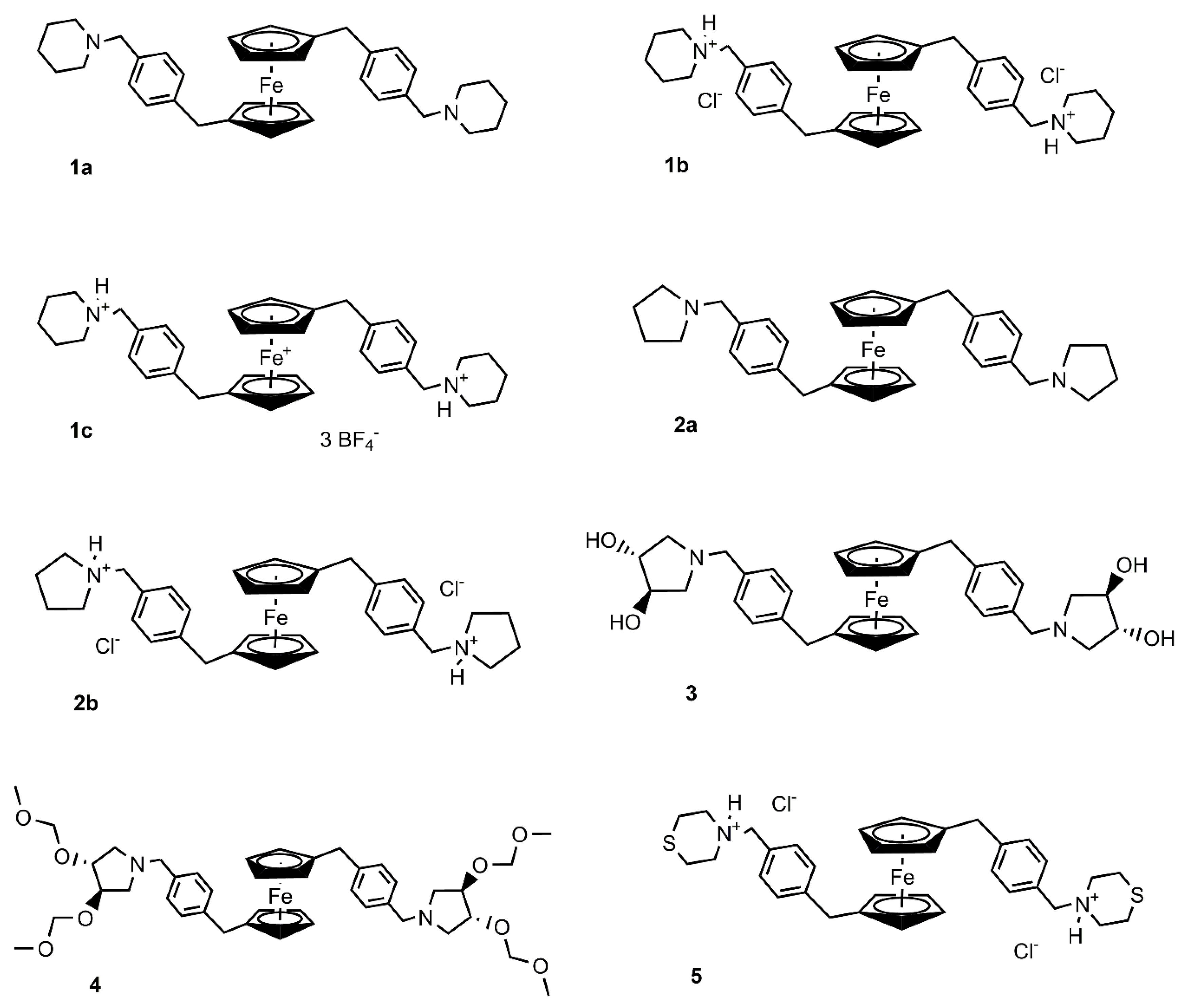
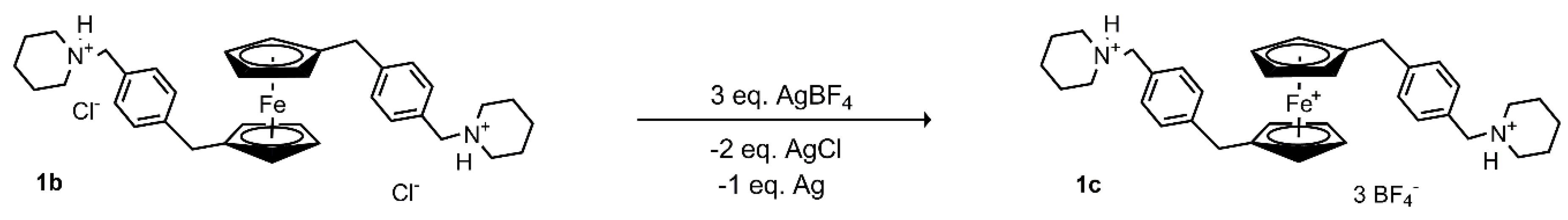
2.1. Preparation and Characterization of Ferrocenes
2.2. Cell Lines and Cultivation
2.3. Cell Viability Assay
2.4. Cell Cycle
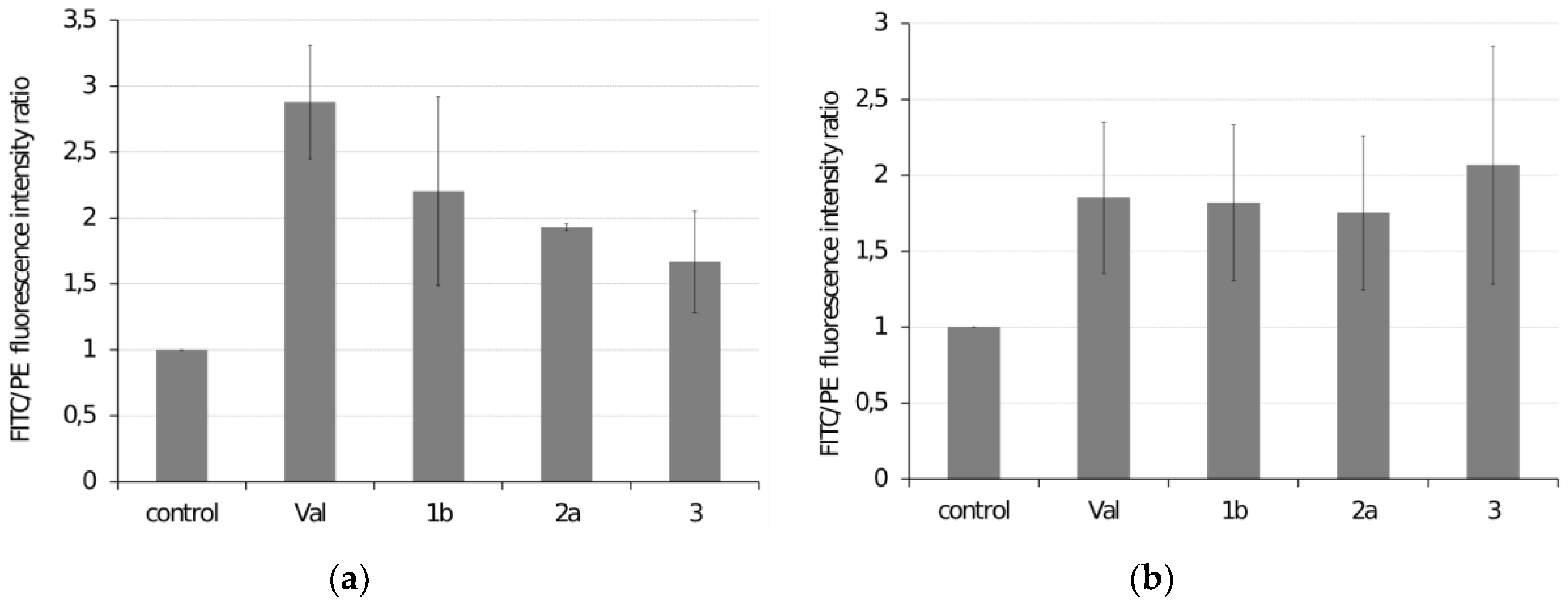
2.5. Reactive Oxygen Species (ROS) Production
2.6. Mitochondrial Membrane Potential Changes
2.7. Annexin V–Fluorescein Isothiocyanate (FITC)/PI Binding Assay
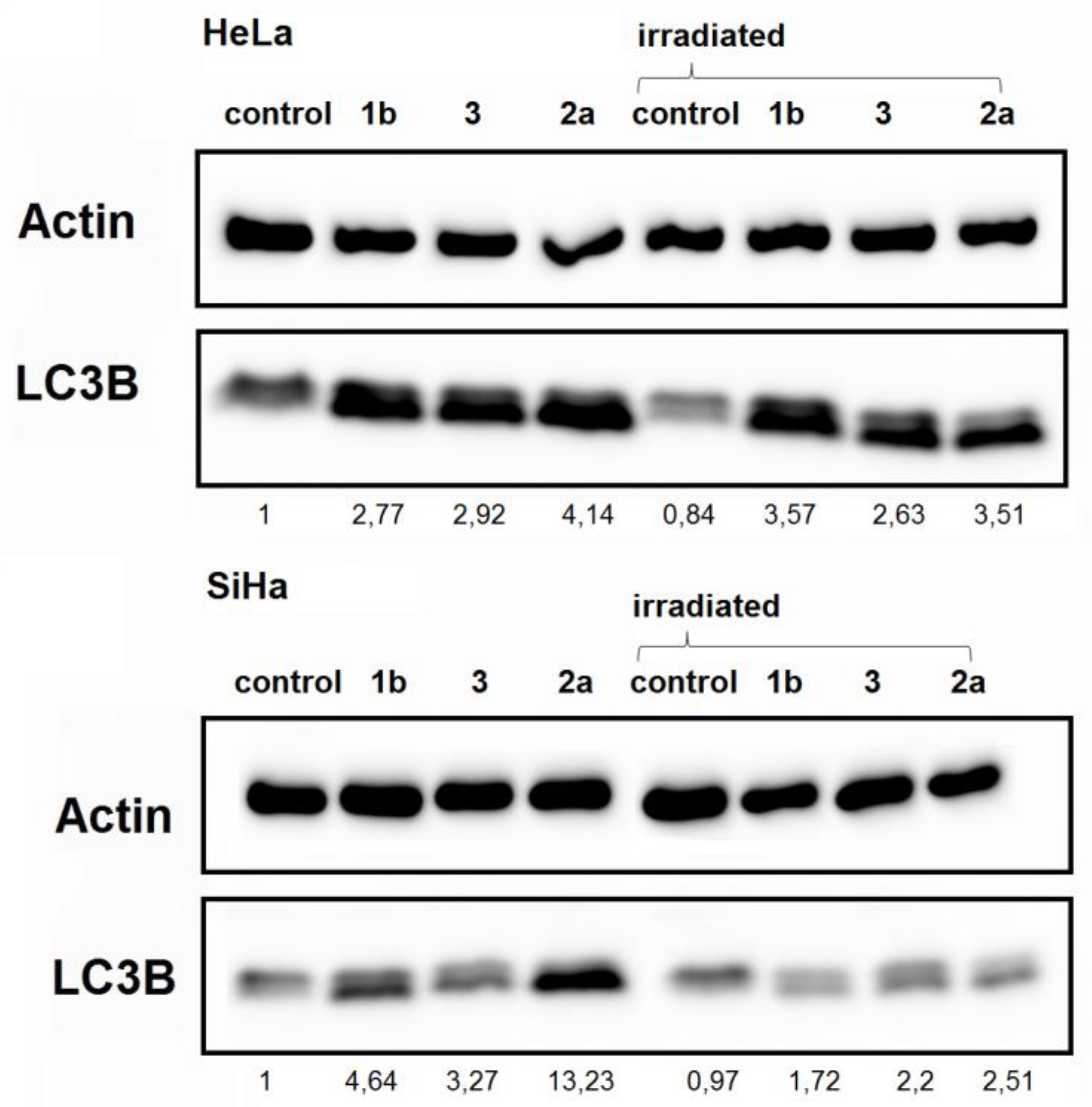
2.8. Western Blot Analysis
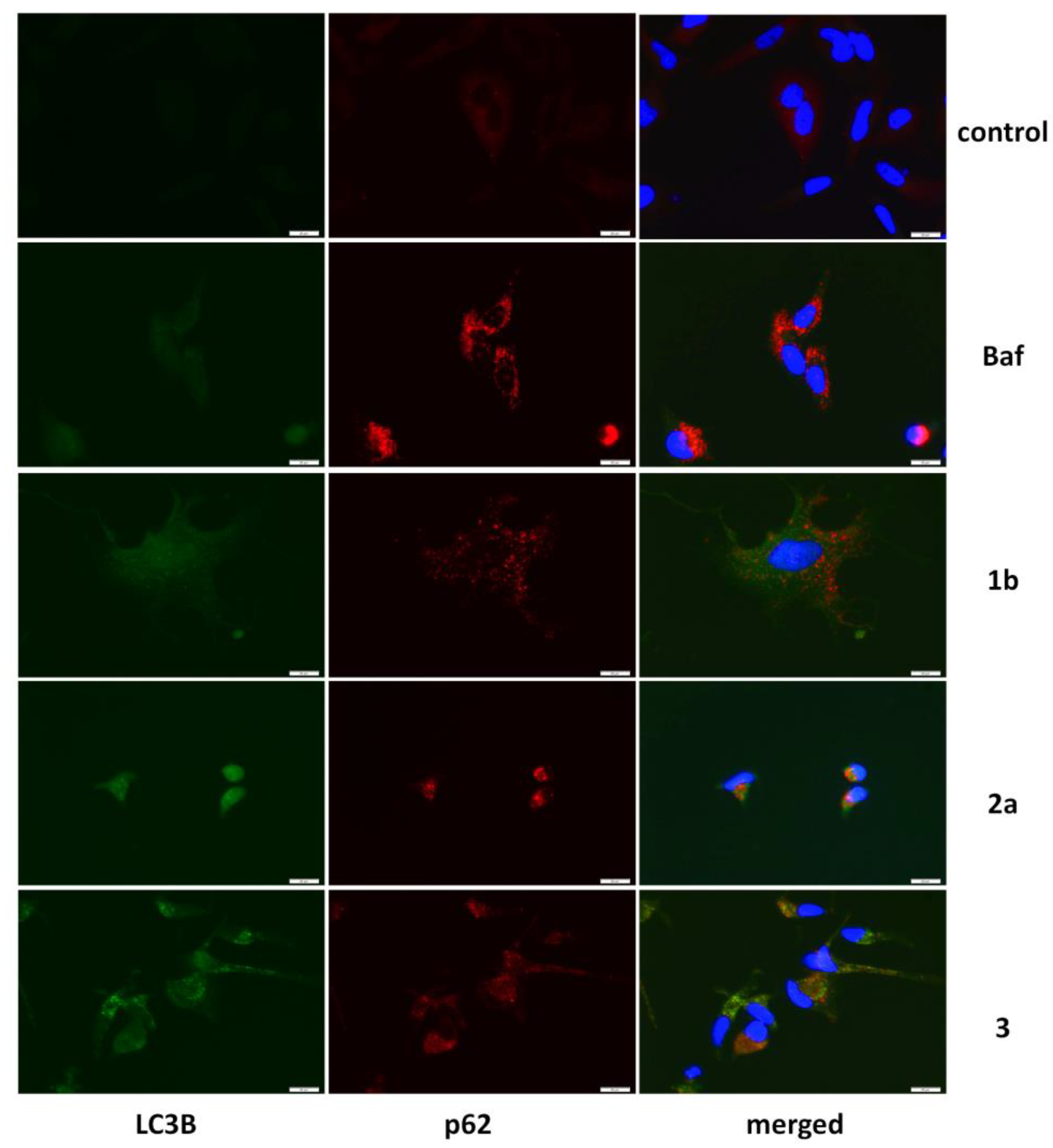
2.9. Immunofluorescence Staining
2.10. Colony Forming Assay (CFA)
2.11. Ionizing Radiation
3. Results
3.1. Cytotoxic Activity
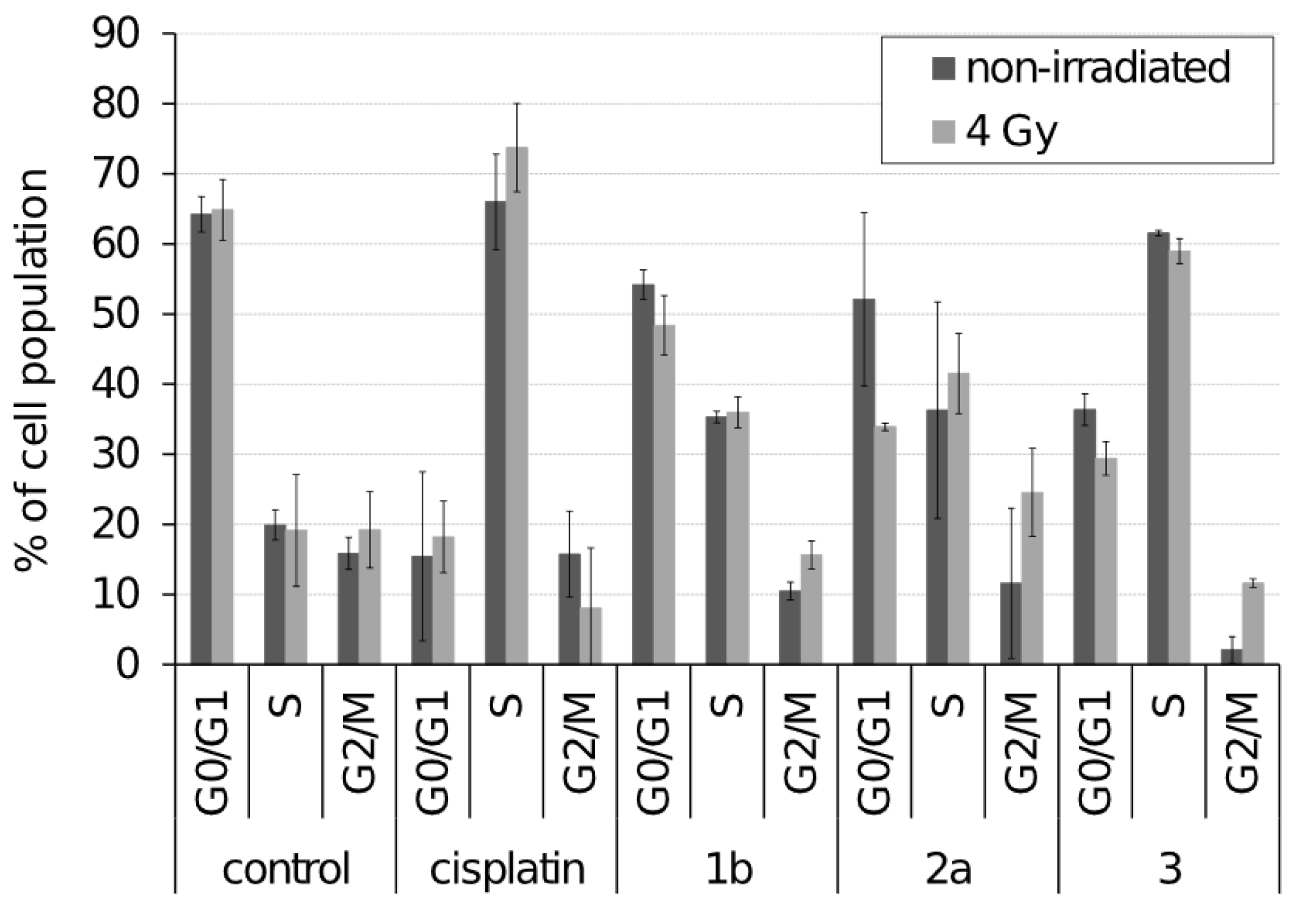
3.2. Effect of Selected Ferrocenes on Cell Cycle
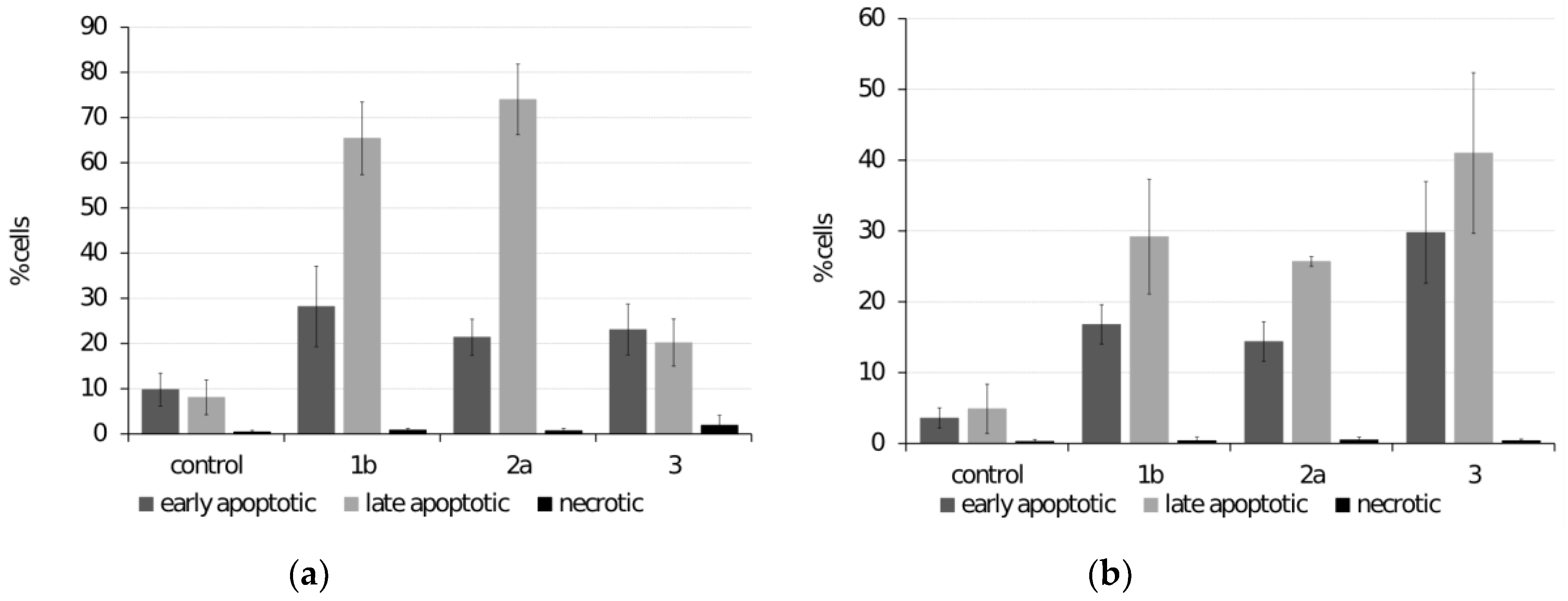
3.3. Analysis of Cell Death
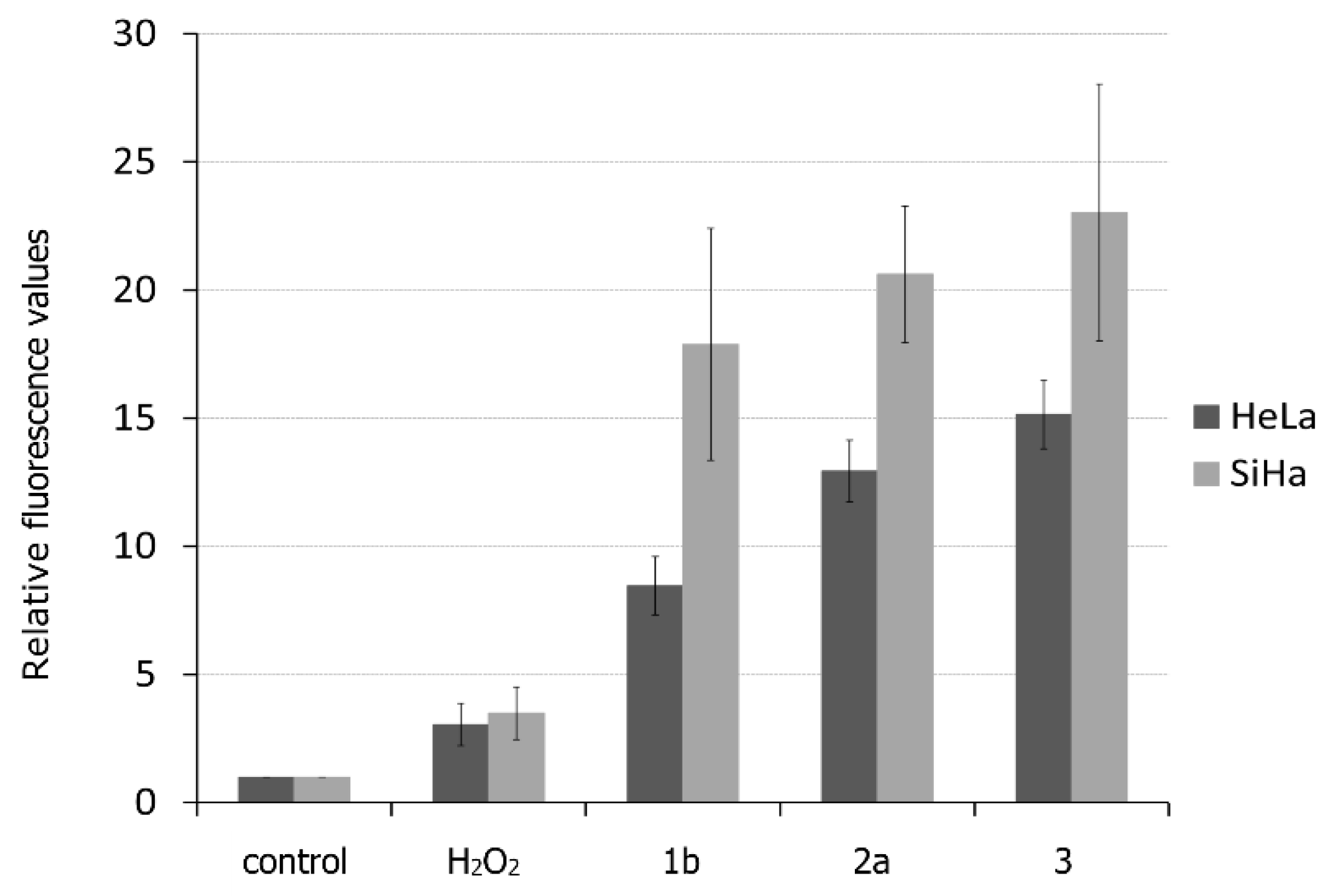
3.3.1. Effect of Selected Ferrocenes on ROS Production
3.3.2. Effect of 1b, 2a, and 3 on Mitochondrial Membrane Potential
3.3.3. Analysis of Apoptosis
3.4. Autophagy Detection
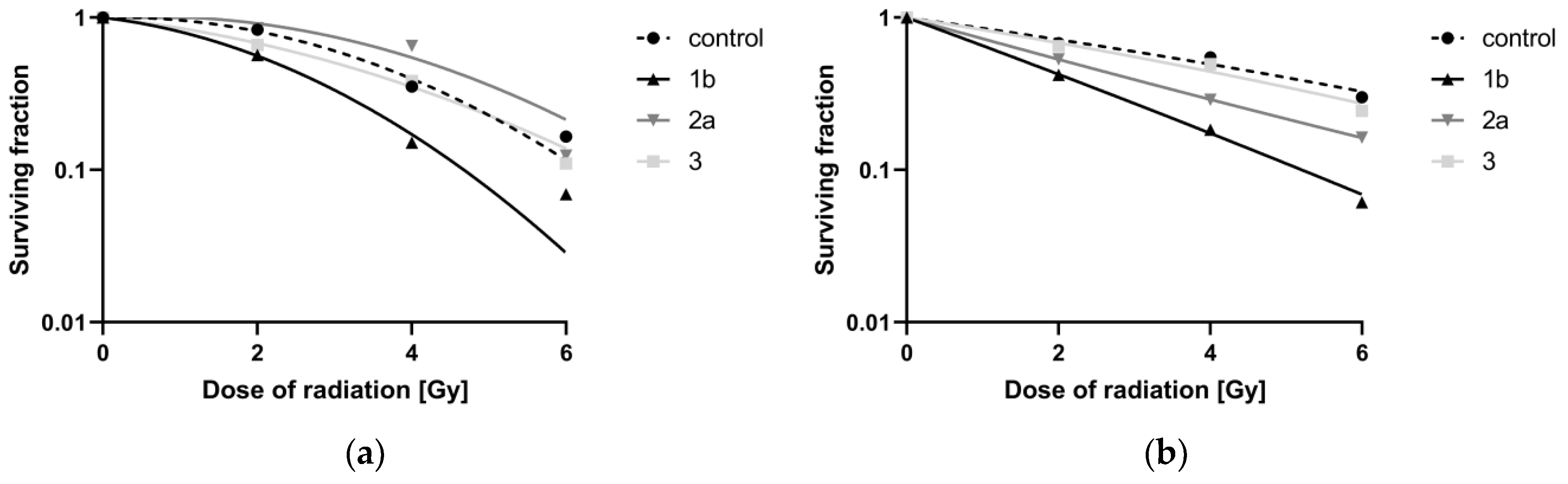
3.5. Sensitivity to Ionizing Radiation
3.5.1. Determination of the Surviving Fraction
3.5.2. Evaluation of Dose-Modifying Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer. Cancer Incidence in Five Continents Time Trends. Available online: http://ci5.iarc.fr/CI5plus/Default.aspx (accessed on 24 June 2018).
- Chovanec, J.; Náležinská, M. Přehled diagnostiky a léčby karcinomu děložního hrdla. Onkologie 2014, 8, 269–274. [Google Scholar]
- Ferlay, J.; Colombet, M.; Bray, F. Cancer Incidence in Five Continents, CI5plus: IARC CancerBase No. 9. Available online: http://ci5.iarc.fr (accessed on 18 October 2019).
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Munoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 262. [Google Scholar] [CrossRef] [PubMed]
- Vale, C.; Tierney, J.F.; Stewart, L.A.; Brady, M.; Dinshaw, K.; Jakobsen, A.; Parmar, M.K.; Thomas, G.; Trimble, T.; Alberts, D.S.; et al. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: A systematic review and meta-analysis of individual patient data from 18 randomized trials. J. Clin. Oncol. 2008, 26, 5802–5812. [Google Scholar]
- Galanski, M.; Jakupec, M.A.; Keppler, B.K. Update of the preclinical situation of anticancer platinum complexes: Novel design strategies and innovative analytical approaches. Curr. Med. Chem. 2005, 12, 2075–2094. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.; Aderibigbe, B.A. Ferrocene-Based Compounds with Antimalaria/Anticancer Activity. Molecules 2019, 24, 3604. [Google Scholar] [CrossRef] [PubMed]
