Oxidative Stress Biomarkers in Erythrocytes of Captive Pre-Juvenile Loggerhead Turtles Following Acute Exposure to Methylmercury
Abstract
:1. Introduction
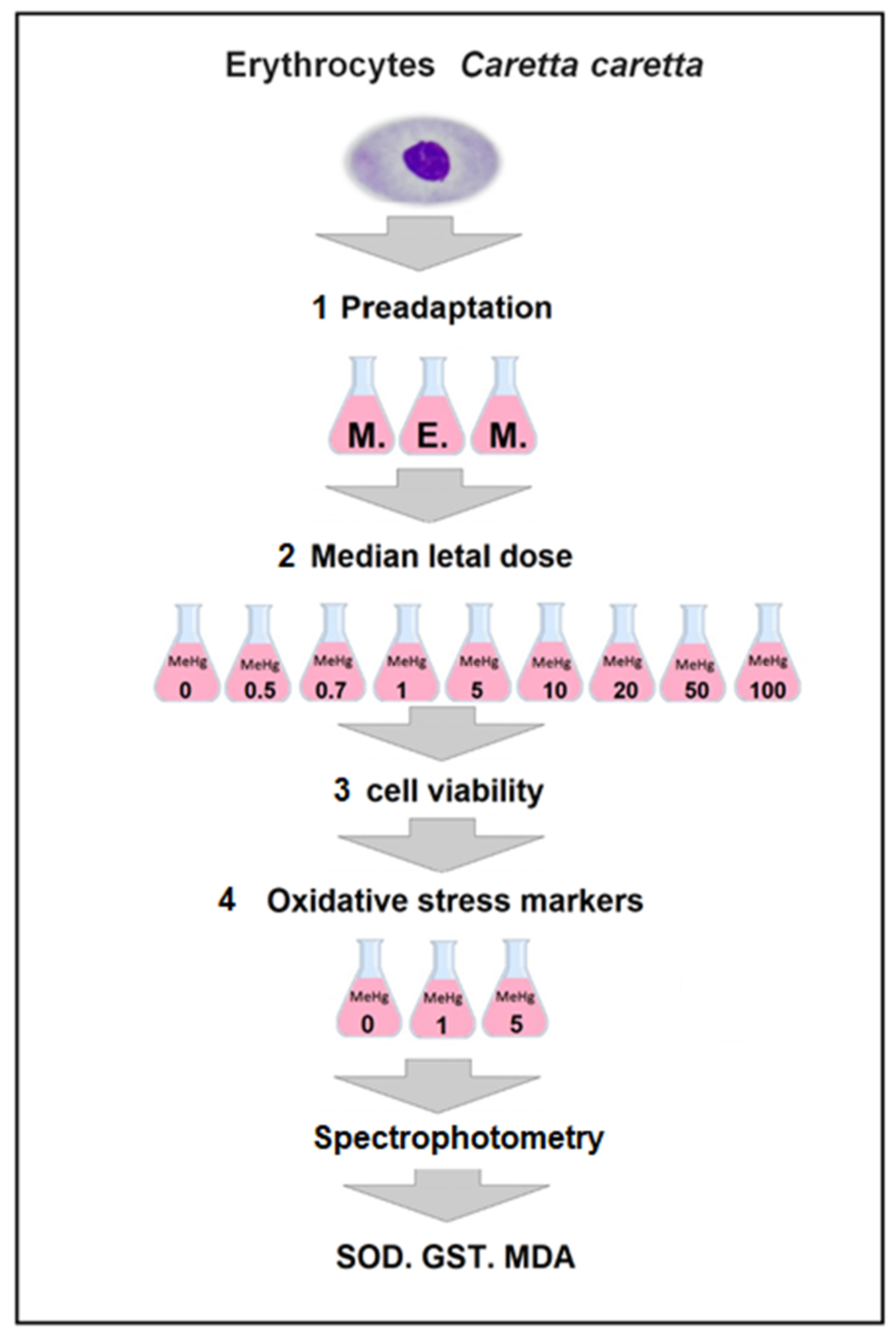
2. Materials and Methods
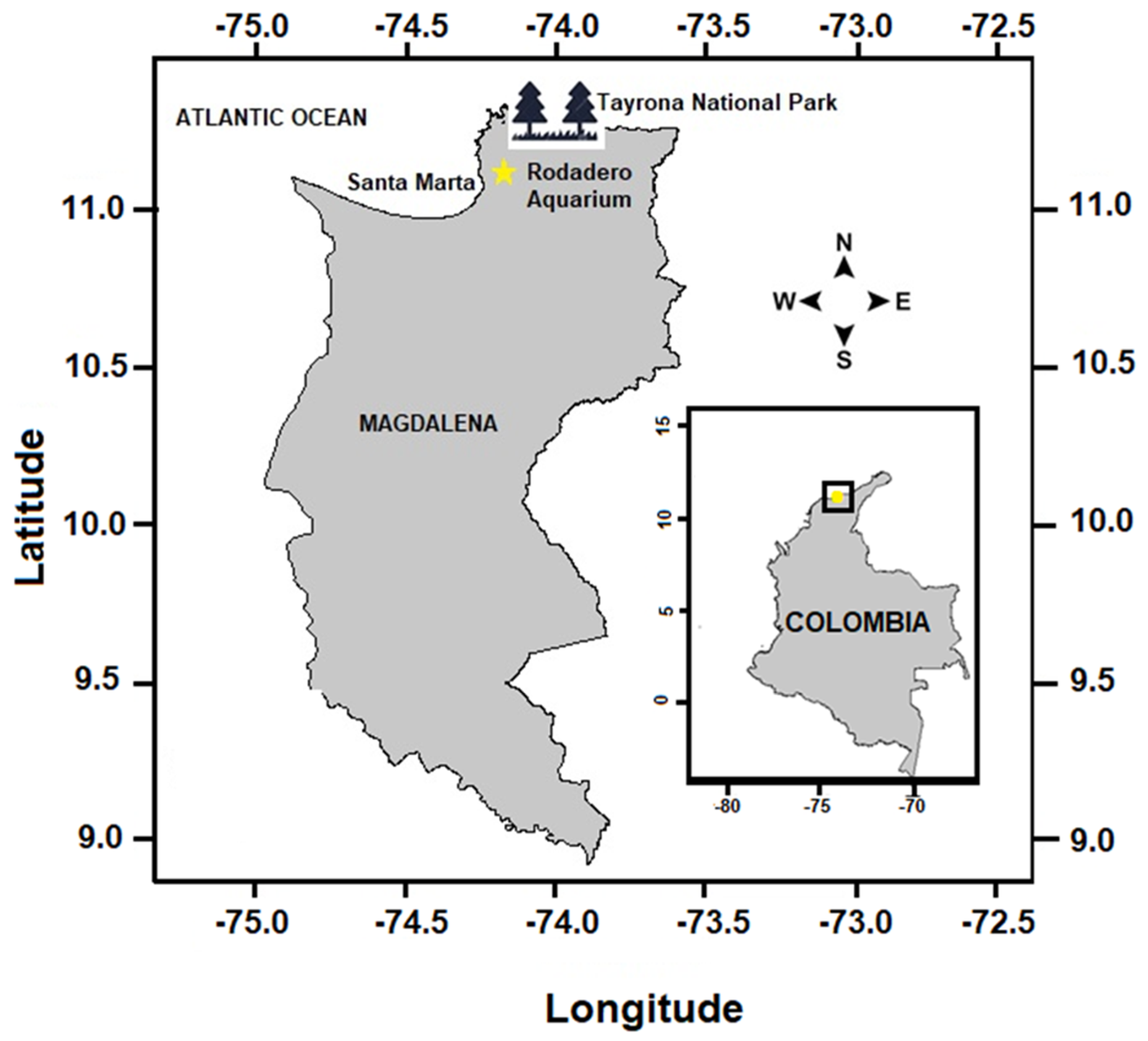
2.1. Study Area, Collection and Analysis of Samples
2.2. Experimental Procedures
2.2.1. Isolation and Pre-Adaptation of Loggerhead Sea Turtles Erythrocytes
2.2.2. Determination of LC50 of Loggerhead Sea Turtles Erythrocytes
2.2.3. Determination of Cell Viability of Loggerhead Sea Turtles RBCs
2.3. Oxidative Stress of Loggerhead Sea Turtles Erythrocytes
2.4. Data Analysis
3. Results
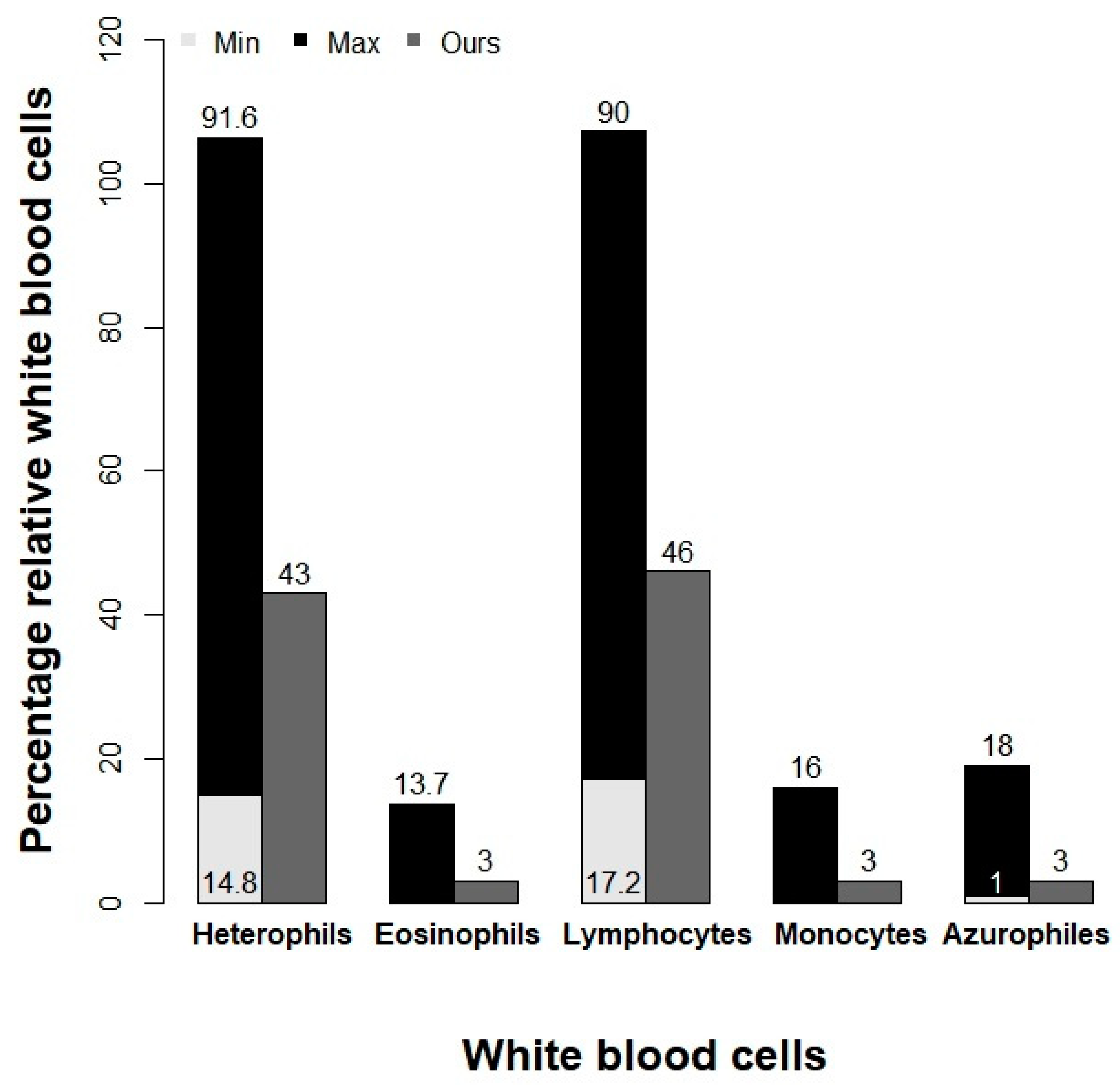
3.1. Blood Count and Health Status of Loggerhead Turtles
3.2. Quantification of Hg in Erythrocytes of Loggerhead Turtles
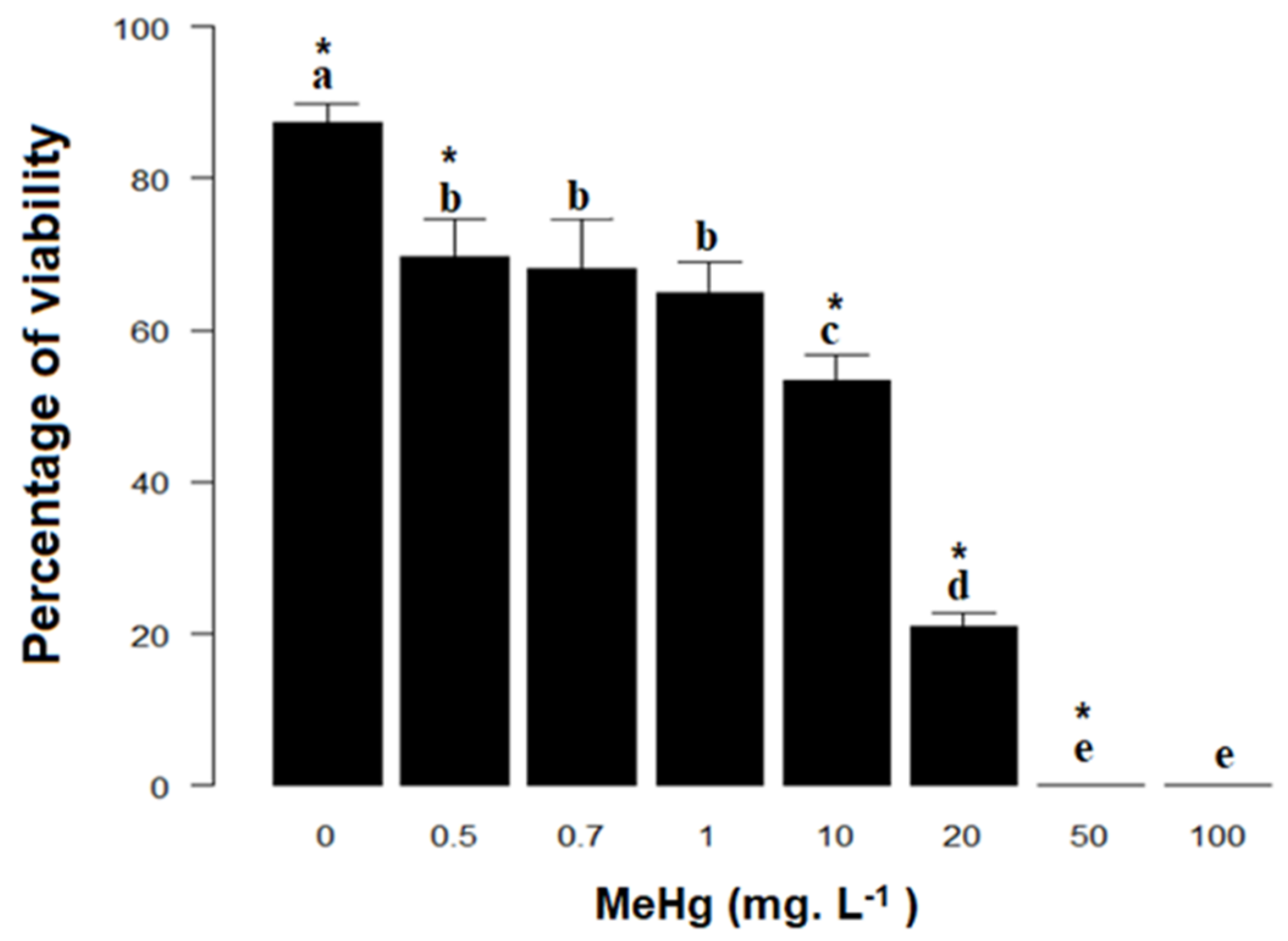
3.3. Lethal Concentration (LC50) and Cell Viability of Erythrocytes of Loggerhead Turtles
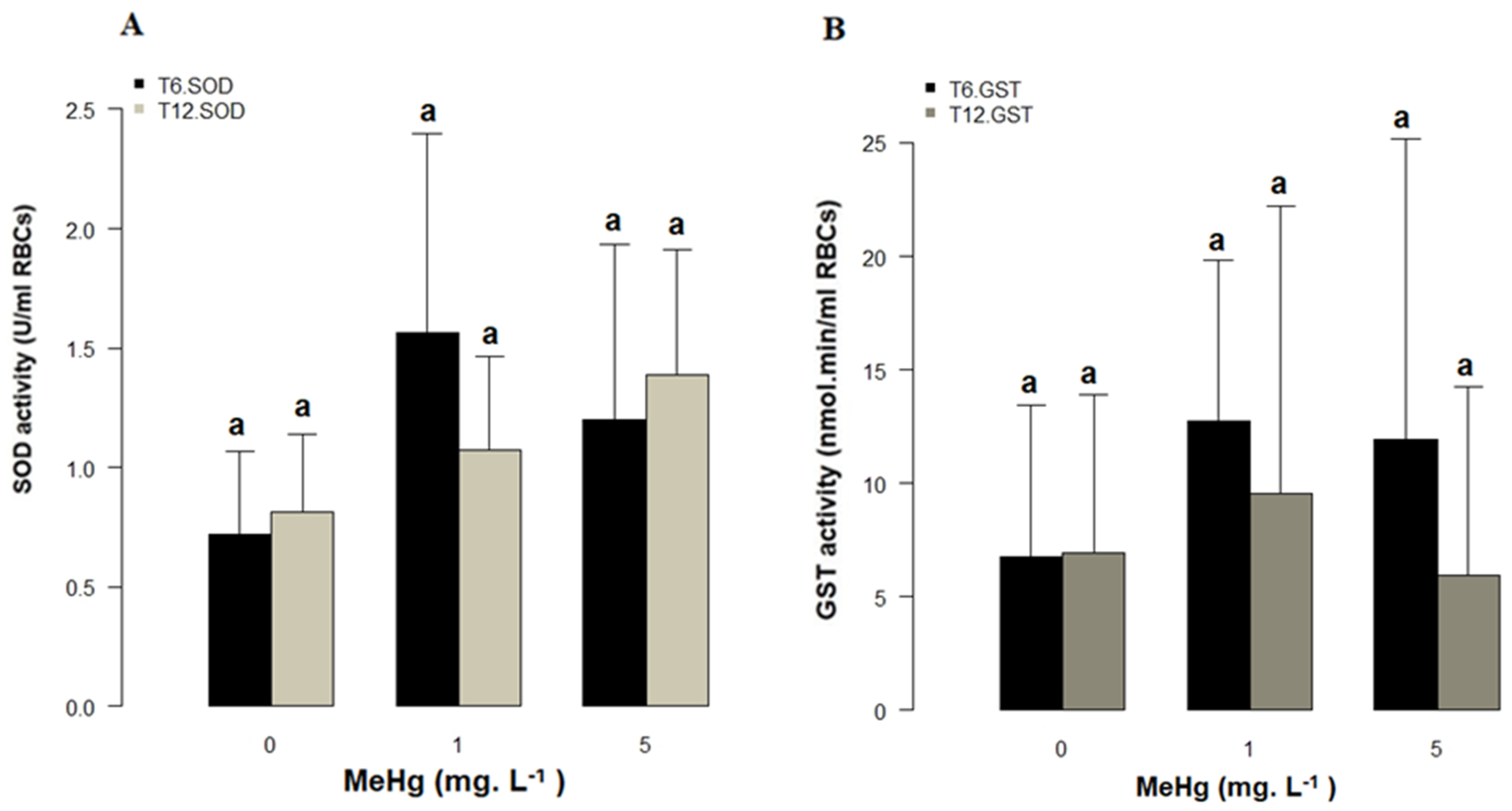
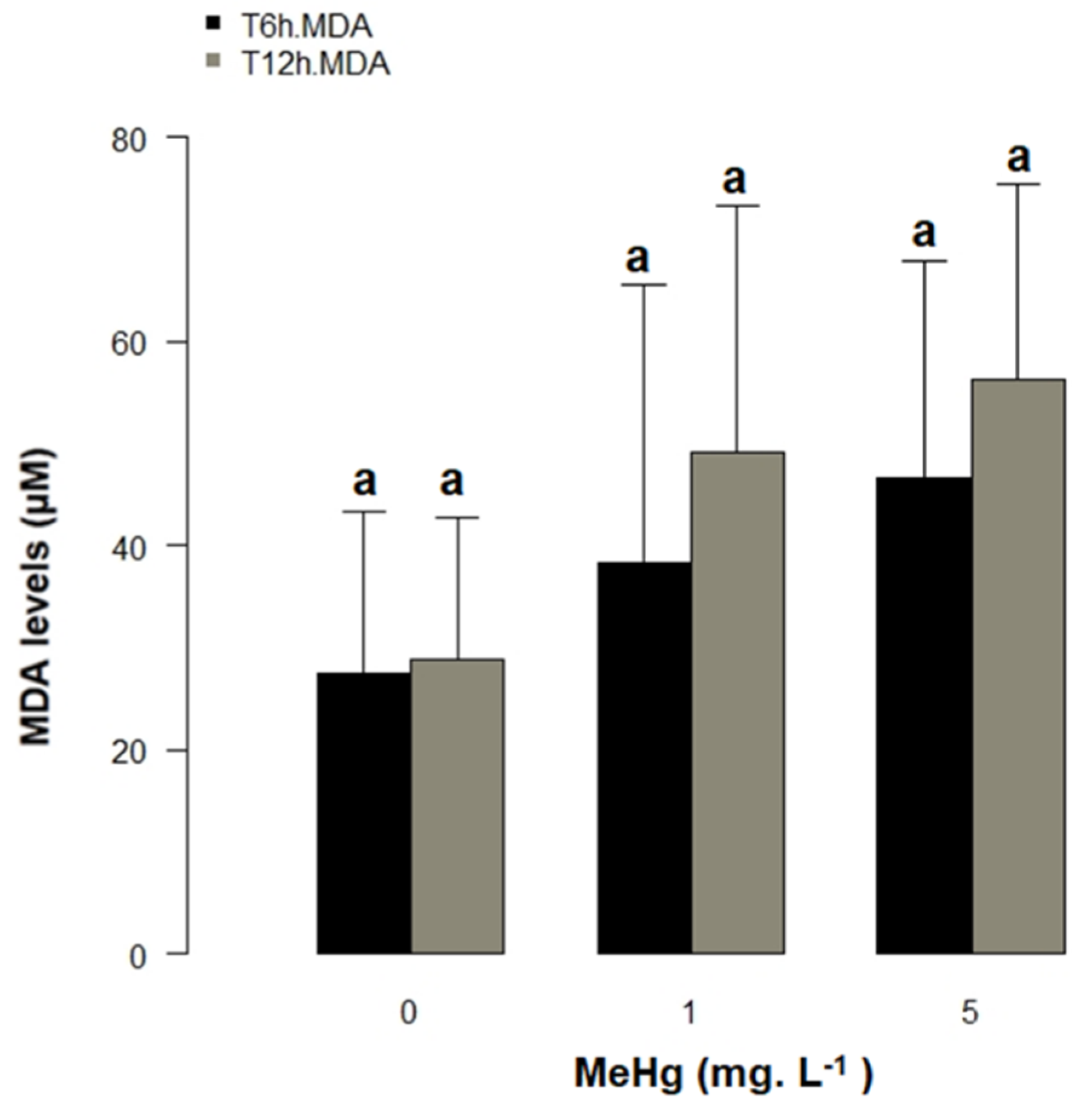
3.4. Bioassay and Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Finlayson, K.A.; Leusch, F.D.L.; Van De Merwe, J.P. The current state and future directions of marine turtle toxicology research. Environ. Int. 2016, 94, 113–123. [Google Scholar] [CrossRef]
- Wallace, B.P.; Dimatteo, A.D.; Hurley, B.J.; Finkbeiner, E.M.; Alan, B.; Chaloupka, M.Y.; Hutchinson, B.J.; Abreu-grobois, F.A.; Marcovaldi, M.A.; Mortimer, J.A.; et al. Regional Management Units for Marine Turtles: A Novel Framework for Prioritizing Conservation and Research across Multiple Scales. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancheros-piliego, D.; Fernández, J.H. AMDAR y PCR-extra-rápida para la identificación de la tortuga cabezona Caretta caretta (Testudines: Cheloniidae) utilizando el gen mitocondrial citocromo c oxidasa I (COI). Univ. Sci. 2013, 18. [Google Scholar] [CrossRef]
- Day, R.D.; Segars, A.L.; Arendt, M.D.; Lee, A.M.; Peden-Adams, M.M. Relationship of blood mercury levels to health parameters in the loggerhead sea turtle (Caretta caretta). Environ. Health Perspect. 2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amorocho, D. Monitoring nesting loggerhead turtles (Caretta caretta) in the central Caribbean coast of Colombia. Mar. Turt. Newsl. 2003, 101, 8–13. [Google Scholar]
- Jule, K.R.; Leaver, L.A.; Lea, S.E.G. The effects of captive experience on reintroduction survival in carnivores: A review and analysis. Biol. Conserv. 2008, 141, 355–363. [Google Scholar] [CrossRef]
- Beyer, W.N.; Meador, J.P. Environmental Contaminants in Wildlife: Interpreting Tissue Concentrations; CRC Press: Boca Raton, FL, USA, 1996; pp. 1–768. [Google Scholar] [CrossRef]
- Ley-quiñónez, C.; Zavala-norzagaray, A.A.; Espinosa-carreón, T.L.; Peckham, H. Baseline heavy metals and metalloid values in blood of loggerhead turtles (Caretta caretta) from Baja California Sur, Mexico. Mar. Pollut. Bull. 2011, 62, 1979–1983. [Google Scholar] [CrossRef]
- Yarsan, E.; Yipel, M. The Important Terms of Marine Pollution “Biomarkers and Biomonitoring. J. Mol. Biomark. Diagn. 2013, 1–4. [Google Scholar] [CrossRef]
- Yipel, M.; Tekeli, İ.O.; İşler, C.T.; Altuğ, M.E. Heavy metal distribution in blood, liver and kidneys of Loggerhead (Caretta caretta) and Green (Chelonia mydas) sea turtles from the Northeast Mediterranean Sea. Mar. Pollut. Bull. 2017, 125, 487–491. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Wang, F.; Luo, Z.; Guo, S.; Strähle, U. Toxicity of mercury: Molecular evidence. Chemosphere 2020, 245. [Google Scholar] [CrossRef]
