Effects of In Situ Co or Ni Doping on the Photoelectrochemical Performance of Hematite Nanorod Arrays
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
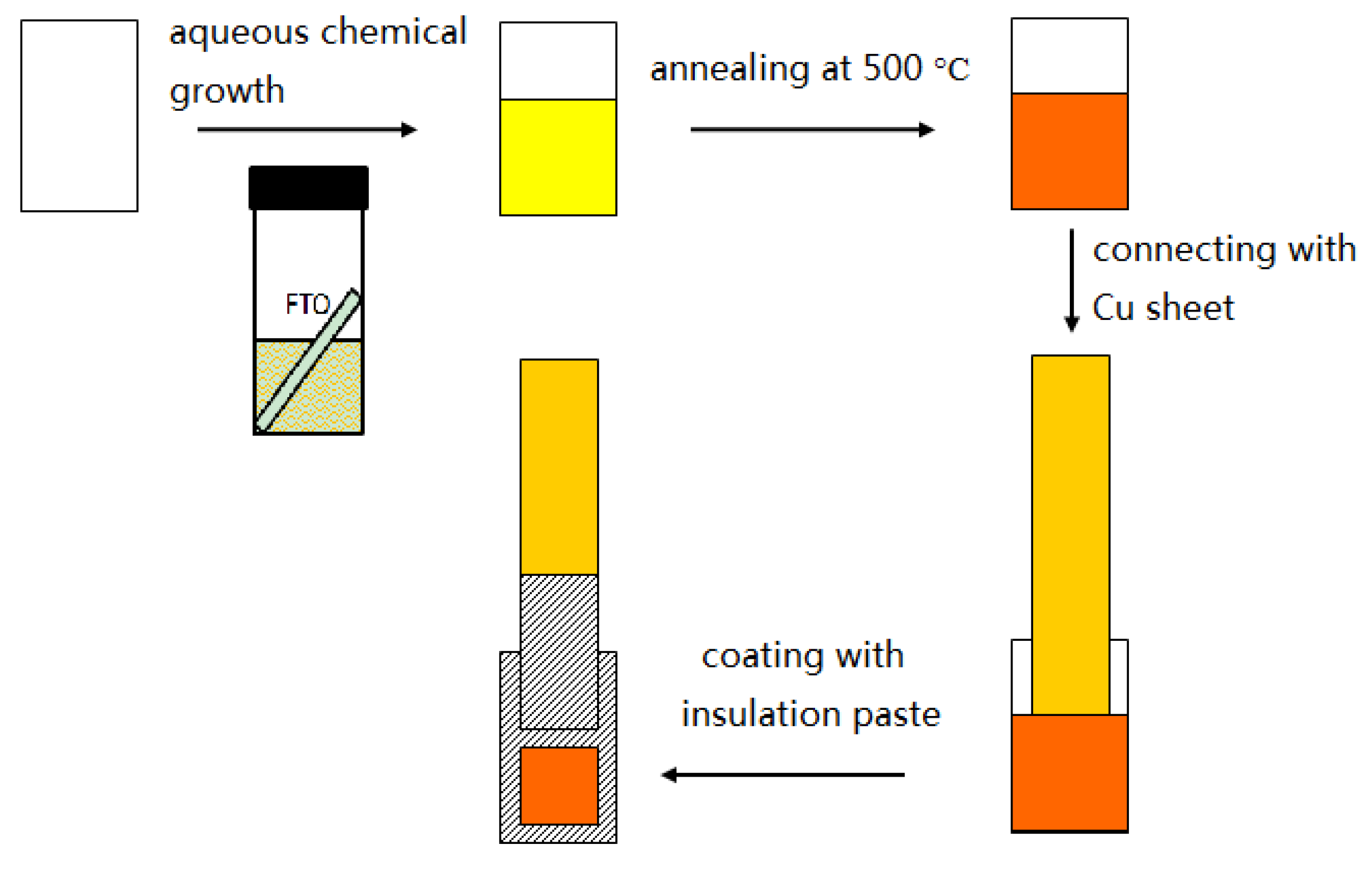
2.1. Materials Preparation
2.2. Materials Characterization
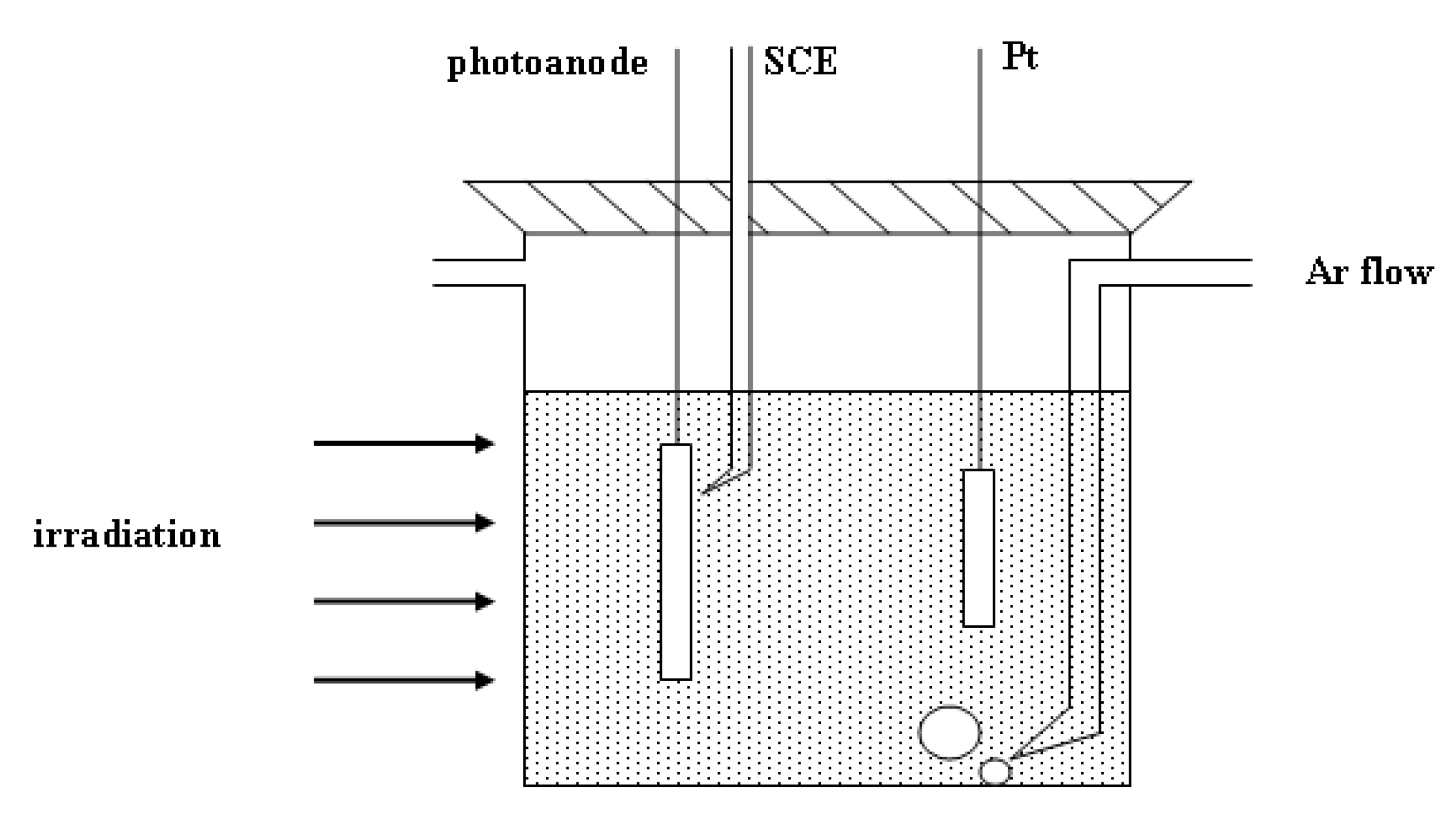
2.3. Photoelectrochemical Measurements
3. Results and Discussion
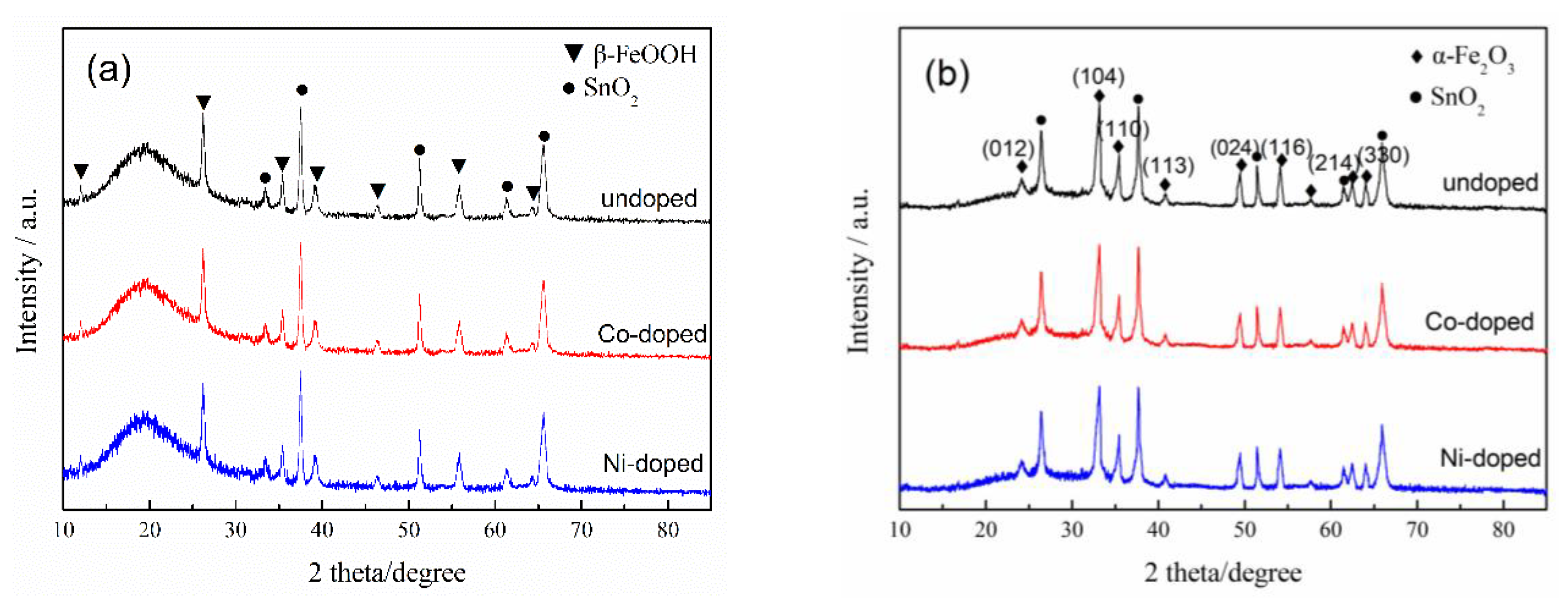
3.1. Effects on Crystalline Phase
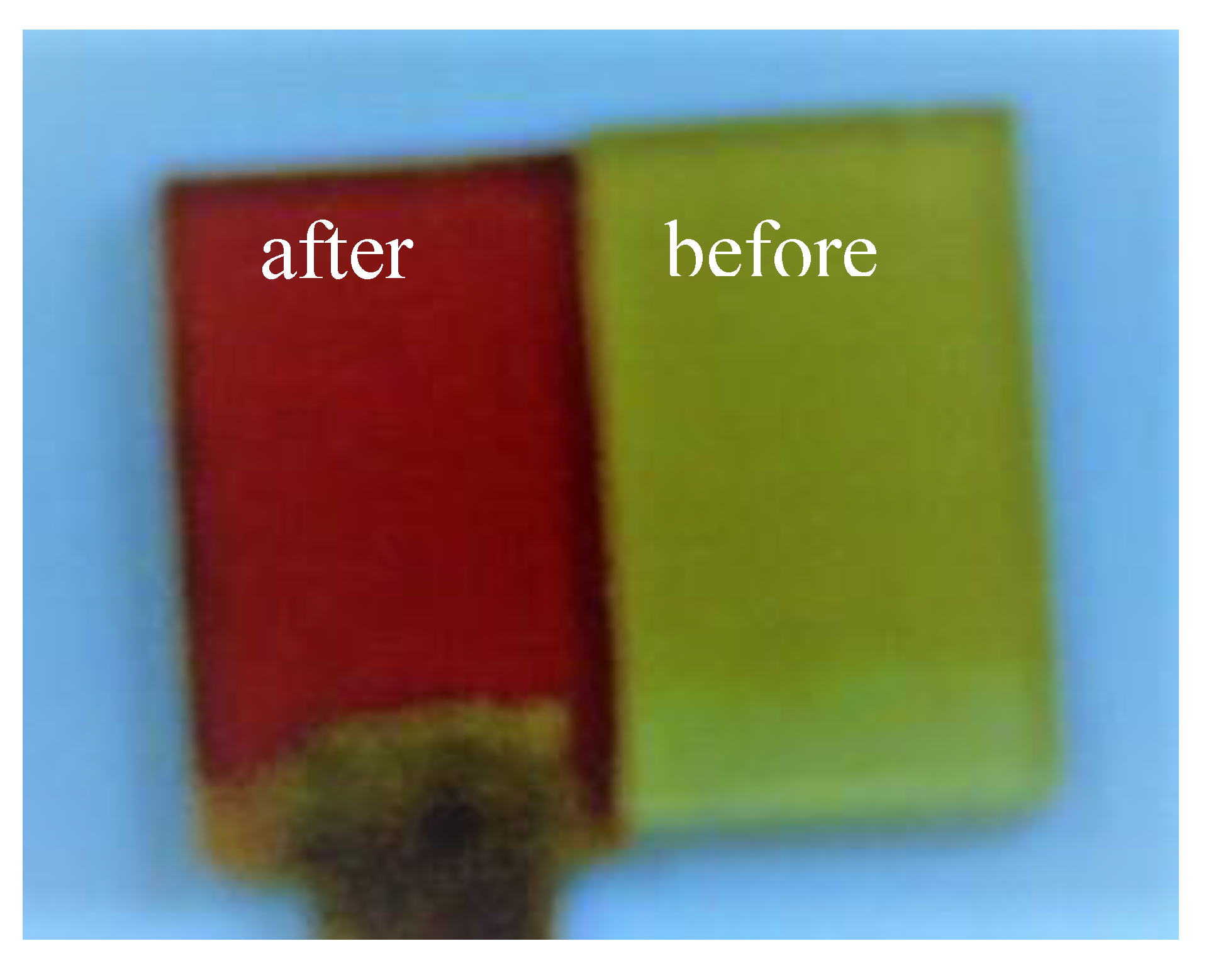
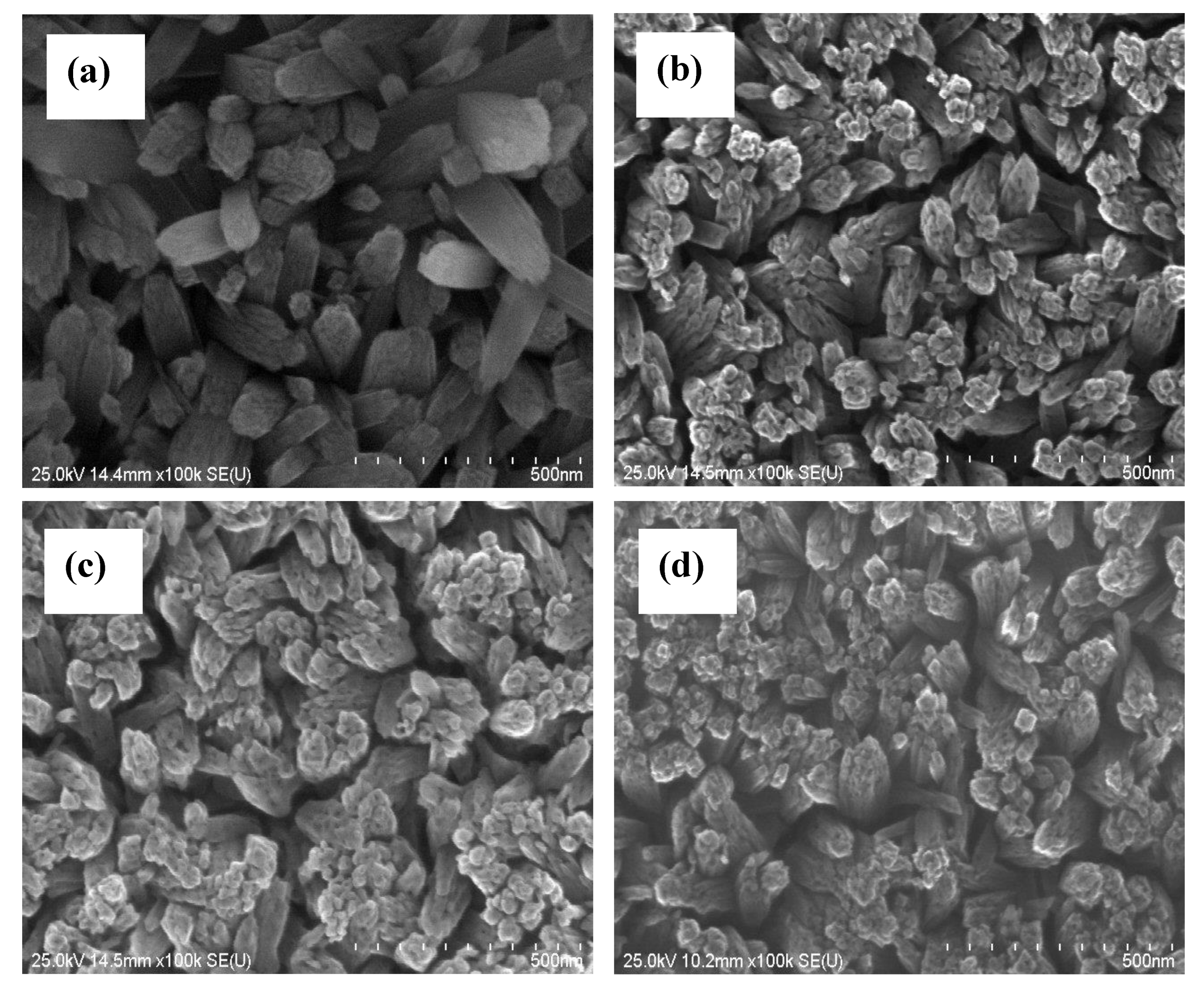
3.2. Effects on Morphology
3.3. Effects on Ultraviolet (UV)–Visible Absorption
- Eg: energy gap in eV between a conduction band and a valence band
- λ: wavelength in nm
- α: absorption coefficient
- hν: the energy of a photon
- B: a constant
- n: a constant 0.5 for direct transition and 2.0 for indirect transition
- A: absorbance
- b: the thickness of light-absorbing substance
- c: the concentration of light-absorbing substance
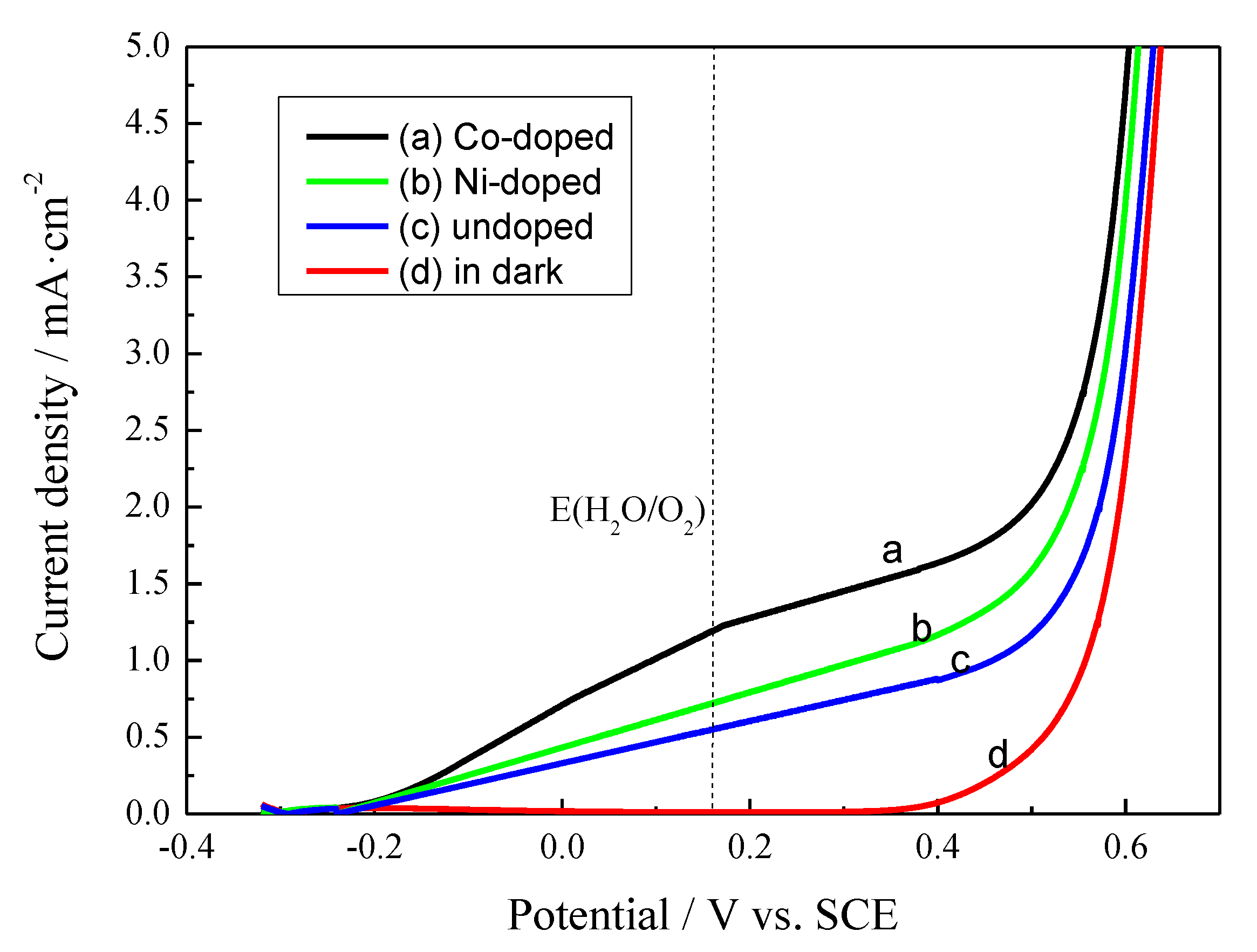
3.4. Effects on Photoelectrochemical Performance
3.4.1. Current-voltage (I-V) Plots
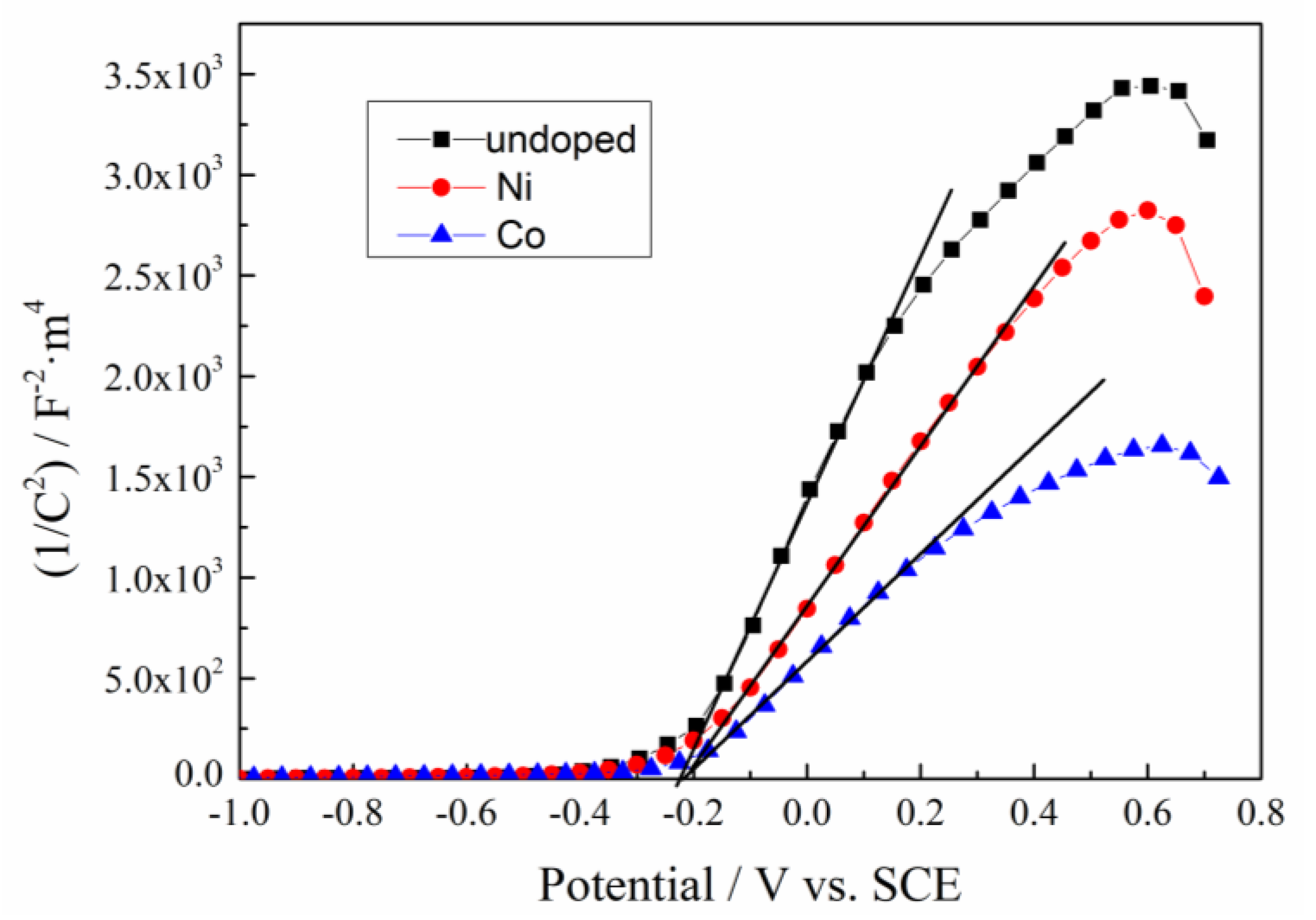
3.4.2. Mott–Schottky Plots
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, F.; Zhu, S.; Xu, Y.; Liang, Q.; Chen, Z. Controlled charge-dynamics in cobalt-doped TiO2 nanowire photoanodes for enhanced photoelectrochemical water splitting. J. Colloid Interface Sci. 2018, 530, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Ding, D.; Li, T.; Ning, C. Black Si-doped TiO2 nanotube photoanode for high-efficiency photoelectrochemical water splitting. RSC Adv. 2018, 8, 5652–5660. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; He, J.; Zhu, H.; Zhou, X.; Li, M.; Yang, Q.; Xu, F. Enhanced photoelectrochemical properties of alpha-Fe2O3 nanoarrays for water splitting. J. Alloy. Compd. 2018, 753, 601–606. [Google Scholar] [CrossRef]