- Sirignano, E.; Saturnino, C.; Botta, A.; Sinicropi, M.S.; Caruso, A.; Pisano, A.; Lappano, R.; Maggiolini, M.; Longo, P. Synthesis, characterization and cytotoxic activity on breast cancer cells of new half-titanocene derivatives. Bioorg. Med. Chem. Lett. 2013, 23, 3458–3462. [Google Scholar] [CrossRef]
- Saturnino, C.; Sirignano, E.; Botta, A.; Sinicropi, M.S.; Caruso, A.; Pisano, A.; Lappano, R.; Maggiolini, M.; Longo, P. New titanocene derivatives with high antiproliferative activity against breast cancer cells. Bioorg. Med. Chem. Lett. 2014, 24, 136–140. [Google Scholar] [CrossRef]
- Saturnino, C.; Barone, I.; Iacopetta, D.; Mariconda, A.; Sinicropi, M.S.; Rosano, C.; Campana, A.; Catalano, S.; Longo, P.; Ando, S. N-heterocyclic carbene complexes of silver and gold as novel tools against breast cancer progression. Future Med. Chem. 2016, 8, 2213–2229. [Google Scholar] [CrossRef]
- Iacopetta, D.; Mariconda, A.; Saturnino, C.; Caruso, A.; Palma, G.; Ceramella, J.; Muia, N.; Perri, M.; Sinicropi, M.S.; Caroleo, M.C.; et al. Novel Gold and Silver Carbene Complexes Exert Antitumor Effects Triggering the Reactive Oxygen Species Dependent Intrinsic Apoptotic Pathway. Chem. Med. Chem. 2017, 12, 2054–2065. [Google Scholar] [CrossRef]
- Alessio, E.; Messori, L. The Deceptively Similar Ruthenium(III) Drug Candidates KP1019 and NAMI-A Have Different Actions. What Did We Learn in the Past 30 Years? Met. Life Sci. 2018, 18, 141. [Google Scholar]
- Ari, F.; Cevatemre, B.; Armutak, E.I.; Aztopal, N.; Yilmaz, V.T.; Ulukaya, E. Apoptosis-inducing effect of a palladium(II) saccharinate complex of terpyridine on human breast cancer cells in vitro and in vivo. Bioorg. Med. Chem. 2014, 22, 4948–4954. [Google Scholar] [CrossRef]
- Carter, R.; Westhorpe, A.; Romero, M.J.; Habtemariam, A.; Gallevo, C.R.; Bark, Y.; Menezes, N.; Sadler, P.J.; Sharma, R.A. Radiosensitisation of human colorectal cancer cells by ruthenium(II) arene anticancer complexes. Sci. Rep. 2016, 6, 20596. [Google Scholar] [CrossRef]
- Mesbahi, A. A review on gold nanoparticles radiosensitization effect in radiation therapy of cancer. Rep. Pract. Oncol. Radiother. J. Greatpoland Cancer Cent. Pozn. Pol. Soc. Radiat. Oncol. 2010, 15, 176–180. [Google Scholar] [CrossRef]