- Kotnik, J.; Horvat, M.; Begu, E.; Shlyapnikov, Y.; Sprovieri, F.; Pirrone, N. Dissolved gaseous mercury (DGM) in the Mediterranean Sea: Spatial and temporal trends. Mar. Chem. 2017, 193, 8–19. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Mercury in canned tuna: White versus light and temporal variation. Environ. Res. 2004, 96, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Parra, J.P.; Betancourt, J.; Espinosa, L.F.; Garay, J. Evolución y estado de la contaminación por metales pesados y compuestos orgánicos en la Bahía de Cartagena, Colombia. Informe Técnico. Inf. Técnico 2011, 1–13. [Google Scholar]
- Tosic, M.; Restrepo, J.D.; Lonin, S.; Izquierdo, A.; Martins, F. Water and sediment quality in Cartagena Bay, Colombia: Seasonal variability and potential impacts of pollution. Estuar. Coast. Shelf Sci. 2019, 216, 187–203. [Google Scholar] [CrossRef]
- Selin, N.E.; Jacob, D.J.; Yantosca, R.M.; Strode, S.; Jaeglé, L.; Sunderland, E.M. Global 3-D land-ocean-atmosphere model for mercury: Present-day versus preindustrial cycles and anthropogenic enrichment factors for deposition. Glob. Biogeochem. Cycles 2008, 22, 1–13. [Google Scholar] [CrossRef]
- Pirrone, N.; Cinnirella, S.; Feng, X.; Finkelman, R.B.; Friedli, H.R.; Leaner, J.; Mason, R.; Mukherjee, A.B.; Stracher, G.B.; Streets, D.G.; et al. Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos. Chem. Phys. 2010, 10, 5951–5964. [Google Scholar] [CrossRef] [Green Version]
- Farina, M.; Aschner, M.; Rocha, J.B.T. Oxidative stress in MeHg-induced neurotoxicity. Toxicol. Appl. Pharmacol. 2011, 256, 405–417. [Google Scholar] [CrossRef] [Green Version]
- Joshi, D.; Kumar, M.D.; Kumar, A.; Sangeeta, S. Reversal of Methylmercury-Induced Oxidative Stress, Lipid Peroxidation, and DNA Damage by the Treatment of N-Acetyl Cysteine: A Protective Approach. J. Environ. Pathol. Toxicol. Oncol. 2014, 33, 167–182. [Google Scholar] [CrossRef]
- Rodríguez, M.; Osuna, A. Efecto de la adición de antioxidantes sobre la motilidad espermática post-criopreservación y fertilidad del semen de peces. Rev. Vet. 2017, 28, 157–164. [Google Scholar]
- Franco, R.; Navarro, G.; Mart, E. Antioxidant defense mechanisms in erythrocytes and in the central nervous system. Antioxidants 2019, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Orson, R.; Brncunn, L. Uptake of methyl mercuric chloride and mercuric chloride by trout: A sturly of uptake pathways into the whotre anirnal and uptake by erythrocytes in vitro. J. Fish. Board Can. 1973, 30, 1293–1299. [Google Scholar] [CrossRef]
- Pagano, M.; Faggio, C. The use of erythrocyte fragility to assess xenobiotic cytotoxicity. Cell Biochem. Funct. 2015, 33, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Massarsky, A.; Abraham, R.; Nguyen, K.C.; Rippstein, P.; Tayabali, A.F.; Trudeau, V.L.; Moon, T.W. Nanosilver cytotoxicity in rainbow trout (Oncorhynchus mykiss) erythrocytes and hepatocytes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 159, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Gabryelak, T.; Piatkowska, M.; Leyko, W.P.G. Seasonal variations in the activities of peroxide metabolism enzymes in erythrocytes of freshwater fish species. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 1983, 75, 383–385. [Google Scholar] [CrossRef]
- Goodchild, C.G.; DuRant, S.E. Fluorescent heme degradation products are biomarkers of oxidative stress and linked to impaired membrane integrity in avian red blood cells. Physiol. Biochem. Zool. 2020, 93, 129–139. [Google Scholar] [CrossRef]
