Featured Application
In this study, we present an innovative new bio-treatment approach for 17α-ethynyestradiol (EE2) using a new macro-encapsulation method, the SBP (Small Bioreactor Platform) technology, for the encapsulation of two bacterial cultures, Rhodococcus zopfii and Pseudomonas putida F1. Our findings demonstrate that SBP-encapsulated cultures might present a practical treatment for steroidal hormones removal in wastewater treatment processes.
Abstract
In this study, we present an innovative new bio-treatment approach for 17α-ethynyestradiol (EE2). Our solution for EE2 decontamination was accomplished by using the SBP (Small Bioreactor Platform) macro-encapsulation method for the encapsulation of two bacterial cultures, Rhodococcus zopfii (R. zopfii ) and Pseudomonas putida F1 (P. putida). Our results show that the encapsulated R. zopffi presented better biodegradation capabilities than P. putida F1. After 24 h of incubation on minimal medium supplemented with EE2 as a sole carbon source, EE2 biodegradation efficacy was 73.8% and 86.5% in the presence of encapsulated P. putida and R. zopfii, respectively. In the presence of additional carbon sources, EE2 biodegradation efficacy was 75% and 56.1% by R. zopfii and P. putida, respectively, indicating that the presence of other viable carbon sources might slightly reduce the EE2 biodegradation efficiency. Nevertheless, in domestic secondary effluents, EE2 biodegradation efficacy was similar to the minimal medium, indicating good adaptation of the encapsulated cultures to sanitary effluents and lack of a significant effect of the presence of other viable carbon sources on the EE2 biodegradation by the two encapsulated cultures. Our findings demonstrate that SBP-encapsulated R. zopfii and P. putida might present a practical treatment for steroidal hormones removal in wastewater treatment processes.
1. Introduction
Increasing use of pharmaceuticals and personal care products, along with population growth, pose new challenges to wastewater treatment that are not met by conventional domestic wastewater treatment plants (DWWTPs). Conventional DWWTPs have been reported to be ineffective barriers to these substances due to their low concentrations and relative resistance to biodegradation or other treatment processes (for example, physical and chemical treatments). Some of these substances may have potentially chronic direct or indirect effects on ecosystems due to endocrine-disrupting chemicals (EDCs) in the aquatic environment (for example, inducing feminization of fish), as well as on public health. EDCs have the ability to mimic, block or compete with endogenous hormones. The presence of such compounds in natural ecosystems is linked to abnormalities observed in wildlife reproduction and development functions [1].
These organic micropollutants (OMPs) can penetrate water bodies, including rivers, lakes and groundwater, and may end up in drinking water through direct discharge or as a result of agricultural irrigation by the treated effluents. For example, 111 types of pharmaceuticals were discovered in DWWTP effluents in Germany, and 23 of them persist in drinking water [2,3,4].
A unique biodegradation model for the synthetic steroid sex hormone 17α-ethynylestradiol (EE2), which is the main ingredient in most oral contraceptive pills, is presented in the present study. EE2 may affect aquatic environments even at an extremely low concentration of 0.1 ng/L [5]. The high biological activity of EE2 is due to its free trafficking through cell membranes and its molecular activity mechanism.
All mammals, including humans, excrete steroid hormones which may be transported to the environment through sewage effluents. It has been postulated that domestic treatment plant effluents are among the main sources for EDCs in natural water bodies [6]. Chang et al. [7] showed that effluents contain various hormones, such as natural androgens (androsterone, epiandrosterone and androstenedione), synthetic progestogens (megestrol acetate, norethindrone and medroxyprogesterone acetate) and natural progestogens (progesterone, 20β-dihydroxy-4-progegnen-3-one, 21α-hydroxyprogesterone, and 17α-hydroxyprogesterone). EE2 is considered to be the main cause for estrogenic activity in wastewater treatment effluents, which also contain the natural estrone (E1), 17β-estradiol (E2) and estriol (E3) [2,3,4]. This endocrine-disrupting chemical has been detected at nanogram per liter levels (Larcher and Yargeau [8]) in both surface waters and domestic wastewater effluents and was reported to cause endocrine disruption and consequently feminization of aquatic organisms [8,9,10].
EE2 removal rates in DWWTPs have been investigated and were found to have a broad range, from 34% to 98%, indicating incomplete removal which leads to the presence of a residual amount of EE2 in the discharging effluents and thus might present an estrogenic activity [8]. EE2 appears to have a slower biodegradation rate than the natural hormones, due to its stable chemical structure [11].
The bacterial metabolic mechanism for EE2 biodegradation is not fully understood. In general, microorganisms degrade steroid hormones via two possible mechanisms: Growth-linked (metabolic) and non-growth-linked (co-metabolic). The co-metabolic mechanism is employed by heterotrophic and nitrifying bacterial strains [12]. Only a few microbial strains that possess the ability to degrade EE2 as a sole carbon and energy source have been reported, including Sphingobacterium sp. JCR5 [13] and Rhodococcus equi [14]. Vader et al. [15] first suggested that ammonium monooxygenase (AMO) can be the key enzyme for EE2 degradation in nitrification tanks. Yi and Harper [16] found a linear relationship between EE2 and NH3 removal. It was found that an enriched nitrifying microbial flora in sludge containing ammonium as its sole energy source could degrade EE2 with a calculated EE2 half-life of around 24 h. Other studies showed that the EE2 half-life in WWTPs is less than 24 h, and its concentration is halved within hours, suggesting that the rate of the EE2 biodegradation mechanism is affected by the bacterial concentration and diversity [17].
Only one previous study, performed by Pauwels et al. [10], investigated the co-metabolic biodegradation of E2 and EE2 by six strains isolated from compost. They reported that EE2 was not degraded even after 100 h when used as a sole carbon source. However, the EE2 degradation rate improved in the presence of E2.
