Evaluation of the Effectiveness of N-Acetylcysteine (NAC) and N-acetylcysteine-cyclodextrins Multi-Composite in Pseudomonas aeruginosa Biofilm Formation
Abstract
:1. Introduction
2. Materials
3. Methods
3.1. Analytical Quantification of N-Acetylcysteine within NACESOLTM
3.2. Chemico-Physical Characterization
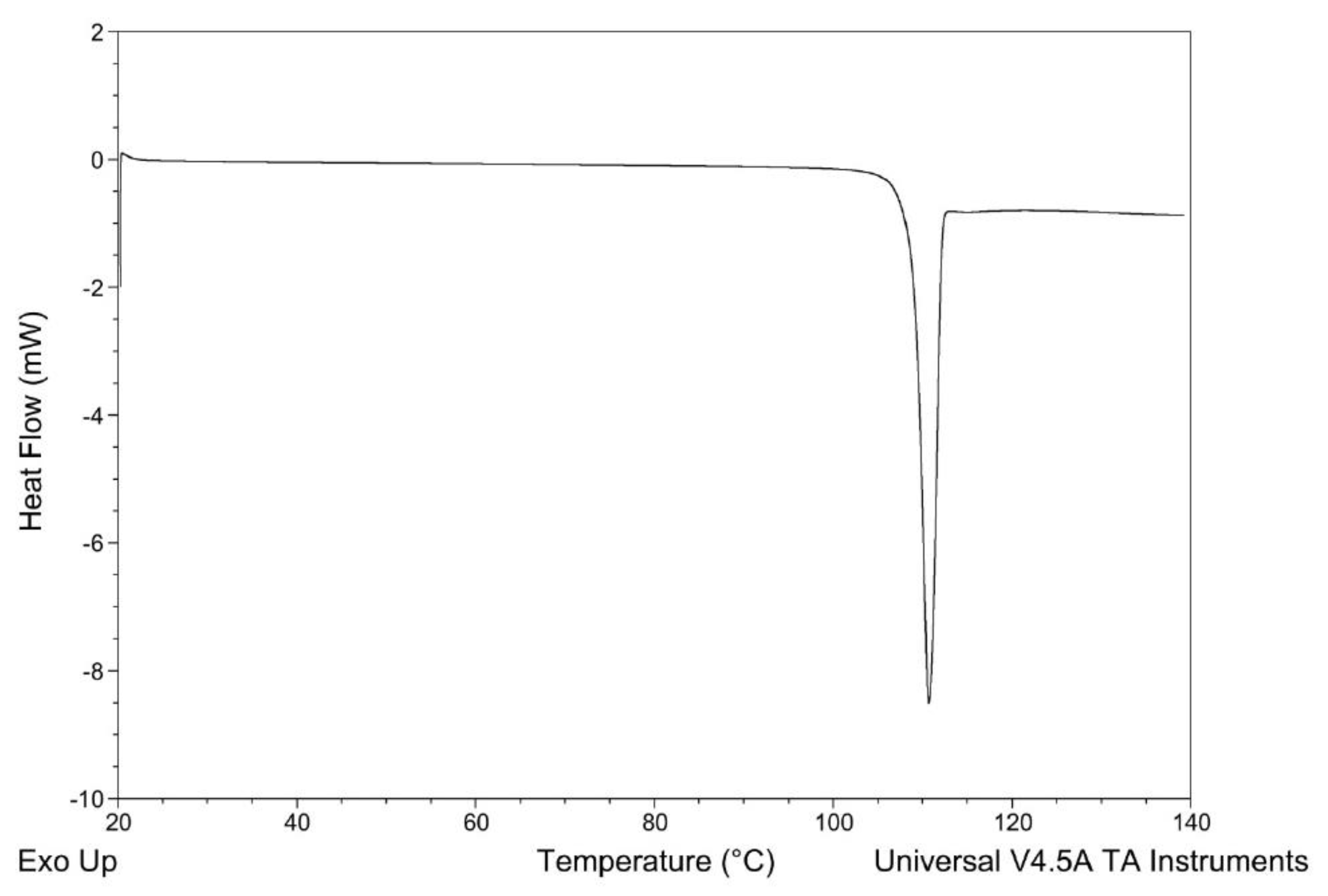
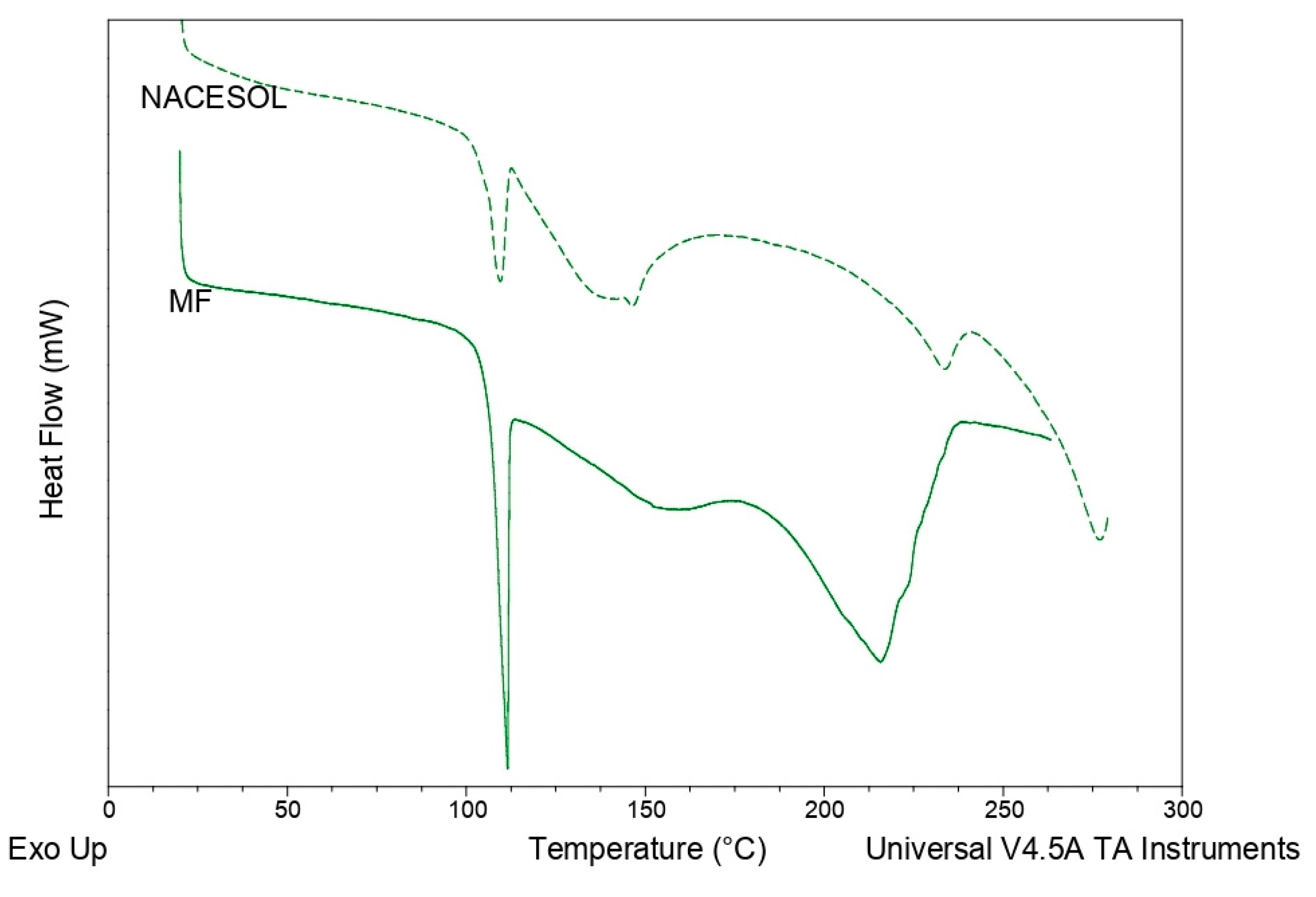
3.2.1. Thermal Analysis: Differential Scanning Calorimetry (DSC)
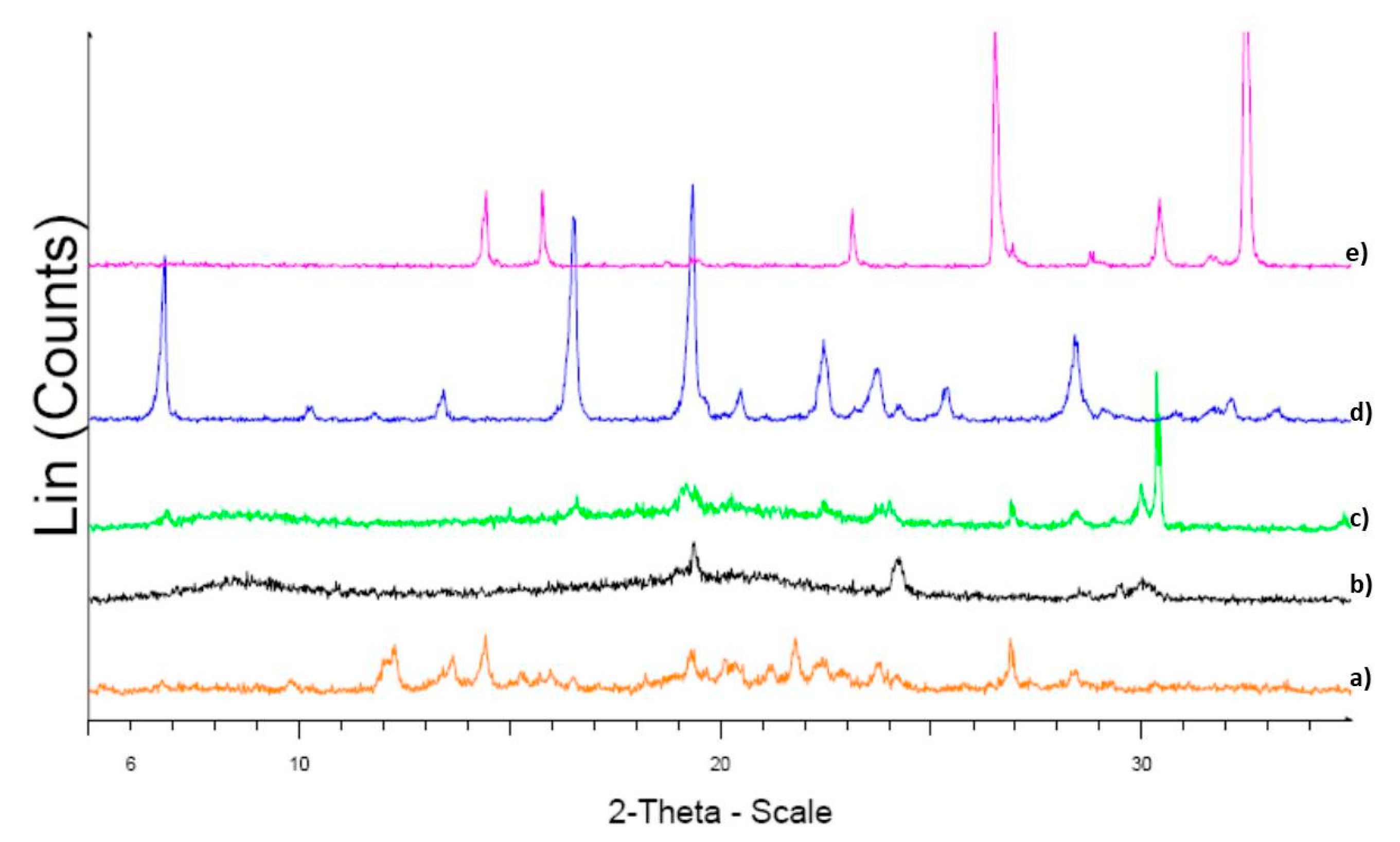
3.2.2. X-ray Powder Diffraction (XRPD) Measurements
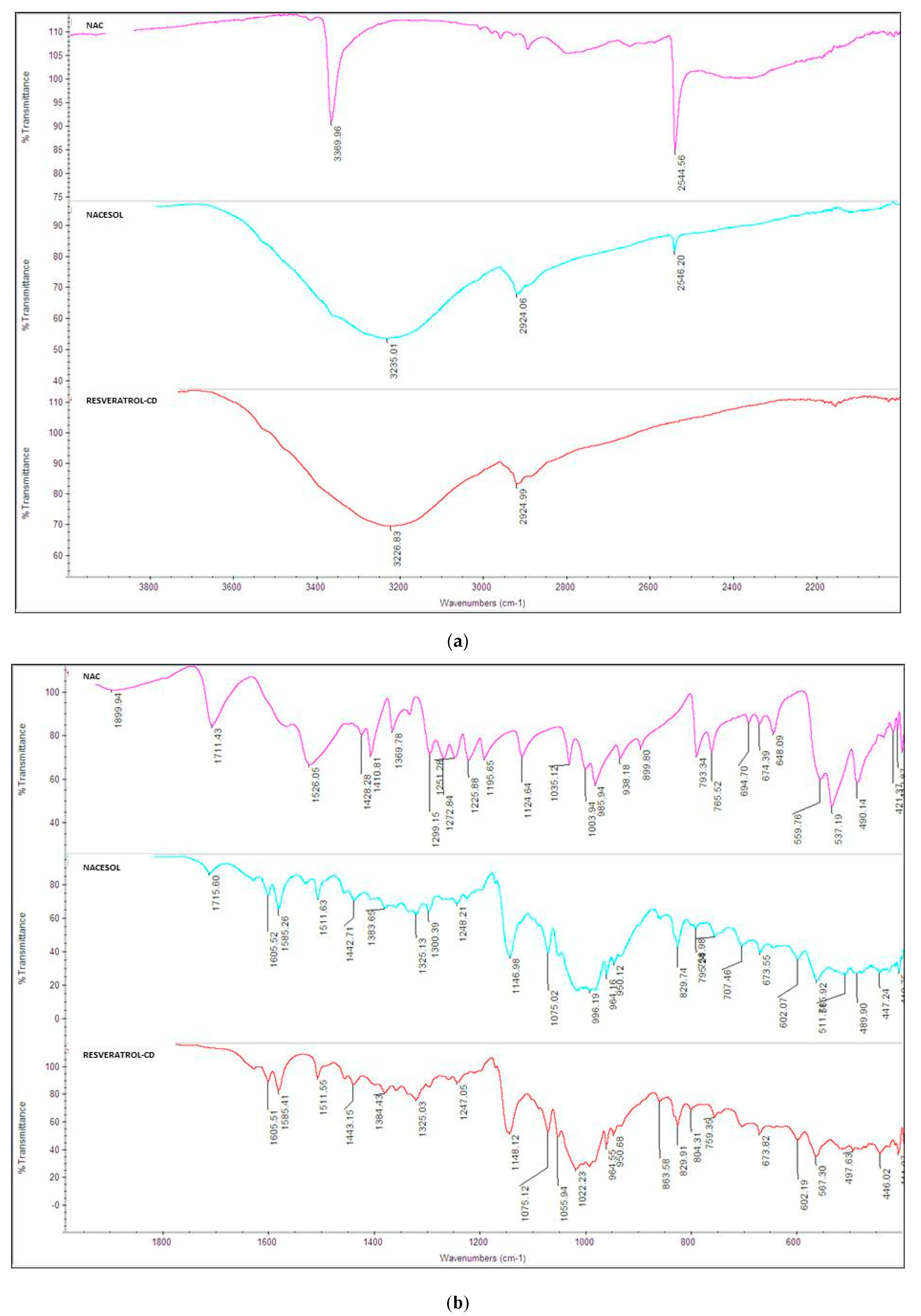
3.2.3. Fourier-Transform Infrared (FTIR) Spectroscopy Measurements
3.3. Biofilm Assay
3.3.1. Microbial Strains and Culture Conditions
3.3.2. Biofilm Growth Protocol
3.3.3. Prevention of Biofilm Formation Assay
3.4. Statistical Analysis
3.5. Scanning Electron Microscopy (SEM)
4. Results
4.1. Analytical Quantification of N-Acetylcysteine within NACESOLTM
4.2. Chemico-Physical Characterization
4.2.1. DSC Measurements
4.2.2. XRPD Measurements