- Velando, A.; Noguera, J.C.; da Silva, A.; Kim, S.Y. Redox-regulation and life-history trade-offs: Scavenging mitochondrial ROS improves growth in a wild bird. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sakuragui, M.M.; Paulino, M.G.; da Silva e Souza, N.E.; Tavares, D.; Terezan, A.P.; Pesenti, E.; Giani, A.; Fernandes, J.B.; Cestari, M.M.; Fernandes, M.N. Crude extract of cyanobacterium Radiocystis fernandoi strain R28 induces anemia and oxidative stress in fish erythrocytes. Toxicon 2019, 169, 18–24. [Google Scholar] [CrossRef]
- Cortés-Gutiérrez, E.I.; García-Salas, J.A.; Dávila-Rodríguez, M.I.; Ceyca-Contreras, J.P.; González-Ramírez, E.G. Evaluation of oxidative DNA damage in pigeon erythrocytes using DNA breakage detection-fluorescence in situ hybridization (DBD-FISH). Biotech. Histochem. 2019, 94, 600–605. [Google Scholar] [CrossRef]
- Burgos-Aceves, M.A.; Lionetti, L.; Faggio, C. Multidisciplinary haematology as prognostic device in environmental and xenobiotic stress-induced response in fish. Sci. Total Environ. 2019, 670, 1170–1183. [Google Scholar] [CrossRef]
- Panghal, A.; Sathua, K.B.; Flora, S.J.S. Gallic acid and MiADMSA reversed arsenic induced oxidative/nitrosative damage in rat red blood cells. Heliyon 2020, 6, e03431. [Google Scholar] [CrossRef]
- Hammami, N.; Athmouni, K.; Lahmar, I.; Ben Abdallah, F.; Belghith, K. Antioxidant potential of Salicornia arabica lipid extract and their protective effect against cadmium induced oxidative stress in erythrocytes isolated from rats. J. Food Meas. Charact. 2019, 13, 2705–2712. [Google Scholar] [CrossRef]
- Agrawal, D.; Sultana, P.; Gupta, G.S.D. Oxidative damage and changes in the glutathione redox system in erythrocytes from rats treated with hexachlorocyclohexane. Food Chem. Toxicol. 1991, 29, 459–462. [Google Scholar] [CrossRef]
- Barraza, A.D.; Komoroske, L.M.; Allen, C.; Eguchi, T.; Gossett, R.; Holland, E.; Lawson, D.D.; LeRoux, R.A.; Long, A.; Seminoff, J.A.; et al. Trace metals in green sea turtles (Chelonia mydas) inhabiting two southern California coastal estuaries. Chemosphere 2019, 223, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Çördük, N.; Doǧru, N.H.; Gül, Ç.; Tosunoǧlu, M. Assessment of nuclear abnormalities in erythrocytes of balkan pond turtle Mauremys rivulata (Valenciennes, 1833) (Testudines: Geoemydidae) from the Biga Stream, Çanakkale, Turkey. Acta Zool. Bulg. 2019, 71, 219–226. [Google Scholar]
- Aguirre, A.A.; Balazs, G.H. Comparative Original Article Blood Biochemistry Values of Green Turtles, Chelonia Mydas, With and Without Fibropapillomatosis. Comp. Haematol. Int. 2000, 10, 132–137. [Google Scholar] [CrossRef]
- Bjorndal, K.A.; Bolten, A.B.; Martins, H.R. Somatic growth model of juvenile loggerhead sea turtles Caretta caretta: Duration of pelagic stage. Mar. Ecol. Prog. Ser. 2000, 202, 265–272. [Google Scholar] [CrossRef]
- Dutton, P.H. Methods for collection and preservation of samples for sea turtle genetic studies. In Proceedings of the International Symposium on Sea Turtle Conservation Genetics, Miami, FL, USA, 1 January 1996. [Google Scholar]
- Finney, D.T. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer Science & Business Media: New York, NY, USA, 2002. [Google Scholar]
- Guisande, C.; Heine, J.; González-DaCosta, J.; García-Roselló, E. RWizard Software; University of Vigo: Vigo, Spain, 2014. [Google Scholar]
- Martins, R.D.P.; Braga, H.D.C.; Aline, P.; Dalmarco, J.B.; De Bem, A.F.; Roberto, A.; Santos, S.; Dafre, A.L.; Pizzolatti, M.G.; Latini, A.; et al. Synergistic neurotoxicity induced by methylmercury and quercetin in mice. Food Chem. Toxicol. 2009, 47, 645–649. [Google Scholar] [CrossRef] [Green Version]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases The First Enzymatic Step In Mercapturic Acid Formation. J. Biol. Chem. 2005, 249, 7130–7139. [Google Scholar]