- Gill, M.R.; Menon, J.U.; Jarman, P.J.; Owen, J.; Skaripa-Koukelli, I.; Able, S.; Thomas, J.A.; Carlisle, R.; Vallis, K.A. (111)In-labelled polymeric nanoparticles incorporating a ruthenium-based radiosensitizer for EGFR-targeted combination therapy in oesophageal cancer cells. Nanoscale 2018, 10, 10596–10608. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, 309–315. [Google Scholar] [CrossRef]
- Chang, M.Y.; Shiau, A.L.; Chen, Y.H.; Chang, C.J.; Chen, H.H.; Wu, C.L. Increased apoptotic potential and dose-enhancing effect of gold nanoparticles in combination with single-dose clinical electron beams on tumor-bearing mice. Cancer Sci. 2008, 99, 1479–1484. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Smilowitz, H.M.; O’Connor, M.J.; Dilmanian, F.A.; Slatkin, D.N. Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomedicine 2013, 8, 1601–1609. [Google Scholar] [CrossRef]
- Teicher, B.A.; Jacobs, J.L.; Cathcart, K.N.; Abrams, M.J.; Vollano, J.F.; Picker, D.H. Some complexes of cobalt(III) and iron(III) are radiosensitizers of hypoxic EMT6 cells. Radiat. Res. 1987, 109, 36–46. [Google Scholar] [CrossRef]
- Joy, A.M.; Goodgame, D.M.; Stratford, I.J. High efficiency of ferricenium salts as radiosensitizers of V79 cells in vitro and the KHT tumor in vivo. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 1053–1056. [Google Scholar] [CrossRef]
- Hodík, T.; Lamač, M.; Červenková Šťastná, L.; Cuřínová, P.; Karban, J.; Skoupilová, H.; Hrstka, R.; Císařová, I.; Gyepes, R.; Pinkas, J. Improving cytotoxic properties of ferrocenes by incorporation of saturated N-heterocycles. J. Organomet. Chem. 2017, 846, 141–151. [Google Scholar] [CrossRef]
- Bartosik, M.; Koubkova, L.; Karban, J.; Cervenkova Stastna, L.; Hodik, T.; Lamac, M.; Pinkas, J.; Hrstka, R. Electrochemical analysis of a novel ferrocene derivative as a potential antitumor drug. Analyst 2015, 140, 5864–5867. [Google Scholar] [CrossRef]
- Kvardova, V.; Hrstka, R.; Walerych, D.; Muller, P.; Matoulkova, E.; Hruskova, V.; Stelclova, D.; Sova, P.; Vojtesek, B. The new platinum(IV) derivative LA-12 shows stronger inhibitory effect on Hsp90 function compared to cisplatin. Mol. Cancer 2010, 9, 147. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Halicka, H.D.; Zhao, H. Analysis of cellular DNA content by flow and laser scanning cytometry. Adv. Exp. Med. Biol. 2010, 676, 137–147. [Google Scholar]
- Koubkova, L.; Vyzula, R.; Karban, J.; Pinkas, J.; Ondrouskova, E.; Vojtesek, B.; Hrstka, R. Evaluation of cytotoxic activity of titanocene difluorides and determination of their mechanism of action in ovarian cancer cells. Investig. New Drugs 2015, 33, 1123–1132. [Google Scholar] [CrossRef]