4.2.3. FT-IR Measurements
4.3. Biofilm Assay
4.3.1. Biofilm Growth Protocol
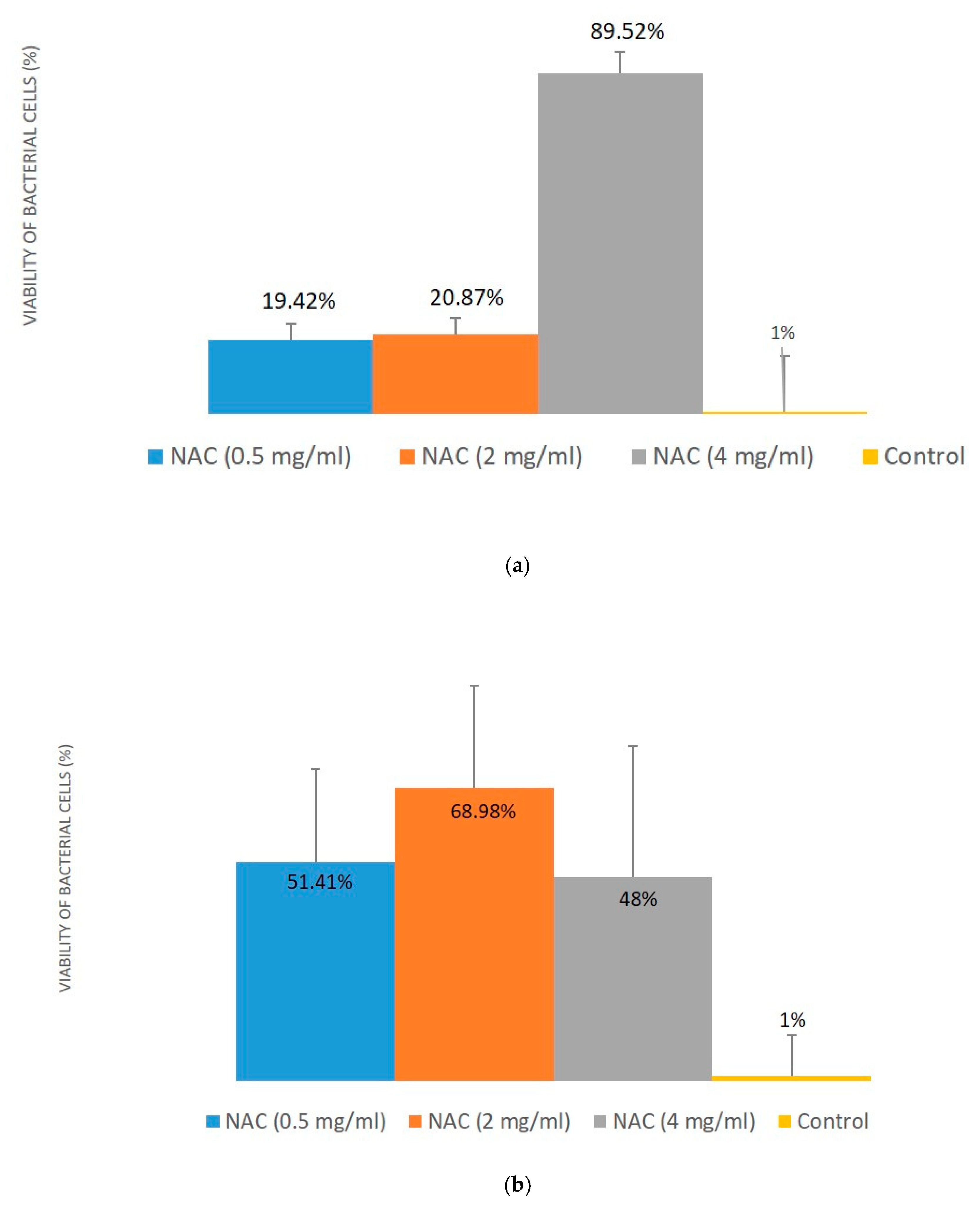
4.3.2. Biofilm Formation Assay
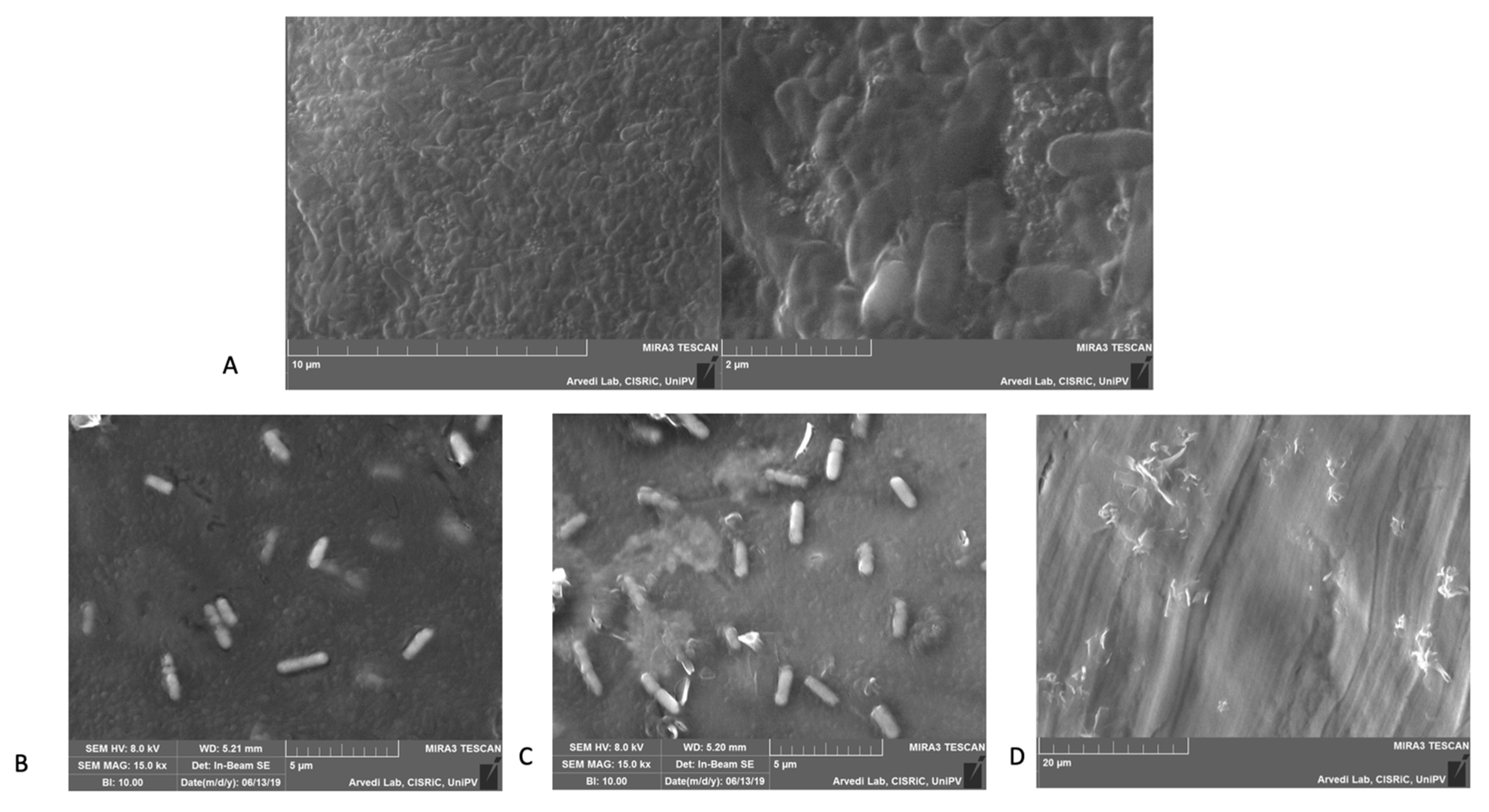
4.4. Scanning Electron Microscope and Elementary Analysis
5. Discussion
- Stabilization to oxidation;
- Protection against degradation of substances or by microorganisms;
- Masking of the smell;
- Catalytic activity of CDs with guest molecule.
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hentzer, M.; Teitzel, G.M.; Balzer, G.J.; Heydorn, A.; Molin, S.; Givskov, M.; Parsek, M.R. Alginate Overproduction Affects Pseudomonas aeruginosa Biofilm Structure and Function. J. Bacteriol. 2001, 183, 5395–5401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjarnsholt, T.; Jensen, P.Ø.; Fiandaca, M.J.; Pedersen, J.; Hansen, C.R.; Andersen, C.B.; Pressler, T.; Givskov, M.; Høiby, N. Pseudomonas aeruginosabiofilms in the respiratory tract of cystic fibrosis patients. Pediatr. Pulmonol. 2009, 44, 547–558. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G. Biofilm formation as mi-crobial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Lewandowski, Z. Structure and function of biofilm. In Biofilm: Recent Advances in Their Study and Control; Harwood Academic Publishers: Amsterdam, The Netherlands, 2000; pp. 1–17. [Google Scholar]
- Donlan, R.M. Role of Biofilms in Antimicrobial Resistance. ASAIO J. 2000, 46, S47–S52. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Chan, R.; Lam, K.; Costerton, J.W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect. Immun. 1980, 28, 546–556. [Google Scholar]
- Heydon, A. Experimental reproducibility in flow-chamber biofilms. Microbiology 2000, 146, 2409–2415. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, S.S.; Kharazmi, A.; Espersen, F.; Høiby, N. Pseudomonas aeruginosa alginate in cystic fibrosis sputum and the inflammatory response. Infect. Immun. 1990, 58, 3363–3368. [Google Scholar] [CrossRef] [Green Version]
- Ryder, C.; Byrd, M.; Wozniak, D.J. Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Curr. Opin. Microbiol. 2007, 10, 644–648. [Google Scholar] [CrossRef] [Green Version]
- Nivens, D.E. Role of alginate and its O acetylation in formation of Pseudomonas aeruginosa mi- crocolonies and biofilms. J. Bacteriol. 2001, 183, 1047–1057. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Conover, M.; Lu, H.; Parsek, M.R.; Bayles, K.; Wozniak, D.J. Assembly and Development of the Pseudomonas aeruginosa Biofilm Matrix. PLoS Pathog. 2009, 5, e1000354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, L.; Kolter, R. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol. Microbiol. 2003, 51, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Tirouvanziam, R.; Conrad, C.K.; Bottiglieri, T.; Herzenberg, L.A.; Moss, R.B.; Herzenberg, L.A. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4628–4633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldini, G. N-Acetylcysteine as an antioxidant and diuslphide bond breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef]