- R Development Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Copenhagen, Denmark, 2017; p. 900051. [Google Scholar]
- Casal, A.B.; Luis, F.L.; Juste, C.; Or, J. Comparative study of hematologic and plasma biochemical variables in Eastern Atlantic juvenile and adult nesting loggerhead sea turtles (Caretta caretta). Vet. Clin. Pathol. 2009, 2, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Deem, S.L.; Norton, T.M.; Mitchell, M.; Segars, A.; Alleman, A.R.; Cray, C.; Poppenga, R.H.; Dodd, M.; Karesh, W.B. Comparison of blood values in foraging, nesting, and stranded loggerhead turtles (Caretta caretta) along the coast of Georgia, USA. J. Wildl. Dis. 2009, 45, 41–56. [Google Scholar] [CrossRef]
- Yang, T.; Haas, H.L.; Patel, S.; Smolowitz, R.; James, M.C.; Williard, A.S. Blood biochemistry and haematology of migrating loggerhead turtles (Caretta caretta) in the Northwest Atlantic: Reference intervals and intra-population comparisons. Conserv. Physiol. 2019, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rousselet, E.; Stacy, N.I.; LaVictoire, K.; Higgins, B.M.; Tocidlowski, M.E.; Flanagan, J.P.; Godard-Codding, C.A. Hematology and plasma biochemistry analytes in five age groups of immature, captive-reared loggerhead sea turtles (Caretta caretta). J. Zoo Wildl. Med. 2013, 44, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Angeles, M.; Morcillo, P.; Cuesta, A. In vitro effects of metals on isolated head-kidney and blood leucocytes of the teleost fi sh Sparus aurata L. and Dicentrarchus labrax L. Fish Shellfish Immunol. 2016, 54. [Google Scholar] [CrossRef]
- Lutz, P.L.; Dunbar-Cooper, A.N.N. The loggerhead sea turtle, Caretta Caretta. Fish. Bull. 1971, 85, 37. [Google Scholar]
- Kelly, T.R.; McNeill, J.B.; Avens, L.; Hall, A.G.; Goshe, L.R.; Hohn, A.A.; Godfrey, M.H.; Nicole Mihnovets, A.; Cluse, W.M.; Harms, C.A. Clinical pathology reference intervals for an in-water population of juvenile loggerhead sea turtle s (Caretta caretta) in Core Sound, North Carolina, USA. PLoS ONE 2015, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Flint, M.; Morton, J.M.; Limpus, C.J.; Patterson-Kane, J.C.; Mills, P.C. Reference intervals for plasma biochemical and hematologic measures in loggerhead sea turtles (Caretta caretta) from Moreton bay Australia. J. Wildl. Dis. 2010, 46, 731–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggiero, M.G.; Ferretti, L.; Glomski, C.; Federico, N. Erythrophagocytosis in Circulating Blood of Loggerhead Turtles Caretta caretta: The Pitting of Heinz Bodies. J. Exp. Zool. 2013. [Google Scholar] [CrossRef]
- Figueres, J. Estudio Sanitario de las Tortugas Terrestres Mediterráneas (Género Testudo) e Implicaciones Para su Conservación; Universidad Autonoma de Barcelona: Barcelona, Spain, 2015; pp. 1–187. [Google Scholar]
- Finlayson, K.A.; Leusch, F.D.L.; Van De Merwe, J.P. Science of the Total Environment Cytotoxicity of organic and inorganic compounds to primary cell cultures established from internal tissues of Chelonia mydas. Sci. Total Environ. 2019, 664, 958–967. [Google Scholar] [CrossRef]
- Olivero-verbel, J.; Caballero-gallardo, K.; Torres, N. International Journal of Environmental Assessment of mercury in muscle of fish from Cartagena Bay, a tropical estuary at the north of Colombia. Int. J. Environ. Health Res. 2009, 5, 37–41. [Google Scholar] [CrossRef]
- Stricks, W.; Kolthoff, I.M. Reactions between Mercuric Mercury and Cysteine and Glutathione. Apparent Dissociation Constants, Heats and Entropies of Formation of Various Forms of Mercuric Mercapto-Cysteine and -Glutathione. J. Am. Chem. Soc. 1953, 75, 5673–5681. [Google Scholar] [CrossRef]