Another metabolic path for indirect EE2 degradation is mediated by manganese-oxidizing bacteria (MnO). In natural environments, manganese-oxidizing microorganisms are able to oxidize Mn2+ to Mn4+, resulting in the formation of biogenic Mn oxides. Sabirova et al. [18] reported that the manganese-oxidizing bacteria Leptothrix discophora and Pseudomonas putida can oxidize Mn2+ to form Mn oxides, which are then used to facilitate the oxidative cleavage of EE2.
Considering the aforementioned information regarding metabolic and co-metabolic consumption of EE2, there might be two major reasons for only partial removal of EE2 from DWWTP effluents: (1) The hydraulic retention time (HRT) within the bioreactor is too short for co-metabolism activity; (2) The bacterial flora that can metabolize EE2 are present in insufficient concentrations.
The proven ability of microorganisms to biodegrade EE2 and other OMPs suggests the potential of using known biochemical paths for OMP treatment, especially in large water bodies. Use of the bioaugmentation treatment approach might present a solution for OMPs treatment, especially by providing a sufficient amount of selective biomass that enables rapid EE2 biodegradation within hours. However, bioaugmentation presents many problems when the suspended culture is introduced into common DWWTPs, since the short HRT (8–24 h) causes dilution of the augmented culture and biomass washout from the bioreactor due to continuous influents. Some protection and immobilization of the bioaugmented culture must therefore be provided in order to prevent loss of the added biomass [19].
A novel encapsulation technology, the ‘small bioreactor platform’ capsules (SBP) (Figure 1), was recently developed. The SBP method is based on macro-encapsulation of a bacterial culture in a confined environment using a microfiltration membrane as a protective barrier. The 3D capsule (2.5 cm long and 0.8 cm diameter) physically separates the suspended microbial culture inside the capsule from the natural microorganisms in the wastewater, while enabling the diffusion of nutrients through the capsule membrane. The capsules are introduced into the WWTP bioreactor or other water bodies such as the secondary clarifier using a perforated cage that enables controlling of the location of the capsules (for example, above the air diffuser), and enables a very simple operation. The SBP capsule creates an appropriate growth environment by providing nutrients and physical protection and preventing competition with the natural microorganisms. This results in rapid biomass acclimation within the SBP capsules. Furthermore, the physical barrier prevents washout of the selective microorganisms from the bioreactor by a continuous outflow [20]. In our recent work on phenol biodegradation using a SBP-encapsulated Pseudomonas putida F1 strain, it was found that the encapsulated culture had a biodegradation rate similar to that of a bacterial suspension (not encapsulated) [21]. As far as we know, this capsule may present an efficient encapsulation matrix in terms of biodegradation rate, which is a significant advantage for OMP degradation in most WWTPs that operate using a short HRT (less than 24 h).
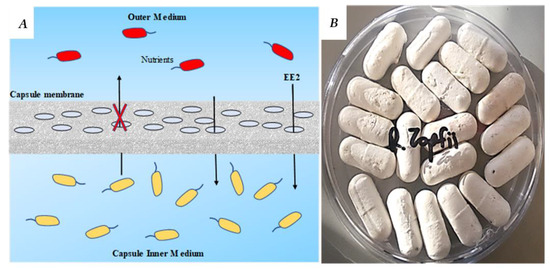
Figure 1.
An illustration (A) and a picture (B) of a small bioreactor platform (SBP) membrane. The membrane allows the transport of dissolved molecules such as organics, nutrients and gases across the microfiltration membrane. (A) Schematic representation showing that the cellulose acetate microfiltration membrane of the SBP capsule holds the inner bacterial culture suspension, while 17α-ethynylestradiol (EE2) (as a model micropollutant in this study) and other nutrients diffuse freely into and out of the capsule body. The red bacteria represent bacteria in the mixed liquor, while the yellow bacteria represent the encapsulated EE2-degrating bacteria. (B) A photograph of SBP capsules encapsulating R. zopfii in a dry state, prior to the activation stage.
In this study, the SBP technology was used to encapsulate selective bacterial cultures that have EE2 biodegradation abilities in order to find a sustainable solution for the removal of OMPs, such as steroid hormones, in wastewater treatment processes. The ability of SBP-encapsulated Rhodococcus zopfii and Pseudomonas putida F1 cultures to biodegrade EE2 in different media, including domestic secondary effluents, was evaluated. The main objective of this study was to gain knowledge that will help design and demonstrate an effective EE2 biodegradation treatment which can be integrated within existing DWWTPs.
2. Materials and Methods
2.1. Bacterial Strains and Media
Based on the literature regarding EE2 biodegradation, we decided to encapsulate two bacterial cultures that exhibit two biodegradation mechanisms: Metabolic and co-metabolic. An EE2-degrading Rhodococcus zopfii (ATCC 51349) strain was purchased from the ATCC culture bank, and the bacterial strain Pseudomonas putida F1 (ATCC 700007) was kindly provided by Prof. Carlos Dosoretz from the Technion–Israel Institute of Technology. The experimental media were composed of minimal salt medium (MSM) as described in Kurzbaum et al. [21], MSM enriched with 2% lysogeny broth (LB) (Sigma, Israel) and secondary effluents sterilized by autoclave. Secondary effluents were taken from a DWWTP (Betaniya, Israel), filtrated (0.45 µm membrane, MF Millipore, Merck) and refrigerated for three days until the experiment. Analytical grade EE2 was purchased from Sigma–Aldrich (Israel). An EE2 stock solution (1000 mg/L) was prepared in methanol and diluted directly into the experimental flasks containing the different experimental media for achieving the required EE2 concentration. The methanol concentration within the test medium was negligible.