- Zhang, R.; Fang, Y.; Chen, T.; Qu, F.; Liu, Z.; Du, G.; Asiri, A.M.; Gao, T.; Sun, X. Enhanced Photoelectrochemical Water Oxidation Performance of Fe2O3 Nanorods Array by S Doping. Acs Sustain. Chem. Eng. 2017, 5, 7502–7506. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.; Dong, Y.; Wu, Q.; Xue, S. Highly efficient hematite films via mid-/ex-situ Sn-doping for photoelectrochemical water oxidation. Int. J. Hydrog. Energy 2017, 42, 16012–16022. [Google Scholar] [CrossRef]
- Wang, B.-S.; Li, R.-Y.; Zhang, Z.-Y.; Xing, W.; Wu, X.-L.; Cheng, G.-A.; Zheng, R.-T. An overlapping ZnO nanowire photoanode for photoelectrochemical water splitting. Catal. Today 2019, 321, 100–106. [Google Scholar] [CrossRef]
- Altaf, C.T.; Yolacan, D.; Sankir, N.D. Decoration of 3D ZnO nanoelectrodes with CuInS2 for solar water splitting. Mater. Lett. 2019, 236, 710–714. [Google Scholar] [CrossRef]
- Song, K.; Ma, Z.; Yang, W.; Hou, H.; Gao, F. Electrospinning WO3 nanofibers with tunable Fe-doping levels towards efficient photoelectrochemical water splitting. J. Mater. Sci. Mater. Electron. 2018, 29, 8338–8346. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Yoo, I.-H.; Eom, K.; Seo, H. Enhancement of photoelectrochemical water splitting response of WO3 by Means of Bi doping. J. Catal. 2018, 357, 127–137. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Seo, H. Influence of molybdenum doping on the structural, optical and electronic properties of WO3 for improved solar water splitting. J. Colloid Interface Sci. 2018, 509, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Sangle, A.L.; Singh, S.; Jian, J.; Bajpe, S.R.; Wang, H.; Khare, N.; MacManus-Driscoll, J.L. Very High Surface Area Mesoporous Thin Films of SrTiO3 Grown by Pulsed Laser Deposition and Application to Efficient Photoelectrochemical Water Splitting. Nano Lett. 2016, 16, 7338–7345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asai, R.; Nemoto, H.; Jia, Q.; Saito, K.; Iwase, A.; Kudo, A. A visible light responsive rhodium and antimony-codoped SrTiO3 powdered photocatalyst loaded with an IrO2 cocatalyst for solar water splitting. Chem. Commun. 2014, 50, 2543–2546. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, W.; Li, D.; Zhang, L.; Jiang, D. Solar Light Driven Pure Water Splitting on Quantum Sized BiVO4 without any Cocatalyst. Acs Catal. 2014, 4, 3498–3503. [Google Scholar] [CrossRef]
- Suarez, C.M.; Hernandez, S.; Russo, N. BiVO4 as photocatalyst for solar fuels production through water splitting: A short review. Appl. Catal. a-Gen. 2015, 504, 158–170. [Google Scholar] [CrossRef]
- Shen, S.; Jiang, J.; Guo, P.; Kronawitter, C.X.; Mao, S.S.; Guo, L. Effect of Cr doping on the photoelectrochemical performance of hematite nanorod photoanodes. Nano Energy 2012, 1, 732–741. [Google Scholar] [CrossRef]
- Mulmudi, H.K.; Mathews, N.; Dou, X.C.; Xi, L.F.; Pramana, S.S.; Lam, Y.M.; Mhaisalkar, S.G. Controlled growth of hematite (alpha-Fe2O3) nanorod array on fluorine doped tin oxide: Synthesis and photoelectrochemical properties. Electrochem. Commun. 2011, 13, 951–954. [Google Scholar] [CrossRef]