- Matarrese, P.; Tinari, A.; Mormone, E.; Bianco, G.A.; Toscano, M.A.; Ascione, B.; Rabinovich, G.A.; Malorni, W. Galectin-1 sensitizes resting human T lymphocytes to Fas (CD95)-mediated cell death via mitochondrial hyperpolarization, budding, and fission. J. Biol. Chem. 2005, 280, 6969–6985. [Google Scholar] [CrossRef]
- Ruan, Q.; Lesort, M.; MacDonald, M.E.; Johnson, G.V. Striatal cells from mutant huntingtin knock-in mice are selectively vulnerable to mitochondrial complex II inhibitor-induced cell death through a non-apoptotic pathway. Hum. Mol. Genet. 2004, 13, 669–681. [Google Scholar] [CrossRef][Green Version]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Starkov, A.A. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N. Y. Acad. Sci. 2008, 1147, 37–52. [Google Scholar] [CrossRef]
- Marchi, S.; Giorgi, C.; Suski, J.M.; Agnoletto, C.; Bononi, A.; Bonora, M.; De Marchi, E.; Missiroli, S.; Patergnani, S.; Poletti, F.; et al. Mitochondria-ros crosstalk in the control of cell death and aging. J. Signal Transduct. 2012, 2012, 329635. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Cossarizza, A.; Ceccarelli, D.; Masini, A. Functional heterogeneity of an isolated mitochondrial population revealed by cytofluorometric analysis at the single organelle level. Exp. Cell Res. 1996, 222, 84–94. [Google Scholar] [CrossRef]
- Di Lisa, F.; Blank, P.S.; Colonna, R.; Gambassi, G.; Silverman, H.S.; Stern, M.D.; Hansford, R.G. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J. Physiol. 1995, 486, 1–13. [Google Scholar] [CrossRef]
- Sick, T.J.; Perez-Pinzon, M.A. Optical methods for probing mitochondrial function in brain slices. Methods 1999, 18, 104–108. [Google Scholar] [CrossRef]
- White, R.J.; Reynolds, I.J. Mitochondrial depolarization in glutamate-stimulated neurons: An early signal specific to excitotoxin exposure. J. Neurosci. 1996, 16, 5688–5697. [Google Scholar] [CrossRef]
- Sekine, C.; Moriyama, Y.; Koyanagi, A.; Koyama, N.; Ogata, H.; Okumura, K.; Yagita, H. Differential regulation of splenic CD8- dendritic cells and marginal zone B cells by Notch ligands. Int. Immunol. 2009, 21, 295–301. [Google Scholar] [CrossRef]
- Galski, H.; Oved-Gelber, T.; Simanovsky, M.; Lazarovici, P.; Gottesman, M.M.; Nagler, A. P-glycoprotein-dependent resistance of cancer cells toward the extrinsic TRAIL apoptosis signaling pathway. Biochem. Pharm. 2013, 86, 584–596. [Google Scholar] [CrossRef]