2.2. Encapsulation of Bacterial Cultures in SBP Capsules
Encapsulation of the bacterial cells was conducted using the SBP technology encapsulation procedure [22]. A cellulose acetate microfiltration membrane was used for encapsulation of the microbial suspension. Prior to the encapsulation procedure, the strains were maintained on nutrient agar (Neogen, USA) and grown on nutrient broth (Neogen, USA) containing 5% trehalose (Sigma, Israel) as a membrane-protective agent for a freeze-drying procedure. In brief, cells were grown to an optical density (OD) of 1 at 30 °C at a 150 rpm shaking speed, followed by a freeze-drying procedure to obtain a dormant bacterial culture in a powder state that can be encapsulated under sterile conditions within the SBP capsules (2.5 cm long and 0.8 cm in diameter, 2 cm3 capsule internal volume). Each SBP capsule contained approximate 1 mg of bacterial powder (dry mass). The encapsulation procedure was conducted by BioCastle Water Technologies Ltd. (Israel) as described in detail in patent application number PCT/IL2010/256 [22]. At this stage, the SBP capsules are in a dry state and the bacteria are not active. In order to activate the encapsulated culture and acclimate it to EE2 utilization as a carbon source, the dry capsules were submerged for 48–72 h in sterile MSM supplemented with EE2 at a final concentration of 0.5 mg/L. During that time, the medium penetrated through the membrane and activated the freeze-dried culture while acclimating it to EE2 consumption as a carbon source (Figure 1).
2.3. EE2 Adsorption to the SBP Capsule Membrane
In order to evaluate the EE2 affinity and adsorption to the SBP capsules, activated SBP capsules were autoclaved for sterilization prior to incubation with MSM solution supplemented with 0.4 mg/L EE2. The treatment group included five sterile SBP capsules, while the control contained the medium only, without any capsules. Samples for EE2 concentration determination were taken before the capsules’ insertion and after 24 h. Both treatment and control experiments were conducted in triplicates. EE2 concentration was determined using the HPLC analytical method described below.
2.4. Experimental Set-Up of EE2 Biodegradation by SBP-Encapsulated Bacteria in MSM Medium and MSM Enriched with 2% LB
The experiment was conducted in triplicates in three 250 mL flasks containing 100 mL MSM solution and three 250 mL flasks containing MSM solution enriched with 2% LB medium. Each flask was supplemented with 0.5 mg/L EE2 and shaken for 2 h, ensuring complete dissolution of the EE2 within the medium. The initial concentration was based on previous published laboratory studies. The flasks were then inoculated with five SBP capsules of one bacterial strain (R. zopfii or P. putida F1) and shaken at 80 rpm and 30 °C. The duration of the biodegradation experiments was 48–72 h. Flasks containing sterile MSM, with and without 2% LB, supplemented with 0.5 mg/L EE2 with no capsules were used as controls (in triplicates). Samples were collected from each flask at different time intervals for analysis of the EE2 concentration using the HPLC analytical method (see below).
2.5. Experimental Set-Up of EE2 Degradation in Sanitary Secondary Effluents
Since the secondary effluents might contain a low EE2 concentration (ng/L), we spiked the medium with a high EE2 concentration, in order to reduce or eliminate the effect of naturally-occurring EE2. The experiment was carried out in triplicates of 100 mL filtrated effluent enriched with 2 mg/L EE2 in 250 mL Erlenmeyer flasks in an incubator shaker at 80 rpm and 30 °C. Each flask was supplemented with five R. zopfii SBP capsules. Sterile controls did not contain any encapsulated bacteria. Samples were collected from each flask at different time intervals for analysis of the EE2 concentration using the HPLC analytical method (see below).
2.6. HPLC-UV Analysis of the EE2 Concentration
Aliquots from the various experimental set-ups were centrifuged and the upper supernatant was taken for HPLC analysis. EE2 analysis was performed using an Agilent 1100-High Performance Liquid Chromatography coupled with an ultraviolet detector set to 223 nm (HPLC-UV). A reverse phase C-18 Kinetex Evo analytical column (100 × 3.0 mm, 2.6 µ) was used at 40 °C. The mobile phase contains a mixture of water:acetoniterile (55:45% v/v). An isocratic method was applied at a flow rate of 0.5 mL/min for 6 min. The injection volume was 60 µL. The calibration curve of EE2 was diluted from an EE2 stock standard (0.4 mg/mL in 50% acetonitrile) to a specific concentration of 1.0, 0.75, 0.50, 0.25, 0.10, 0.05 mg/L.
2.7. Water Quality Parameters
Water quality parameters of the secondary DWWTP effluents used in this present study were measured according to Standard Methods [23] and are presented in Table 1.

Table 1.
Characterization of the secondary effluents from the DWWTP (Betaniya, Israel) used in this study.
3. Results and Discussion
3.1. SBP-Encapsulated Bacterial Culture Characterization over Time
Prior to examination of R. zopfii’s ability to biodegrade EE2, we explored the viability and adaptation of the encapsulated culture for long-term survival in the confined environment provided by the SBP capsule. R. zopfii is a member of the Actinobacteria phylum. Rhodococcus spp. are defined as Gram-positive to Gram-variable, aerobic, and nonmotile. Their cell morphology varies during different stages of their growth cycle. These bacteria grow in filaments and exhibit extensive branching and hyphal growth before fragmenting into an irregular rod-shape or coccoid units. Some can be found as cocci that later turn into short rods, while others continue transforming into long filamentous rods or start branching out.
R. zopfii cells were cultivated in LB medium (30 °C, 150 rpm), and created suspended colonies of 1–2 mm diameter after a few days of incubation. Microscopic observation of the grown culture was conducted, presenting a Gram-positive filament growth state and the presence of Gram-positive cocci that are assumed to be released from the mother filaments (data not shown).