- Mohapatra, S.K.; John, S.E.; Banerjee, S.; Misra, M. Water Photooxidation by Smooth and Ultrathin α-Fe2O3 Nanotube Arrays. Chem. Mater. 2009, 21, 3048–3055. [Google Scholar] [CrossRef]
- Lindgren, T.; Wang, H.; Beermann, N.; Vayssieres, L.; Hagfeldt, A.; Lindquist, S.-E. Aqueous photoelectrochemistry of hematite nanorod array. Sol. Energy Mater. Sol. Cells 2002, 71, 231–243. [Google Scholar] [CrossRef]
- Vayssieres, L.; Beermann, N.; Lindquist, S.E.; Hagfeldt, A. Controlled aqueous chemical growth of oriented three-dimensional crystalline nanorod arrays: Application to iron(III) oxides. Chem. Mater. 2001, 13, 233–235. [Google Scholar] [CrossRef]
- Mao, A.; Shin, K.; Kim, J.K.; Wang, D.H.; Han, G.Y.; Park, J.H. Controlled Synthesis of Vertically Aligned Hematite on Conducting Substrate for Photoelectrochemical Cells: Nanorods versus Nanotubes. Acs Appl. Mater. Interfaces 2011, 3, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.; Cesar, I.; Grätzel, M. New Benchmark for Water Photooxidation by Nanostructured α-Fe2O3 Films. J. Am. Chem. Soc. 2006, 128, 15714–15721. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.; Han, G.Y.; Park, J.H. Synthesis and photoelectrochemical cell properties of vertically grown α-Fe2O3 nanorod arrays on a gold nanorod substrate. J. Mater. Chem. 2010, 20, 2247–2250. [Google Scholar] [CrossRef]
- Souza, F.L.; Lopes, K.P.; Nascente, P.A.P.; Leite, E.R. Nanostructured hematite thin films produced by spin-coating deposition solution: Application in water splitting. Sol. Energy Mater. Sol. Cells 2009, 93, 362–368. [Google Scholar] [CrossRef]
- Lassoued, A.; Lassoued, M.S.; Garcia-Granda, S.; Dkhil, B.; Ammar, S.; Gadri, A. Synthesis and characterization of Ni-doped alpha-Fe2O3 nanoparticles through co-precipitation method with enhanced photocatalytic activities. J. Mater. Sci. Mater. Electron. 2018, 29, 5726–5737. [Google Scholar] [CrossRef]