- Seguier, S.; Tartour, E.; Guerin, C.; Couty, L.; Lemitre, M.; Lallement, L.; Folliguet, M.; El Naderi, S.; Terme, M.; Badoual, C.; et al. Inhibition of the differentiation of monocyte-derived dendritic cells by human gingival fibroblasts. PLoS ONE 2013, 8, e70937. [Google Scholar] [CrossRef]
- Azad, M.B.; Chen, Y.; Gibson, S.B. Regulation of autophagy by reactive oxygen species (ROS): Implications for cancer progression and treatment. Antioxid. Redox Signal. 2009, 11, 777–790. [Google Scholar] [CrossRef]
- Cordani, M.; Donadelli, M.; Strippoli, R.; Bazhin, A.V.; Sanchez-Alvarez, M. Interplay between ROS and Autophagy in Cancer and Aging: From Molecular Mechanisms to Novel Therapeutic Approaches. Oxid. Med. Cell. Longev. 2019, 2019, 8794612. [Google Scholar] [CrossRef]
- Yamamoto, A.; Tagawa, Y.; Yoshimori, T.; Moriyama, Y.; Masaki, R.; Tashiro, Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct. Funct. 1998, 23, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Thames, H.D., Jr.; Rasmussen, S.L. A test for dose-modifying factors. Radiat. Res. 1978, 76, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, Y.; Kigawa, J.; Itamochi, H.; Kanamori, Y.; Shimada, M.; Takahashi, M.; Terakawa, N. Cisplatin-resistant HeLa cells are resistant to apoptosis via p53-dependent and -independent pathways. Jpn. J. Cancer Res. 1999, 90, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Solomides, C.; Parekh, H.; Simpkins, F.; Simpkins, H. Cisplatin resistance in human cervical, ovarian and lung cancer cells. Cancer Chemother. Pharm. 2015, 75, 1217–1227. [Google Scholar] [CrossRef]
- Skoupilova, H.; Bartosik, M.; Sommerova, L.; Pinkas, J.; Vaculovic, T.; Kanicky, V.; Karban, J.; Hrstka, R. Ferrocenes as new anticancer drug candidates: Determination of the mechanism of action. Eur. J. Pharm. 2020, 867, 172825. [Google Scholar] [CrossRef]
- Velma, V.; Dasari, S.R.; Tchounwou, P.B. Low Doses of Cisplatin Induce Gene Alterations, Cell Cycle Arrest, and Apoptosis in Human Promyelocytic Leukemia Cells. Biomark. Insights 2016, 11, 113–121. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Keyomarsi, K. Role of cell cycle in mediating sensitivity to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Cruet-Hennequart, S.; Villalan, S.; Kaczmarczyk, A.; O’Meara, E.; Sokol, A.M.; Carty, M.P. Characterization of the effects of cisplatin and carboplatin on cell cycle progression and DNA damage response activation in DNA polymerase eta-deficient human cells. Cell Cycle 2009, 8, 3039–3050. [Google Scholar] [CrossRef]