- Zhao, T. N-acetylcysteine inhibit biofilms produced by Research article Pseudomonas aeruginosa. BMC Microbiol. 2010, 10, 140. [Google Scholar] [CrossRef] [Green Version]
- Olofosson, A.C. N-Acetyl-L-Cysteine affects growth, extracellular polysaccharide production, and bacterial biofilm formation on solid surface. Appl. Environ. Microbiol. 2003, 69, 4814–4822. [Google Scholar] [CrossRef] [Green Version]
- Carli, F. Co-Grinding Process for the Preparation of a Ternary Composition. Patent Number WO 03/097012 A1, 27 November 2003. [Google Scholar]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 4117–4129. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Calheiros, L. SEM analysis of surface impact on biofilm antibiotic treatment. Scanning 2017, 2017, 2960194. [Google Scholar]
- Archakam, S.C. Quantitative determination of N-acetylcysteine by RP-HPLC method in bulk and parenteral inijection. Asian J. Pharm. Pharmacol. 2018, 4, 702–705. [Google Scholar] [CrossRef]
- Ceresa, C.; Fracchia, L.; Williams, M.; Banat, I.M.; De Rienzo, M.A.D. The effect of sophorolipids against microbial biofilms on medical-grade silicone. J. Biotechnol. 2019, 309, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.P.; Cerqueira, M.A.; Faustino, A.; Azeredo, J. Use of newly isolated phages for control of Pseudomonas aeruginosa PAO1 and ATCC 10145 biofilms. Res. Microbiol. 2011, 162, 798–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Stewart, P.S. Biofilm removal caused by chemical treatments. Water Res. 2000, 34, 4229–4233. [Google Scholar] [CrossRef]
- Taglietti, A. Antibiofilm activity pf monolayer of silver nanoparticles anchored to an amino-silanized glass surface. Biomaterial 2014, 35, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Bakich, S.L. Protocol for Simulated Natural Biofilm Formation. Patent Number EP1053299A1, 12 February 1999. [Google Scholar]
- Li, X.-H.; Kim, S.-K.; Lee, J.-H. Anti-biofilm effects of anthranilate on a broad range of bacteria. Sci. Rep. 2017, 7, 8604. [Google Scholar] [CrossRef]