Figure S1 shows a microscopic observation of the encapsulated R. zopfii. The encapsulated culture exhibited an adaptation to the confined SBP environment. Eleven days after the activation point, where the SBP-encapsulated culture was incubated in MSM medium enriched with EE2, it presented large filamentous colonies (50–1000 µm), which are similar to the structure of colonies that were observed in the suspended state of the culture. Moreover, it seems that the culture has three growth states in the internal medium of the capsule: Filamentous colonies with extensive branching and hyphal growth; single irregular rod-shaped filament units; and single Gram-positive cocci cells. The encapsulated R. zopfii culture formed a floc particles structure and an advanced stage of fragmentation into coccoid elements was also observed. The inner suspension of the SBP capsule contained a mean of 2.88 × 108 CFU/mL R. zopfii at the end of the capsules activation stage.
A similar procedure was employed for the encapsulated P. putida F1 strain. This bacterial strain was also resistant to the freeze-drying procedure and culture assimilation was observed within the SBP capsules, with bacterial counts of at least 3.38 × 108 CFU/mL. This strain was studied previously for its ability to degrade phenolic compounds as an encapsulated culture and was described in detail in Kurzbaum et al. [21].
3.2. EE2 Adsorption to the SBP Capsule Membrane
We conducted an adsorption test by using sterile capsules in order to verify that EE2 concentration reduction is a result of bacterial metabolic activity and not a result of passive adsorption to SBP capsule components. The control system without any SBP capsules exhibited an EE2 concentration of 0.37 ± 0.04 mg/L and 0.44 ± 0.03 mg/L (T = 0 h and T = 24 h, respectively). The test system, which contained five sterile SBP capsules, showed a similar result, with 0.39 ± 0.06 and 0.34 ± 0.04 mg/L (T = 0 h and T = 24 h, respectively) of EE2. No significant difference was observed between the groups (t-test, p > 0.05, n = 6). These results show that the capsule’s cellulose acetate membrane and other internal components (dead biomass) have no affinity for the EE2 molecules and that the reduction in the EE2 concentration due to passive adsorption is negligible.
3.3. EE2 Biodegradation in Minimal Salt Medium (MSM)
We examined the ability of the SBP-encapsulated R. zopfii and P. putida F1 cultures to biodegrade EE2 as the sole carbon source. Figure 2A presents the EE2 biodegradation rate in MSM medium by the two encapsulated cultures. The initial EE2 concentration was 0.415 mg/L for the control system, 0.459 mg/L for the R. zopfii system and 0.45 mg/L for the P. putida system. Both R. zopfii and P. putida treatments showed an overall reduction of over 70% within 24 h, while the sterile control did not change the hormone concentration. After 48 h, the EE2 concentration in the R. zopfii system decreased to 0.044 mg/L, whereas no further decrease was observed in the P. putida system, indicating a final biodegradation of about 90.4% and 72.7%, respectively, demonstrating that the SBP-encapsulated R. zopfii showed a better biodegradation ability compared to encapsulated P. putida.
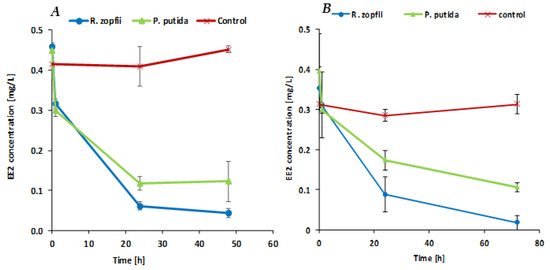
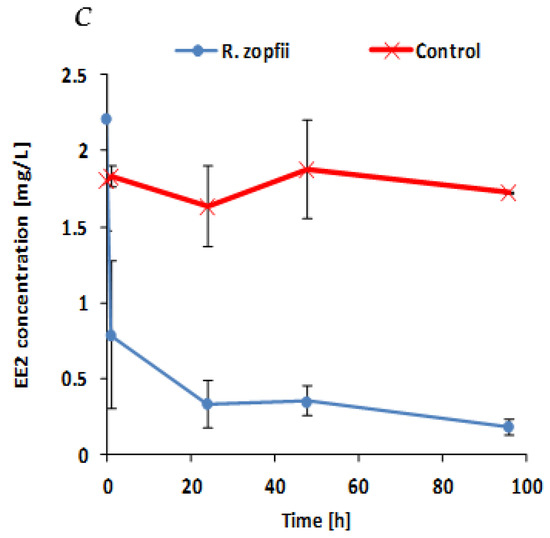
Figure 2.
(A) EE2 biodegradation in minimal salt medium (MSM) medium supplemented with EE2 as the sole carbon source. All experiments were conducted in triplicates. Points represent means of triplicates and bars represent standard deviations. (B) EE2 biodegradation in MSM medium enriched with 2% lysogeny broth (LB) solution providing an additional source for hydrocarbons and nutrients. All experiments were conducted in triplicates. Points represent means of triplicates and bars represent standard deviations. (C) EE2 biodegradation by R. zopfii SBP capsules in sanitary secondary effluents that contain some residual organic matter and other substances (~88 mg/L COD, ~20 mg/L BOD). All experiments were conducted in triplicates. Points represent means of triplicates and bars represent standard deviations.