- Wagner, J.M.; Karnitz, L.M. Cisplatin-induced DNA damage activates replication checkpoint signaling components that differentially affect tumor cell survival. Mol. Pharm. 2009, 76, 208–214. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, W.; Ke, W.; Chen, W.; He, C.; Ge, Z. Multifunctional Polymeric Micelles with Amplified Fenton Reaction for Tumor Ablation. Biomacromolecules 2018, 19, 1990–1998. [Google Scholar] [CrossRef]
- Liu, C.; Chen, W.; Qing, Z.; Zheng, J.; Xiao, Y.; Yang, S.; Wang, L.; Li, Y.; Yang, R. In Vivo Lighted Fluorescence via Fenton Reaction: Approach for Imaging of Hydrogen Peroxide in Living Systems. Anal. Chem. 2016, 88, 3998–4003. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990, 186, 1–85. [Google Scholar] [PubMed]
- Acevedo-Morantes, C.Y.; Meléndez, E.; Singh, S.P.; Ramírez-Vick, J.E. Cytotoxicity and Reactive Oxygen Species Generated by Ferrocenium and Ferrocene on MCF7 and MCF10A Cell Lines. J. Cancer Sci. Ther. 2012, 4, 271–275. [Google Scholar]
- Gasser, G.; Ott, I.; Metzler-Nolte, N. Organometallic anticancer compounds. J. Med. Chem. 2011, 54, 3–25. [Google Scholar] [CrossRef]
- Mooney, A.; Tiedt, R.; Maghoub, T.; O’Donovan, N.; Crown, J.; White, B.; Kenny, P.T. Structure--activity relationship and mode of action of N-(6-ferrocenyl-2-naphthoyl) dipeptide ethyl esters: Novel organometallic anticancer compounds. J. Med. Chem. 2012, 55, 5455–5466. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Gandhi, V. ROS-activated anticancer prodrugs: A new strategy for tumor-specific damage. Ther. Deliv. 2012, 3, 823–833. [Google Scholar] [CrossRef]
- Podolski-Renić, A.; Bösze, S.; Dinić, J.; Kocsis, L.; Hudecz, F.; Csámpai, A.; Pešić, M. Ferrocene-Cinchona Hybrids with Triazolyl-chalcone Linker Act as Prooxidants and Sensitize Human Cancer Cell Lines to Paclitaxel. Metallomics 2017, 9, 1132–1141. [Google Scholar] [CrossRef]
- Kovjazin, R.; Eldar, T.; Patya, M.; Vanichkin, A.; Lander, H.M.; Novogrodsky, A. Ferrocene-induced lymphocyte activation and anti-tumor activity is mediated by redox-sensitive signaling. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2003, 17, 467–469. [Google Scholar]
- van Staveren, D.R.; Metzler-Nolte, N. Bioorganometallic chemistry of ferrocene. Chem. Rev. 2004, 104, 5931–5985. [Google Scholar] [CrossRef]