- Greco, C.; Martincic, I.; Gusinjac, A.; Kaláb, M.; Yang, A.-F.; Ramírez-Arcos, S. Staphylococcus epidermidis forms biofilms under simulated platelet storage conditions. Transfusion 2007, 47, 1143–1153. [Google Scholar] [CrossRef]
- Maurice, N.M.; Bedi, B.; Sadikot, R.T. Pseudomonas aeruginosa Biofilms: Host Response and Clinical Implications in Lung Infections. Am. J. Respir. Cell Mol. Boil. 2018, 58, 428–439. [Google Scholar] [CrossRef]
- Del Valle, M. Cyclodextrins and theri uses: A review. Process Biochem. 2003, 39, 1033–1046. [Google Scholar] [CrossRef]
- La Placa, M. I Principi di Microbiologia Medica, 12th ed.; Esculapio: Bologna, Italy, 2010. [Google Scholar]
- El Abed, S. Scanning Electron Microscopy (SEM) and enviromental SEM: Suitable tools for study of adhesion stage and biofilm formation. Scanning Electron Microsc. 2012, 35, 717–730. [Google Scholar] [CrossRef] [Green Version]


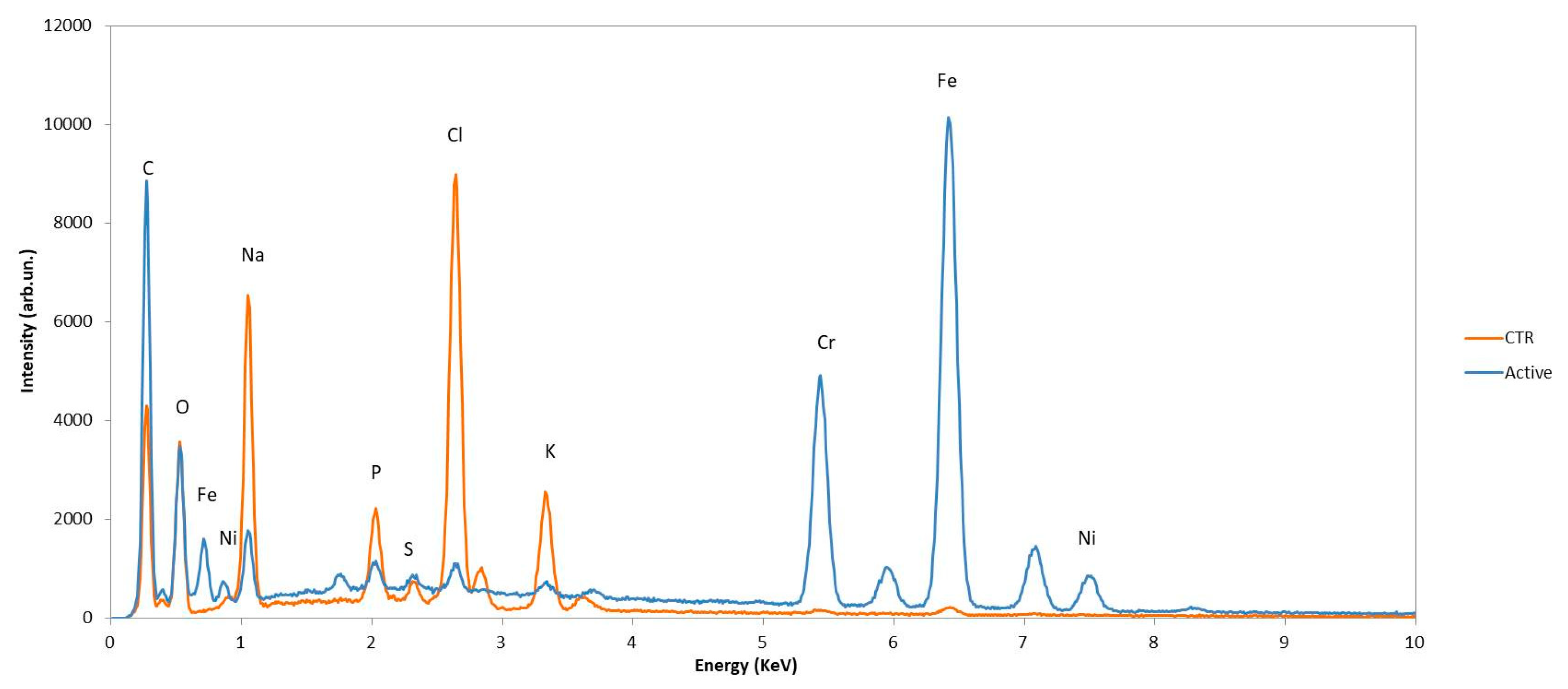
| S Content (%) | S.D. | |
|---|---|---|
| CTR | 0.43 | 0.018 |