- Weed, R.; Eber, J.; Biology, R. Interaction of Mercury with Human Erythrocytes. J. Gen. Physiol. 1962, 45, 395–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naganuma, A.; Koyama, Y.; Imura, N. Behavior of Methylmercury in Mammalian Erythrocytes. Toxicol. Appl. Pharmacol. 1980, 410, 405–410. [Google Scholar] [CrossRef]
- Farina, M.; Aschner, M. Methylmercury-Induced Neurotoxicity: Focus on Pro-oxidative Events and Related Consequences. In Neurotoxicity of Metals; Aschner, M., Costa, L.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 267–286. ISBN 978-3-319-60189-2. [Google Scholar]
- Zapata, L.M.; Bock, B.C.; Yaneth, L.; Palacio, J.A. Ecotoxicology and environmental fafety application of the micronucleus test and comet assay in Trachemys callirostris erythrocytes as a model for in situ genotoxic monitoring. Ecotoxicol. Environ. Saf. 2016, 127, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Cocci, P.; Capriotti, M.; Mosconi, G.; Palermo, F.A. Effects of endocrine disrupting chemicals on estrogen receptor alpha and heat shock protein 60 gene expression in primary cultures of loggerhead sea turtle (Caretta caretta) erythrocytes ☆. Environ. Res. 2017, 158, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Wang, M.; Wang, W.; Alonso Aguirre, A.; Lu, Y. Validation of an in vitro cytotoxicity test for four heavy metals using cell lines derived from a green sea turtle (Chelonia mydas). Cell Biol. Toxicol. 2010, 26, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Wang, Y.; Lu, Y. In vitro evaluation of inorganic and methyl mercury mediated cytotoxic effect on neural cells derived from different animal species. J. Environ. Sci. 2016, 41, 138–145. [Google Scholar] [CrossRef]
- Young, J.L.; Wise, S.S.; Xie, H.; Zhu, C.; Fukuda, T.; Wise, J.P. Comparative cytotoxicity and genotoxicity of soluble and particulate hexavalent chromium in human and hawksbill sea turtle (Eretmochelys imbricata) skin cells. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 178, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Kaska, Y.; Furness, R.W. Heavy metals in marine turtle eggs and hatchlings in the mediterranean. Zool. Middle East 2001, 24, 127–132. [Google Scholar] [CrossRef]
- Kampalath, R.; Gardner, S.C.; Méndez-Rodríguez, L.; Jay, J.A. Total and methylmercury in three species of sea turtles of Baja California Sur. Mar. Pollut. Bull. 2006, 52, 1816–1823. [Google Scholar] [CrossRef]
- Mcgraw, K.J.; Cohen, A.A.; Costantini, D.; Ho, P. The ecological significance of antioxidants and oxidative stress: A marriage between mechanistic and functional perspectives. Funct. Ecol. 2010, 2000, 947–949. [Google Scholar] [CrossRef]
- Labrada-martagón, V.; Rodríguez, P.A.T.; Méndez-rodríguez, L.C.; Zenteno-savín, T. Comparative Biochemistry and Physiology, Part C Oxidative stress indicators and chemical contaminants in East Paci fi c green turtles (Chelonia mydas) inhabiting two foraging coastal lagoons in the Baja California peninsula. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2011, 154, 65–75. [Google Scholar] [CrossRef]
- Perrault, J.R.; Schmid, J.R.; Walsh, C.J.; Yordy, J.E.; Tucker, A.D. Brevetoxin exposure, superoxide dismutase activity and plasma protein electrophoretic profiles in wild-caught Kemp’s ridley sea turtles (Lepidochelys kempii) in southwest Florida. Harmful Algae 2014, 37, 194–202. [Google Scholar] [CrossRef]
- Finlayson, K.A.; Leusch, F.D.L.; Van De Merwe, J.P. Primary green turtle (Chelonia mydas) skin fibroblasts as an in vitro model for assessing genotoxicity and oxidative stress. Aquat. Toxicol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Isaji, Y.; Okochi, M.; Horio, F.; Honda, H. Use of erythrocyte adhesion assay to predict the risk of diabetic complications. Biochem. Eng. J. 2009, 43, 178–184. [Google Scholar] [CrossRef]