- Kment, Š.; Sivula, K.; Naldoni, A.; Sarmah, S.P.; Kmentová, H.; Kulkarni, M.; Rambabu, Y.; Schmuki, P.; Zbořil, R. FeO-based nanostructures and nanohybrids for photoelectrochemical water splitting. Prog. Mater. Sci. 2020, 110, 100632. [Google Scholar] [CrossRef]
- Bouhjar, F.; Mollar, M.; Chourou, M.L.; Mari, B.; Bessais, B. Hydrothermal synthesis of nanostructured Cr-doped hematite with enhanced photoelectrochemical activity. Electrochim. Acta 2018, 260, 838–846. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Xu, J.; Situ, Y.; Huang, H. Morphology-controlled synthesis of Ti-doped alpha-Fe2O3 nanorod arrays as an efficient photoanode for photoelectrochemical applications. Res. Chem. Intermed. 2018, 44, 2365–2378. [Google Scholar] [CrossRef]
- Zheng, D.; He, X.; Xu, W.; Lu, X. Self-surface-passivation of titanium doped hematite photoanode for efficient solar water and formaldehyde oxidation. Mater. Res. Bull. 2017, 96, 354–359. [Google Scholar] [CrossRef]
- Kaambre, T.; Vanags, M.; Parna, R.; Kisand, V.; Ignatans, R.; Kleperis, J.; Sutka, A. Yttrium-doped hematite photoanodes for solar water splitting: Photoelectrochemical and electronic properties. Ceram. Int. 2018, 44, 13218–13225. [Google Scholar] [CrossRef]
- Singh, A.P.; Saini, N.; Mehta, B.R.; Carraro, G.; Barreca, D. Hematite Thin Film Photoanodes for Visible Light Water Photooxidation: Effects of Zn Doping and Hydrogen Treatment. J. Nanosci. Nanotechnol. 2017, 17, 8959–8966. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, L.; Huang, X.; Chen, T.; Qu, F.; Liu, Z.; Du, G.; Asiri, A.M.; Sun, X. Se doping: An effective strategy toward Fe2O3 nanorod arrays for greatly enhanced solar water oxidation. J. Mater. Chem. A 2017, 5, 12086–12090. [Google Scholar] [CrossRef]