Interestingly, we did not expect P. putida to be able to use EE2 as the sole carbon source, since it was reported that the EE2 breakdown mechanism by P. putida is related to co-metabolism, and occurs via the ammonium monooxygenase (AMO) mechanism and/or nitrification [12]. This result can be explained by three possible mechanisms: (1) The bacteria had completed their growth phase by consuming the available carbon sources and nutrients that were provided by the capsule itself during the activation phase and entered into a stationary phase. EE2 could therefore be co-metabolically degraded by the P. putida capsules for energy production and was not utilized for growth-supporting mechanisms. (2) The AMO mechanism can use EE2 in the presence of a high ammonia concentration, during the nitrification process. The MSM medium contains both Mn+2 and ammonium, indicating possible involvement of either nitrification by the ammonia-oxidizing and/or manganese-oxidizing process which exist in P. putida strains [18,24,25]. However, the ability of P. putida F1 to use EE2 as a sole carbon source requires further research in order to elucidate this mechanism.
3.4. EE2 Biodegradation in Minimal Salt Medium Enriched with Hydrocarbons and Nutrients (MSM + 2%LB)
This experimental section was conducted to determine whether the presence of other available carbon sources and nutrients have an inhibitory effect on the EE2 biodegradation rate. A medium that is enriched with various nutrients might simulate a domestic effluent that contains some residual biochemical oxygen demand (BOD), nitrogen and phosphorus. Figure 2B presents the EE2 biodegradation rate of encapsulated R. zopfii and P. putida F1 in MSM medium enriched with 2% LB. After 1 h of incubation, the EE2 concentration decreased significantly only in the P. putida test system. EE2 concentration reductions after 1 h were 11.6% for the R. zopfii system and 24.5% for the P. putida system. The significant EE2 reduction in the two test systems occurred during the first 24 h of incubation. Our results indicate that SBP-encapsulated R. zopfii and P. putida F1 can successfully biodegrade EE2 molecules within 24 h, from an initial concentration of 0.352 mg/L and 0.396 mg/L to 0.088 mg/L (75%) and 0.174 mg/L (56.1%), respectively. At the final incubation time point, after 72 h, the EE2 concentration decreased to a final biodegradation efficacy of 94.9% and 73.1% in the R. zopfii and the P. putida systems, respectively. The EE2 concentration in the control system after 72 h was not significantly different from the starting point. These results indicate that most of the EE2 molecules were consumed during the first 24 h of incubation, even in the presence of other hydrocarbons and nutrients. Again, it is shown that the R. zopfii capsules biodegrade EE2 faster than the P. putida capsules. This shows that the encapsulated culture may be a good candidate for biological treatment of EE2 in future studies, in pilot studies or in laboratory studies for treating effluents, since effluents contain a significant amount of available organic matter.
Table 2 presents a literature summary of EE2 biodegradation by a suspended R. zopfii culture in the presence of other carbon sources. Yoshimoto et al. [14] presented a biodegradation rate similar to the rate found in the present study, indicating that the SBP encapsulation method did not have an inhibitory effect on the EE2 reduction rate. Moreover, the SBP-encapsulated R. zopfii presented superior biodegradation results regarding its kinetic and efficiency parameters compared to the findings reported by Larcher and Yargeau [8].

Table 2.
Selected literature results for EE2 biodegradation efficacy by Rhodococcus species metabolic activity in the presence of other carbon sources.
O’Grady et al. [9] reported that EE2 is partially biodegraded by three Rhodococcus strains in the presence of co-substrates. In their work, Rhodococcus equi removed up to 39% of the initial EE2 concentration (1.4 mg/L) after 65 h, whereas no significant EE2 removal was observed using R. rhodochrous and R. zopfii. On the contrary, our results present an almost complete biodegradation efficacy of 94.9% after 72 h of incubation with MSM + 2% LB and 91.7% after 96 h in secondary effluents (~88 mg/L COD).
Table 3 presents a literature summary of EE2 biodegradation by P. putida in the presence of other carbon sources. Those studies presented various EE2 biodegradation rates, from hours to days, indicating that medium composition might play a central role in EE2 biodegradation by the co-metabolic mechanism. Our results revealed the unexpected phenomenon that encapsulated P. putida cells are able to consume EE2 as the sole carbon source. In addition, it was not expected that the biodegradation rate in a medium that was not enriched with hydrocarbons and nutrients would present a faster biodegradation rate compared to the same medium that contained hydrocarbons and nutrients (73.8.1% vs. 56.1%, at T = 72 h, respectively). Since EE2 biodegradation in MSM medium presents better results, it can be assumed that a stationary encapsulated P. putida culture in the presence of ammonia and Mn+2 might use EE2 as a major carbon source or a sole carbon source for metabolic activity and efficient energy generation. This was also shown in the studies of Sabirova et al. [18] and Tran et al. [26], which showed that P. putida grown in a Mn+2-enriched medium presented almost complete EE2 biodegradation (over 90%) after a short incubation time (17–48 h).

Table 3.
Selected literature results for EE2 biodegradation efficacy by co-metabolic activity.
3.5. EE2 Biodegradation in Domestic Secondary Effluents
Since R. zopfii presented superior EE2 biodegradation results in both tested media, it was further selected for an additional experiment with secondary effluents from a sanitary wastewater treatment plant. The effluent water quality parameters are presented in Table 1. Figure 2C presents the EE2 biodegradation rate by R. zopfii SBP capsules in the secondary effluents. Unexpectedly, and contrary to the results obtained with the other media, most of the EE2 reduction occurred during the first hours of incubation, from 2.2 mg/L to 0.786 mg/L (64.24% reduction). After 24 h of incubation, the EE2 concentration was 0.33 mg/L (85% reduction) and after 96 h of incubation, the EE2 concentration decreased to 0.18 mg/L (91.8% reduction). Control without capsules did not change the EE2 concentration during the experimental interval. These results indicate that R. zopfii SBP capsules can be used as an EE2 removal treatment in secondary effluents, since most of the EE2 biodegradation occurred during the first hours of incubation (64.24% reduction). Moreover, R. zopfii is considerably more adapted to the secondary effluent medium, since it presents superior EE2 consumption during the first hour of incubation compared to MSM and MSM + 2%LB media (31.2% and 11.7% reduction, respectively). Thus, encapsulated R. zopfii does not need an adaptation stage (lag phase) in a secondary effluent environment and its response to the presence of EE2 in the medium is almost instantaneous. This may be explained by the fact that the purchased R. zopfii isolate used in this study originates in an aerated wastewater bioreactor [28].