- Schara, M.; Nemec, M.; Falnoga, I.; Kobal, A.B.; Kveder, M.; Svetek, J. The action of mercury on cell membranes. Cell. Mol. Biol. Lett. 2001, 6, 299–304. [Google Scholar] [PubMed]
- Cortés-Gómez, A.A.; Romero, D.; Girondot, M. The current situation of inorganic elements in marine turtles: A general review and meta-analysis. Environ. Pollut. 2017, 229, 567–585. [Google Scholar] [CrossRef]
- Salvarani, P.I.; Vieira, L.R.; Ku-Peralta, W.; Morgado, F.; Osten, J.R. Von Oxidative stress biomarkers and organochlorine pesticides in nesting female hawksbill turtles Eretmochelys imbricata from Mexican coast (Punta Xen, Mexico). Environ. Sci. Pollut. Res. 2018, 25, 23809–23816. [Google Scholar] [CrossRef]
- Berntssen, M.H.G.; Aatland, A.; Handy, R.D. Chronic dietary mercury exposure causes oxidative stress, brain lesions, and altered behaviour in Atlantic salmon (Salmo salar) parr. Aquat. Toxicol. 2003, 65. [Google Scholar] [CrossRef]
- Zaman, K.; Macgill, R.S.; Johnson, J.E.; Ahmad, S.; Pardini, R.S. An Insect Model for Assessing Mercury Toxicity: Effect of Mercury on Antioxidant Enzyme Activities of the Housefly (Musca domestica) and the Cabbage Looper Moth (Trichoplusia ni). Arch. Environ. Contam. Toxicol. 1994, 118, 114–118. [Google Scholar] [CrossRef]
- Hirota, Y.; Yamaguchi, S.; Shimojoh, N.; Sano, K.I. Inhibitory effect of methylmercury on the activity of glutathione peroxidase. Toxicol. Appl. Pharmacol. 1980, 176, 174–176. [Google Scholar] [CrossRef]
- Kumagai, Y.; Shinyashiki, M.; Shimojo, N. Alterations in Superoxide Dismutase Isozymes by Methylmercury. Appl. Organomet. Chem. 1997, 11, 635–643. [Google Scholar] [CrossRef]
- Lund, B.; Miller, M.; Woodst, S. Studies on Hg (II) -Induced H2O2 formation and oxidative stress in vivo and in vitro in rat. Biochmdcal Phamwcology. 2017, 45, 2017–2024. [Google Scholar]
- Yee, S.; Choi, B. Methylmercury poisoning induces oxidative stress in the mouse brain. Exp. Mol. Pathol. 1994, 60, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W. The Toxicology of Mercury and Its Chemical Compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Stier, A.; Reichert, S.; Criscuolo, F.; Bize, P. Red blood cells open promising avenues for longitudinal studies of ageing in laboratory, non-model and wild animals. Exp. Gerontol. 2015, 71, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Nakai, K.; Nakai, K. Hemolysis and morphological changes in rat erythrocytes with mercurials. Jpn. J. Pharmacol. 1977, 27, 413–419. [Google Scholar] [CrossRef]
- Lillig, C.H.; Holmgren, A. Thioredoxin and Related Molecules–From Biology to Health and Disease. Antioxid. Redox Signalin 2006, 9, 25–47. [Google Scholar] [CrossRef]
- Hall, A.; Karplus, P.A.; Poole, L.B. Typical 2-Cys peroxiredoxins - Structures, mechanisms and functions. FEBS J. 2009, 276, 2469–2477. [Google Scholar] [CrossRef] [Green Version]
- Perkins, A.; Nelson, K.J.; Parsonage, D.; Poole, L.B.; Karplus, P.A. Peroxiredoxins: Guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem. Sci. 2015, 40, 435–445. [Google Scholar] [CrossRef] [Green Version]
- Arnér, E.S.J. Focus on mammalian thioredoxin reductases—Important selenoproteins with versatile functions. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2009, 1790, 495–526. [Google Scholar] [CrossRef]
- Winterbourn, C.C. The Biological Chemistry of Hydrogen Peroxide, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 528, ISBN 9780124058811. [Google Scholar]