- Chang, H.W.; Fu, Y.M.; Lee, W.Y.; Lu, Y.R.; Huang, Y.C.; Chen, J.L.; Chen, C.L.; Chou, W.C.; Chen, J.M.; Lee, J.F.; et al. Visible light-induced electronic structure modulation of Nb- and Ta-doped alpha-Fe2O3 nanorods for effective photoelectrochemical water splitting. Nanotechnology 2018, 29. [Google Scholar] [CrossRef] [PubMed]
- Bemana, H.; Rashid-Nadimi, S. Effect of sulfur doping on photoelectrochemical performance of hematite. Electrochim. Acta 2017, 229, 396–403. [Google Scholar] [CrossRef]
- Luo, Z.; Li, C.; Liu, S.; Wang, T.; Gong, J. Gradient doping of phosphorus in Fe2O3 nanoarray photoanodes for enhanced charge separation. Chem. Sci. 2017, 8, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Zuo, F.; Dagg, A.; Feng, P. A Three-Dimensional Branched Cobalt-Doped α-Fe2O3 Nanorod/MgFe2O4 Heterojunction Array as a Flexible Photoanode for Efficient Photoelectrochemical Water Oxidation. Angew. Chem. 2013, 125, 1286–1290. [Google Scholar] [CrossRef]
- Suresh, R.; Vijayalakshmi, L.; Stephen, A.; Narayanan, V. Hydrothermal Synthesis and Characterization of Cobalt Doped α-Fe2O3. AIP Conf. Proc. 2010, 1276, 362–367. [Google Scholar] [CrossRef]
- Wang, J.; Du, C.; Peng, Q.; Yang, J.; Wen, Y.; Shan, B.; Chen, R. Enhanced photoelectrochemical water splitting performance of hematite nanorods by Co and Sn co-doping. Int. J. Hydrog. Energy 2017, 42, 29140–29149. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, G.; Wheeler, D.A.; Zhang, J.Z.; Li, Y. Sn-Doped Hematite Nanostructures for Photoelectrochemical Water Splitting. Nano Lett. 2011, 11, 2119–2125. [Google Scholar] [CrossRef]