Table 4 summarizes the EE2 biodegradation efficiency in the various types of media examined in this study. It is important to note that other EE2 biodegradation studies used suspended cultures (Table 2 and Table 3). Our results present one of the best EE2 biodegradation rates, thus suggesting that a confined environment, such as the SBP capsule, might provide superior environmental conditions for the R. zopfii culture.

Table 4.
EE2 reduction (%) over time by the encapsulated R. zopfii and P. putida in the three experimental media (MSM, MSM + 2% LB, secondary effluent).
3.6. Technology Implementation for Micropollutant Treatment in WWTPs
We determined EE2 reduction at ppm (mg/L) concentrations, which are approximately 3–6 orders of magnitude greater than the levels observed in wastewater and surface water [2,3,4]. This has also occurred in other studies where the studied EE2 concentrations were higher in order to quantify biodegradation and adsorption during the biological removal of EE2 as mentioned in Table 2 and Table 3 [14,29]. While the selected concentrations of EE2 do not represent the actual EE2 concentrations found in WWTPs and other aquatic habitats, the results of this study provide a good indication of their potential reduction using the proposed encapsulation technology. It has been reported that chemicals which are present in low concentrations (µg/L and ng/L) may exhibit a different biodegradation behavior than when they are present in high concentrations (mg/L) [30]. Alexander [31] reported that biodegradation is often favored at low concentrations and Forrez et al. [28] reported that EE2 removal efficiency within WWTPs secondary effluents enriched with 100 ng/L EE2 exhibits similar results to those obtained within the µg/L range (75–85% concentration reduction).
Many studies have focused on the efficiency of different treatment methods for OMP reduction in wastewater. It is known that the chemical and physical treatment approaches require substantial input of resources (energy, chemical agents) to reach the desired reduction of the target micropollutants. Some national initiatives for OMP management were launched recently (e.g., Germany and Switzerland). However, no truly ground-breaking solution has been developed to date.
Three major technologies are currently offered for removal of OMPs: Advanced oxidation processes (AOPs) such as ozonation and UV/H2O2, powdered activated carbon (PAC) and reverse osmosis (RO). AOP may present as a useful tool for OMP breakdown and treatment. However, it may also create a new secondary metabolite that might exhibit higher cytotoxicity, thus creating a water safety issue. Moreover, this treatment is considered expensive and perhaps not suitable for the treatment of large water bodies. PAC and RO are not considered as a treating tool for molecule breakdown. They concentrate and transfer the OMPs to a solid phase (PAC) or to RO leachate. This means that the RO and PAC pellets still require additional treatment for disposing of these contaminants prior to their discharge into the environment. We also need to recognize that the aforementioned treatment approaches are not selective. They therefore treat not only the EE2 molecules, but the whole water body (such as in a RO process). This fact increases their energy requirements in order to exclude low concentrations of selective OMP molecules from the entire water body. For example, small and non-polar molecules (500 Da) such as hydrophobic endocrine-disrupting chemicals (EDCs) can diffuse through the RO membrane [32]. High desalting RO membranes effectively retained 90% of the semi-volatile organic compounds (SVOC) and 80% of the VOC. However, low desalting membranes retained only 20% of the VOC as bromoform and dibromochloromethane [33]. Small hydrophilic contaminants such as corrosion inhibitors (benzotriazole), pharmaceuticals, artificial sweeteners such as acesulfame and saccharin, and industrial chemicals can pass the RO membrane into wastewater effluents. Low concentrations of pesticides such as metolachlor and propiconazole and pharmaceuticals such as lamotrigine and tramadol were also detected. Even some large and hydrophobic chemicals can pass through the RO membrane. RO treatment is therefore ineffective for these molecules [32].
The absence of a cost-effective chemical–physical treatment for decontamination of large water bodies from OMPs emphasizes the advantages of using the biological approach. In order to comprise a cost-effective and safe treatment, a bio-selective treatment should be implemented in the wastewater treatment bioreactor as an additive treatment or at the secondary clarifier stage as a polisher treatment. The biological treatment approach using selective bacterial cultures targeting OMPs as a carbon source may present as a good strategy for OMP removal without the need to treat the entire organic matter present in the water body.
As seen in this study, dissolved contaminants in the water are expected to traffic from the water into the SBP capsule’s internal medium. When the concentration of the contaminants inside the capsule decreases due to degradation and mineralization of the molecules to carbon dioxide by the bacteria, this will cause a concentration gradient that will increase the diffusion of the contaminant into the SBP capsule, thus creating an efficient biodegradation process. We are therefore presenting a treatment method that does not necessitate transporting the entire water body through a treatment medium, which is necessary when using RO and PAC media. The SBP treatment approach is expected to reduce most of the associated energy investment for water-circulating processes such as RO and PAC.
In the present study we demonstrate that a bioaugmentation treatment may present a useful solution that enables treatment of target OMPs instead of using a filtration treatment approach (RO or PAC). The SBP technology can be implemented inside a host DWWTP bioreactor in perforated cages as presented in our previous study [34], in order to remove OMPs. Moreover, it can also be implemented inside the secondary sedimentation tank for OMPs removal. There is thus no need to change the infrastructure for using this technology. The secondary effluent results demonstrate significant EE2 removal (64%) after 1 h of incubation, indicating that bioaugmentation can be implemented within WWTP secondary clarifiers for reducing the EE2 concentration in the effluents. We estimate that EE2 bio-treatment kinetics and efficiency over time should be increased to over 90% reduction after 6 h of contact in order to comprise a practical solution that can be integrated in the secondary clarifier tank. Future research should explore EE2 biodegradation by various encapsulated (homogenetic or hetrogenetic) cultures in different aquatic environments in order to find the right combination that will enable a practical bio-treatment solution for reducing OMP concentrations in effluents.