- Santos, M.M.; Bastos, P.; Catela, I.; Zalewska, K.; Branco, L.C. Recent Advances of Metallocenes for Medicinal Chemistry. Mini Rev. Med. Chem. 2017, 17, 771–784. [Google Scholar] [CrossRef]
- Satoh, T.; Enokido, Y.; Aoshima, H.; Uchiyama, Y.; Hatanaka, H. Changes in mitochondrial membrane potential during oxidative stress-induced apoptosis in PC12 cells. J. Neurosci. Res. 1997, 50, 413–420. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Wu, Y.L.; Tashiro, S.; Onodera, S.; Ikejima, T. Reactive oxygen species contribute to oridonin-induced apoptosis and autophagy in human cervical carcinoma HeLa cells. Acta. Pharm. Sin. 2011, 32, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Elena-Real, C.A.; Diaz-Quintana, A.; Gonzalez-Arzola, K.; Velazquez-Campoy, A.; Orzaez, M.; Lopez-Rivas, A.; Gil-Caballero, S.; De la Rosa, M.A.; Diaz-Moreno, I. Cytochrome c speeds up caspase cascade activation by blocking 14-3-3epsilon-dependent Apaf-1 inhibition. Cell Death Dis. 2018, 9, 365. [Google Scholar] [CrossRef]
- Niioka, T.; Uno, T.; Yasui-Furukori, N.; Takahata, T.; Shimizu, M.; Sugawara, K.; Tateishi, T. Pharmacokinetics of low-dose nedaplatin and validation of AUC prediction in patients with non-small-cell lung carcinoma. Cancer Chemother. Pharm. 2007, 59, 575–580. [Google Scholar] [CrossRef]
- Jaouen, G.; Vessieres, A.; Top, S. Ferrocifen type anti cancer drugs. Chem. Soc. Rev. 2015, 44, 8802–8817. [Google Scholar] [CrossRef]
- Cortes, R.; Tarrado-Castellarnau, M.; Talancon, D.; Lopez, C.; Link, W.; Ruiz, D.; Centelles, J.J.; Quirante, J.; Cascante, M. A novel cyclometallated Pt(II)-ferrocene complex induces nuclear FOXO3a localization and apoptosis and synergizes with cisplatin to inhibit lung cancer cell proliferation. Metallomics 2014, 6, 622–633. [Google Scholar] [CrossRef]
- Wlassoff, W.A.; Albright, C.D.; Sivashinski, M.S.; Ivanova, A.; Appelbaum, J.G.; Salganik, R.I. Hydrogen peroxide overproduced in breast cancer cells can serve as an anticancer prodrug generating apoptosis-stimulating hydroxyl radicals under the effect of tamoxifen-ferrocene conjugate. J. Pharm. Pharm. 2007, 59, 1549–1553. [Google Scholar] [CrossRef]
- Ivanova, D.; Gronemeyer, H.; de Lera, A.R. Design and stereoselective synthesis of retinoids with ferrocene or N-butylcarbazole pharmacophores that induce post-differentiation apoptosis in acute promyelocytic leukemia cells. Chem. Med. Chem. 2011, 6, 1518–1529. [Google Scholar] [CrossRef]