- Cherepy, N.J.; Liston, D.B.; Lovejoy, J.A.; Hongmei, D.; Zhang, J.Z. Ultrafast studies of photoexcited electron dynamics in gamma- and alpha-Fe2O3 semiconductor nanoparticles. J. Phys. Chem. B 1998, 102, 770–776. [Google Scholar] [CrossRef]
- Kennedy, J.H. Photooxidation of Water at α-Fe2O3 Electrodes. J. Electrochem. Soc. 1978, 125, 709–714. [Google Scholar] [CrossRef]
- Vayssieres, L.; Sathe, C.; Butorin, S.M.; Shuh, D.K.; Nordgren, J.; Guo, J.H. One-dimensional quantum-confinement effect in alpha-Fe2O3 ultrafine nanorod arrays. Adv. Mater. 2005, 17, 2320–2323. [Google Scholar] [CrossRef]
- Glasscock, J.A.; Barnes, P.R.F.; Plumb, I.C.; Savvides, N. Enhancement of photoelectrochemical hydrogen production from hematite thin films by the introduction of Ti and Si. J. Phys. Chem. C 2007, 111, 16477–16488. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, S.; Song, W.; Zhou, P.; Ji, H.; Ma, W.; Hao, W.; Chen, C.; Zhao, J. Nonmetal P-doped hematite photoanode with enhanced electron mobility and high water oxidation activity. Energy Environ. Sci. 2015, 8, 1231–1236. [Google Scholar] [CrossRef]
- Suryanto, B.H.R.; Wang, Y.; Hocking, R.K.; Adamson, W.; Zhao, C. Overall electrochemical splitting of water at the heterogeneous interface of nickel and iron oxide. Nat. Commun. 2019, 10, 5599. [Google Scholar] [CrossRef]
- Malviya, K.D.; Dotan, H.; Shlenkevich, D.; Tsyganok, A.; Mor, H.; Rothschild, A. Systematic comparison of different dopants in thin film hematite (α-Fe2O3) photoanodes for solar water splitting. J. Mater. Chem. A 2016, 4, 3091–3099. [Google Scholar] [CrossRef]