| ACTIVE | 0.24 | 0.095 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerini, M.; Perugini, P.; Grisoli, P. Evaluation of the Effectiveness of N-Acetylcysteine (NAC) and N-acetylcysteine-cyclodextrins Multi-Composite in Pseudomonas aeruginosa Biofilm Formation. Appl. Sci. 2020, 10, 3466. https://doi.org/10.3390/app10103466
Guerini M, Perugini P, Grisoli P. Evaluation of the Effectiveness of N-Acetylcysteine (NAC) and N-acetylcysteine-cyclodextrins Multi-Composite in Pseudomonas aeruginosa Biofilm Formation. Applied Sciences. 2020; 10(10):3466. https://doi.org/10.3390/app10103466
Chicago/Turabian StyleGuerini, Marta, Paola Perugini, and Pietro Grisoli. 2020. "Evaluation of the Effectiveness of N-Acetylcysteine (NAC) and N-acetylcysteine-cyclodextrins Multi-Composite in Pseudomonas aeruginosa Biofilm Formation" Applied Sciences 10, no. 10: 3466. https://doi.org/10.3390/app10103466
APA StyleGuerini, M., Perugini, P., & Grisoli, P. (2020). Evaluation of the Effectiveness of N-Acetylcysteine (NAC) and N-acetylcysteine-cyclodextrins Multi-Composite in Pseudomonas aeruginosa Biofilm Formation. Applied Sciences, 10(10), 3466. https://doi.org/10.3390/app10103466