- Zhang, Y.; Mi, K.; Ding, X.; Li, Y.; Wang, T.; Dou, T.; Ding, J.; Wei, W. Characterization of a classical 2-cysteine peroxiredoxin1 gene from Chinese soft-shelled turtle Pelodiscus sinensis with its potent antioxidant properties and putative immune response. Dev. Comp. Immunol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.L.; Chew, E.; Hashemy, S.I.; Lu, J. Inhibition of the Human Thioredoxin System. J. Biol. Chem. 2008, 283, 11913–11923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farina, M.; Avila, D.S.; Da Rocha, J.B.T.; Aschner, M. Metals, oxidative stress and neurodegeneration: A focus on iron, manganese and mercury. Neurochem. Int. 2013, 62, 575–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maniero, M.Á.; Wuilloud, R.G.; Callegari, E.A.; Smichowski, P.N.; Fanelli, M.A. Metalloproteomics analysis in human mammary cell lines treated with inorganic mercury. J. Trace Elem. Med. Biol. 2020, 58, 126441. [Google Scholar] [CrossRef]
- Liu, D.L.; Zhao, L.X.; Zhang, S.; Du, J.R. Peroxiredoxin 1-mediated activation of TLR4/NF-κB pathway contributes to neuroinflammatory injury in intracerebral hemorrhage. Int. Immunopharmacol. 2016, 41, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Poynton, R.A.; Hampton, M.B. Peroxiredoxins as biomarkers of oxidative stress. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 906–912. [Google Scholar] [CrossRef]
- Immenschuh, S.; Baumgart-Vogt, E. Peroxiredoxins, oxidative stress, and cell proliferation. Antioxid. Redox Signal. 2005, 7, 768–777. [Google Scholar] [CrossRef]
- Walsh, C.J.; Cocilova, C.; Restivo, J.; Flewelling, L.; Milton, S. Immune function in Trachemys scripta following exposure to a predominant brevetoxin congener, PbTx-3, as a model for potential health impacts for sea turtles naturally exposed to brevetoxins. Ecotoxicol 2019, 28, 1085–1104. [Google Scholar] [CrossRef]
- Krivoruchko, A.; Storey, K.B. Activation of antioxidant defenses in response to freezing in freeze-tolerant painted turtle hatchlings. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2010. [Google Scholar] [CrossRef]
- Storey, K.B. Reptile freeze tolerance: Metabolism and gene expression. Cryobiology 2006, 52, 1–16. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, J.; Li, Y.; Zou, Y.; Lu, B.; Chen, Y.; Ma, Y.; Xu, H. Evaluation of differentially expressed immune-related genes in intestine of Pelodiscus sinensis after intragastric challenge with lipopolysaccharide based on transcriptome analysis. Fish Shellfish Immunol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Branco, V.; Ramos, P.; Canário, J.; Lu, J.; Holmgren, A.; Carvalho, C. Biomarkers of adverse response to mercury: Histopathology versus thioredoxin reductase activity. BioMed Res. Int. 2012, 2012. [Google Scholar] [CrossRef]
- Robitaille, S.; Mailloux, R.J.; Man, H. Methylmercury alters glutathione homeostasis by inhibiting glutaredoxin 1 and enhancing glutathione biosynthesis in cultured human astrocytoma cells. Toxicol. Lett. 2016, 256, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Campos, F.; Vendrell, I.; Berenguer, J.; Barzi, M. Probucol Increases Glutathione Peroxidase-1 Activity and Displays Long-Lasting Protection against Methylmercury Toxicity in Cerebellar Granule Cells. Toxicol. Sci. 2009, 112, 416–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, C.M.L.; Lu, J.; Zhang, X.; Arne, E.S.J.; Effects, A. Effects of selenite and chelating agents on mammalian thioredoxin reductase inhibited by mercury: Implications for treatment of mercury poisoning. FASEB J. 2010, 25, 370–381. [Google Scholar] [CrossRef] [Green Version]