- Guo, J.Y.; Chen, H.Y.; Mathew, R.; Fan, J.; Strohecker, A.M.; Karsli-Uzunbas, G.; Kamphorst, J.J.; Chen, G.; Lemons, J.M.; Karantza, V.; et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011, 25, 460–470. [Google Scholar] [CrossRef]
- Yonekawa, T.; Thorburn, A. Autophagy and cell death. Essays Biochem. 2013, 55, 105–117. [Google Scholar]
| Comp. | Ca Ski | SiHa | HeLa | RPE-1 | HEK 293 |
|---|---|---|---|---|---|
| Cisplatin | 35.1 ± 8.7 | 26.6 ± 4.1 | 28.2 ± 5.9 | 46.8 ± 6.0 | 25.3 ± 8.6 |
| 1a | 69.4 ± 8.6 | 17.1 ± 3.4 | 61.5 ± 2.9 | 82.8 ± 3.7 | >100 |
| 1b | 6.2 ± 2.1 | 9.9 ± 2.2 | 6.4 ± 1.6 | 5.1 ± 0.8 | 35.6 ± 6.2 |
| 1c | 12.5 ± 0.6 | 18.2 ± 3.1 | 10.6 ± 2.5 | 11.0 ± 2.4 | 16.7 ± 2.0 |
| 2a | 8.3 ± 2.3 | 14.1 ± 3.4 | 7.3 ± 1.2 | 4.7 ± 0.1 | 6.2 ± 1.6 |
| 2b | 15.0 ± 1.0 | 30.2 ± 2.9 | 13.0 ± 0.6 | 8.2 ± 0.4 | 5.4 ± 0.6 |
| 3 | 7.6 ± 1.3 | 11.1 ± 1.5 | 6.5 ± 0.4 | 8.5 ± 1.1 | 9.5 ± 1.0 |
| 4 | >100 | >100 | >100 | >100 | >100 |
| 5 | >100 | 91.8 ± 9.4 | 7.7 ± 1.1 | 7.4 ± 2.0 | 3.0 ± 0.3 |
| HeLa Cells | SiHa Cells | ||||||
|---|---|---|---|---|---|---|---|
| Conc. | 1b | 2a | 3 | Conc. | 1b | 2a | 3 |
| 0.5 μM | 0.9 ± 0.08 | 1.0 ± 0.1 | 0.9 ± 0.08 | 1 μM | 1.4 ± 0.19 | 1.3 ± 0.07 | 0.9 ± 0.08 |
| 1 μM | 1.6 ± 0.13 | 0.9 ± 0.2 | 1.2 ± 0.08 | 2 μM | 1.8 ± 0.5 | 1.4 ± 0.17 | 1.2 ± 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skoupilova, H.; Rak, V.; Pinkas, J.; Karban, J.; Hrstka, R. The Cytotoxic Effect of Newly Synthesized Ferrocenes against Cervical Carcinoma Cells Alone and in Combination with Radiotherapy. Appl. Sci. 2020, 10, 3728. https://doi.org/10.3390/app10113728
Skoupilova H, Rak V, Pinkas J, Karban J, Hrstka R. The Cytotoxic Effect of Newly Synthesized Ferrocenes against Cervical Carcinoma Cells Alone and in Combination with Radiotherapy. Applied Sciences. 2020; 10(11):3728. https://doi.org/10.3390/app10113728
Chicago/Turabian StyleSkoupilova, Hana, Vladimir Rak, Jiri Pinkas, Jindrich Karban, and Roman Hrstka. 2020. "The Cytotoxic Effect of Newly Synthesized Ferrocenes against Cervical Carcinoma Cells Alone and in Combination with Radiotherapy" Applied Sciences 10, no. 11: 3728. https://doi.org/10.3390/app10113728
APA StyleSkoupilova, H., Rak, V., Pinkas, J., Karban, J., & Hrstka, R. (2020). The Cytotoxic Effect of Newly Synthesized Ferrocenes against Cervical Carcinoma Cells Alone and in Combination with Radiotherapy. Applied Sciences, 10(11), 3728. https://doi.org/10.3390/app10113728