4. Conclusions
Our study results present the ability of SBP-encapsulated R. zopfii and P. putida to biodegrade EE2 within a relatively short period of time, with an emphasis on a better biodegradation rate in a secondary effluent medium. This study shows that the SBP encapsulation method provides a selective and highly effective biological treatment tool which can be easily implemented in the WWTP infrastructure. It overcomes three main obstacles that previously caused the failure of biological bioaugmentation treatments: It enables the achievement of long-term selective biomass implementation inside WWTPs, it controls the location of the culture (for example, in a net or perforated box) and it yields a relatively rapid biodegradation rate which can be synchronized with the HRT of WWTPs. To date, most studies exploring the biodegradation of low EE2 concentrations demonstrated a relatively low degradation rate in the range of hours to days using a suspended R. zopfii culture. However, use of a suspended additive culture is impractical in domestic wastewater treatment plants. In this study it was shown that SBP-encapsulated R. zopfii cultures exhibit a faster EE2 biodegradation rate compared to other studies, in particular in the secondary effluent medium. Future studies are required in order to elucidate the environmental conditions which impact the encapsulated cultures during the micropollutant biodegradation process and the mechanisms involved in this treatment approach.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/10/1/336/s1. Light microscope images of encapsulated R. zopfii culture morphologies in activated SBP microfiltration capsules over time.
Author Contributions
Conceptualization, O.M. and E.K.; methodology, O.M., E.K. and D.A.; formal analysis, A.K.; investigation, Y.R., V.C.-Y. and M.E.K.; resources, O.M. and E.K.; data curation, Y.R., V.C.-Y., O.M and E.K.; writing—original draft preparation, O.M. and E.K.; writing—review and editing, O.M., D.A., H.M. and E.K.; visualization, E.K.; supervision, O.M. and E.K.; project administration, O.M.; funding acquisition, O.M. and E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Israeli Ministry of Science, Space and Technology (MOST) [grant number 3-12375]. Additionally, this research was also funded by Kinneret Research Fund.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Muller, M.; Rabenoelina, F.; Balaguer, P.; Patureau, D.; Lemenach, K.; Budzinski, H.; Barceló, D.; de Alda, M.L.; Kuster, M.; Delgenès, J.-P.; et al. Chemical and biological analysis of endocrine-disrupting hormones and estrogenic activity in an advanced sewage treatment plant. Environ. Toxicol. Chem. 2008, 27, 1649–1658. [Google Scholar] [CrossRef]
- Avisar, D.; Lester, Y.; Ronen, D. Sulfamethoxazole contamination of a deep phreatic aquifer. Sci. Total Environ. 2009, 407, 4278–4282. [Google Scholar] [CrossRef]
- Carballa, M.; Omil, F.; Lema, J.M.; Llompart, M.; García-Jares, C.; Rodríguez, I.; Gómez, M.; Ternes, T. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res. 2004, 38, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Gago-Ferrero, P.; Gros, M.; Ahrens, L.; Wiberg, K. Impact of on-site, small and large scale wastewater treatment facilities on levels and fate of pharmaceuticals, personal care products, artificial sweeteners, pesticides, and perfluoroalkyl substances in recipient waters. Sci. Total Environ. 2017, 601, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Cajthaml, T.; Křesinová, Z.; Svobodová, K.; Sigler, K.; Řezanka, T. Microbial transformation of synthetic estrogen 17α-ethinylestradiol. Environ. Pollut. 2009, 157, 3325–3335. [Google Scholar] [CrossRef] [PubMed]
- Baronti, C.; Curini, R.; D’Ascenzo, G.; Di Corcia, A.; Gentili, A.; Samperi, R. Monitoring natural and synthetic estrogens at activated sludge sewage treatment plants and in a receiving river water. Environ. Sci. Technol. 2000, 34, 5059–5066. [Google Scholar] [CrossRef]
- Chang, H.; Wan, Y.; Wu, S.; Fan, Z.; Hu, J. Occurrence of androgens and progestogens in wastewater treatment plants and receiving river waters: Comparison to estrogens. Water Res. 2011, 45, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Larcher, S.; Yargeau, V. Biodegradation of 17α-ethinylestradiol by heterotrophic bacteria. Environ. Pollut. 2013, 173, 17–22. [Google Scholar] [CrossRef]
- O’Grady, D.; Evangelista, S.; Yargeau, V. Removal of Aqueous 17α-Ethinylestradiol by Rhodococcus Species. Environ. Eng. Sci. 2009, 26, 1393–1400. [Google Scholar] [CrossRef]
- Pauwels, B.; Wille, K.; Noppe, H.; De Brabander, H.; Van de Wiele, T.; Verstraete, W.; Boon, N. 17α-ethinylestradiol cometabolism by bacteria degrading estrone, 17β-estradiol and estriol. Biodegradation 2008, 19, 683–693. [Google Scholar] [CrossRef]