- Shinyashiki, M.; Kumagai, Y.; Homma-Takeda, S.; Nagafune, J.; Shimojo, N. Selective inhibition of the mouse brain Mn-SOD by methylmercury. Environ. Toxicol. Pharmacol. 1996, 2, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Toyama, T.; Sumi, D.; Shinkai, Y.; Yasutake, A.; Taguchi, K.; Tong, K.I.; Yamamoto, M.; Kumagai, Y. Cytoprotective role of Nrf2 / Keap1 system in methylmercury toxicity. Biochem. Biophys. Res. Commun. 2007, 363, 645–650. [Google Scholar] [CrossRef]
- Messer, R.L.W.; Lockwood, P.E.; Tseng, W.Y.; Edwards, K.; Shaw, M.; Caughman, G.B.; Lewis, J.B.; Wataha, J.C.; College, M. Mercury (II) alters mitochondrial activity of monocytes at sublethal doses via oxidative stress mechanisms. J. Biomed. Mater. Res. Part B 2005, 257–263. [Google Scholar] [CrossRef]
- Wataha, J.C.; Lewis, J.B.; Mccloud, V.V.; Shaw, M.; Omata, Y.; Lockwood, P.E.; Messer, R.L.W.; Hansen, J.M. Effect of mercury (II) on Nrf2, thioredoxin reductase-1 and thioredoxin-1 in human monocytes. Dent. Mater. 2007, 24, 765–772. [Google Scholar] [CrossRef]

| ID of Turtles | Weight (Kg) | SCL (cm) | ACL (cm) |
|---|---|---|---|
| UJTL-CC1 | 3.0 | 31 | 28 |
| UJTL-CC3 | 2.9 | 30 | 28 |
| UJTL-CC5 | 2.6 | 29 | 24 |
| UJTL-CC7 | 2.3 | 25 | 22 |
| UJTL-CC8 | 3.1 | 32 | 29 |
| HEMOGRAM | ||||||
|---|---|---|---|---|---|---|
| Variable | Mean | SD | Min | Max | Reference | Unit |
| ERYTHROCYTES | 1.42 | 0.13 | 1.21 | 1.61 | 1.60–70.0 | 105 Cells/μL |
| HEMOGLOBIN | 3 | 0.59 | 2.1 | 3.7 | 3.9–9.5 | g/L |
| HEMATOCRIT | 6.98 | 1.20 | 5 | 8.4 | 9.5–36.0 | (%) |
| M.V.C | 488.5 | 49.97 | 413.2 | 560 | 257.1–1188 | Femtoliters |
| H.C.M. | 210.2 | 30.37 | 174 | 247 | 157.7–216.3 | Picograms |
| C.M.H.C. | 43.2 | 5.91 | 37 | 54 | 24.1–32.4 | (g%) |
| PLASMATIC PROTEIN | 9.74 | 1.23 | 8.5 | 12 | 1.2–5.0 | g/L |
| ALBUMIN | 6.72 | 0.89 | 5.4 | 8 | 0.8–2.2 | g/L |
| GLOBULINS | 3.02 | 1.44 | 1.8 | 5.6 | 0.2–3.3 | g/L |
| THROMBOCYTES | 24 | 12.65 | 3 | 42 | Cells/μL | |
| LEUCOCYTES | 1.83 | 0.14 | 1.59 | 1.99 | 1.760–23.60 | 103 Cells/μL |
| HETEROPHILE | 42 | 2.37 | 38 | 45 | 14.8-91.6 | (%) |
| BASOPHILS | 3.6 | 0.8 | 3 | 5 | 0–16 | (%) |
| EOSINOPHILS | 3 | 1.09 | 2 | 5 | 0-13.7 | (%) |
| LYMPHOCYTES | 45 | 3.03 | 41 | 49 | 17.2-90 | (%) |
| MONOCYTES | 3.4 | 1.85 | 1 | 6 | 0-16.1 | (%) |
| AZUROPHILES | 3 | 1.41 | 1 | 5 | 1-8 | (%) |
| ABSOLUTE PARAMETERS | ||||||
| LYMPHOCYTES | 820 | 50 | 747 | 892 | 429.8–11034.3 | Cells/μL |
| HETEROPHILE | 790 | 60 | 692 | 856 | 1308.1–8400.9 | Cells/μL |
| EOSINOPHILS | 50 | 20 | 32 | 98 | 13.6–2580.2 | Cells/μL |
| MONOCYTES | 60 | 40 | 18 | 117 | 7.2–2796.6 | Cells/μL |
| BASOPHILS | 60 | 20 | 0.048 | 0.1 | 0.064–0.928 | 103Cells/μL |
| AZUROPHILES | 60 | 30 | 15.9 | 99.5 | 78.4–729.9 | Cells/μL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Fernández, J.; López-Barrera, E.A.; Mariño-Ramírez, L.; Rodríguez-Becerra, P.; Pinzón-Velasco, A. Oxidative Stress Biomarkers in Erythrocytes of Captive Pre-Juvenile Loggerhead Turtles Following Acute Exposure to Methylmercury. Appl. Sci. 2020, 10, 3602. https://doi.org/10.3390/app10103602
Hernández-Fernández J, López-Barrera EA, Mariño-Ramírez L, Rodríguez-Becerra P, Pinzón-Velasco A. Oxidative Stress Biomarkers in Erythrocytes of Captive Pre-Juvenile Loggerhead Turtles Following Acute Exposure to Methylmercury. Applied Sciences. 2020; 10(10):3602. https://doi.org/10.3390/app10103602
Chicago/Turabian StyleHernández-Fernández, Javier, Ellie Anne López-Barrera, Leonardo Mariño-Ramírez, Pilar Rodríguez-Becerra, and Andrés Pinzón-Velasco. 2020. "Oxidative Stress Biomarkers in Erythrocytes of Captive Pre-Juvenile Loggerhead Turtles Following Acute Exposure to Methylmercury" Applied Sciences 10, no. 10: 3602. https://doi.org/10.3390/app10103602