| Samples | Photocurrent (mA/cm2) | Potential (V vs. RHE) | Reference No. |
|---|---|---|---|
| undoped | 0.00498 | 0.85 | 16 |
| Cr-doped | 0.00903 | 0.85 | 16 |
| undoped | 0.72 | 1.23 | 4 |
| S-doped | 1.42 | 1.23 | 5 |
| undoped | 0.58 | 1.23 | 5 |
| Sn-doped | 1.54 | 1.23 | 6 |
| Y-doped | 0.0022 | 0.442 | 30 |
| P-doped | 3 | 1.23 | 44 |
| Sn-doped | 1.86 | 1.23 | 39 |
| undoped | 0.523 | 0 | 17 |
| Undoped | 0.61 | 1.27 | Present work |
| Ni-doped | 0.79 | 1.27 | Present work |
| Co-doped | 1.28 | 1.27 | Present work |
| Samples | Slope | Charge Carrier Density (m−3) | Flat-Band Potential Efb (V) |
|---|---|---|---|
| undoped | 6.04 × 103 | 1.65 × 1025 | −0.226 |
| Co-doped | 2.66 × 103 | 3.74 × 1025 | −0.220 |
| Ni-doped | 3.98 × 103 | 2.50 × 1025 | −0.218 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, F.; Li, X. Effects of In Situ Co or Ni Doping on the Photoelectrochemical Performance of Hematite Nanorod Arrays. Appl. Sci. 2020, 10, 3567. https://doi.org/10.3390/app10103567
Cheng F, Li X. Effects of In Situ Co or Ni Doping on the Photoelectrochemical Performance of Hematite Nanorod Arrays. Applied Sciences. 2020; 10(10):3567. https://doi.org/10.3390/app10103567
Chicago/Turabian StyleCheng, Feng, and Xiuwei Li. 2020. "Effects of In Situ Co or Ni Doping on the Photoelectrochemical Performance of Hematite Nanorod Arrays" Applied Sciences 10, no. 10: 3567. https://doi.org/10.3390/app10103567
APA StyleCheng, F., & Li, X. (2020). Effects of In Situ Co or Ni Doping on the Photoelectrochemical Performance of Hematite Nanorod Arrays. Applied Sciences, 10(10), 3567. https://doi.org/10.3390/app10103567