- Cicek, N.; Londry, K.; Oleszkiewicz, J.A.; Wong, D.; Lee, Y. Removal of selected natural and synthetic estrogenic compounds in a Canadian full-scale municipal wastewater treatment plant. Water Environ. Res. 2007, 79, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-P.; Deeb, R.A.; Chu, K.-H. Microbial degradation of steroidal estrogens. Chemosphere 2013, 91, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Haiyan, R.; Shulan, J.; ud din Ahmad, N.; Dao, W.; Chengwu, C. Degradation characteristics and metabolic pathway of 17α -ethynylestradiol by Sphingobacterium sp. JCR5. Chemosphere 2007, 66, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, T.; Nagai, F.; Fujimoto, J.; Mizukoshi, H.; Makino, T.; Saino, H.; Sawada, H.; Omura, H.; Watanabe, K.; Kimura, K. Degradation of Estrogens by Rhodococcus zopfii and Rhodococcus equi isolates from activated sludge in wastewater treatment plants. Appl. Environ Microbiol. 2004, 70, 5283–5289. [Google Scholar] [CrossRef]
- Vader, J.S.; van Ginkel, C.G.; Sperling, F.M.G.M.; de Jong, J.; de Boer, W.; de Graaf, J.S.; van der Most, M.; Stokman, P.G.W. Degradation of ethinyl estradiol by nitrifying activated sludge. Chemosphere 2000, 41, 1239–1243. [Google Scholar] [CrossRef]
- Yi, T.; Harper, W.F. The link between nitrification and biotransformation of 17α-ethinylestradiol. Environ. Sci. Technol. 2007, 41, 4311–4316. [Google Scholar] [CrossRef]
- Yu, C.-P.; Roh, H.; Chu, K.-H. 17β-Estradiol-Degrading Bacteria Isolated from Activated Sludge. Environ. Sci. Technol. 2007, 41, 486–492. [Google Scholar] [CrossRef]
- Sabirova, J.S.; Cloetens, L.F.F.; Vanhaecke, L.; Forrez, I.; Verstraete, W.; Boon, N. Manganese-oxidizing bacteria mediate the degradation of 17α-ethinylestradiol. Microb. Biotechnol. 2008, 1, 507–512. [Google Scholar] [CrossRef]
- Cassidy, M.B.; Lee, H.; Trevors, J.T. Environmental applications of immobilized microbial cells: A review. J. Ind. Microbiol. 1996, 16, 79–101. [Google Scholar] [CrossRef]
- Menashe, O.; Kurzbaum, E. Small-bioreactor platform technology as a municipal wastewater additive treatment. Water Sci. Technol. 2014, 69, 504–510. [Google Scholar] [CrossRef]
- Kurzbaum, E.; Raizner, Y.; Cohen, O.; Suckeveriene, R.Y.; Kulikov, A.; Hakimi, B.; Iasur Kruh, L.; Armon, R.; Farber, Y.; Menashe, O. Encapsulated Pseudomonas putida for phenol biodegradation: Use of a structural membrane for construction of a well-organized confined particle. Water Res. 2017, 121, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Menashe, O. Microorganism comprising particles and uses of same. U.S. Patent Application Number PCT/IL2010/000256, 28 October 2010. [Google Scholar]
- Baird, R.B.; Eaton, A.D.; Rice, E.W. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF): Washington, WA, USA, 2017. [Google Scholar]
- Hennebel, T.; De Gusseme, B.; Boon, N.; Verstraete, W. Biogenic metals in advanced water treatment. Trends Biotechnol. 2009, 27, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic manganese oxides: properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef]
- Tran, T.N.; Kim, D.-G.; Ko, S.-O. Synergistic effects of biogenic manganese oxide and Mn(II)-oxidizing bacterium Pseudomonas putida strain MnB1 on the degradation of 17 α-ethinylestradiol. J. Hazard. Mater. 2018, 344, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Forrez, I.; Carballa, M.; Boon, N.; Verstraete, W. Biological removal of 17α-ethinylestradiol (EE2) in an aerated nitrifying fixed bed reactor during ammonium starvation. J. Chem. Technol. Biotechnol. 2009, 84, 119–125. [Google Scholar] [CrossRef]
- Stoecker, M.A.; Herwig, R.P.; Staley, J.T. Rhodococcus zopfii sp. nov., a Toxicant-Degrading Bacterium. Int. J. Syst. Evol. Microbiol. 1994, 44, 106–110. [Google Scholar] [CrossRef][Green Version]
- Weber, S.; Leuschner, P.; Kämpfer, P.; Dott, W.; Hollender, J. Degradation of estradiol and ethinyl estradiol by activated sludge and by a defined mixed culture. Appl. Microbiol. Biotechnol. 2005, 67, 106–112. [Google Scholar] [CrossRef]
- Ren, Y.X.; Nakano, K.; Nomura, M.; Chiba, N.; Nishimura, O. Effects of bacterial activity on estrogen removal in nitrifying activated sludge. Water Res. 2007, 41, 3089–3096. [Google Scholar] [CrossRef]
- Alexander, M. Biodegradation of organic chemicals. Environ. Sci. Technol. 1985, 19, 106–111. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Ricart, M.; Köck-Schulmeyer, M.; Guasch, H.; Bonnineau, C.; Proia, L.; de Alda, M.L.; Sabater, S.; Barceló, D. Pharmaceuticals and pesticides in reclaimed water: Efficiency assessment of a microfiltration–reverse osmosis (MF–RO) pilot plant. J. Hazard. Mater. 2015, 282, 165–173. [Google Scholar] [CrossRef]
- Agenson, K.O.; Oh, J.-I.; Urase, T. Retention of a wide variety of organic pollutants by different nanofiltration/reverse osmosis membranes: controlling parameters of process. J. Memb. Sci. 2003, 225, 91–103. [Google Scholar] [CrossRef]
- Menashe, O.; Kurzbaum, E. A novel bioaugmentation treatment approach using a confined microbial environment: a case study in a Membrane Bioreactor wastewater treatment plant. Environ. Technol. 2016, 37, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).