Abstract
The deterioration of the cement that bonds the cap, pin, and porcelain shell of the porcelain insulators can be observed by three-dimensional computed tomography (3D-CT), a non-destructive test. When porcelain insulators are used for a long duration, the size of the pores existing in the cement increase as the cement expands due to the alkali-silica reaction (ASR). It is possible to visually confirm the corrosion of caps and pins. The degree of corrosion is divided into four noticeable stages (I–IV), and deterioration of cement includes one of the four stages of corrosion. The standard time of replacement of insulator vs. continued use is presented. As a result of X-ray diffraction (XRD) analysis, Fe is oxidized to Fe2O3 at 36, 43, 54, and 70 degrees. The corrosion in cement is caused by factors including the value of pH, oxygen content, and temperature. For the caps and pins, although a zinc coating is applied to prevent the corrosion of iron, corrosion of zinc is caused by substances present in the external environment. When the zinc coating begins to corrode, the corrosion of the Fe component of the cap and pin accelerates, causing mechanical/electrical problems.
Keywords:
3D-CT; alkali-silica reaction; corrosion; insulator; power transmission; X-ray diffraction 1. Introduction
National and international standards have been adapted for manufacturing of 154 kV insulators, specifying the electrical and mechanical performance requirements that the insulators must meet. Size, insulation, electrical and mechanical strength are important for service compatibility in various countries. The design and material of a porcelain insulator varies according to the processing method. American National Standards Institute (ANSI) and the Canadian Standards Association (CSA) worked hard to reconcile these variations, and after a considerable time, the International Electrochemical Commission (IEC) standard achieved consensus and was accepted commercially all over the world [1,2]. This study aims to investigate the effects of aging on porcelain insulators and provide guidelines for the diagnosis of the extent of damage caused by aging within metal and cement. At present, the lifespan of a porcelain insulator device is predicted to be from 30 to 60 years or more [3]. Once such an insulator is installed and ready for use, it should be able to serve the customer without interruption. It is very difficult to replace all the insulators on a line at once because of safety concerns. Currently, replacement is only carried out if external damage is visible. Since we cannot accurately predict the life expectancy of the porcelain insulator, it is necessary to instead track age-related changes in the porcelain structure. As data from aging studies accumulate, life expectancy may become predictable.
The lifespan of insulators varies considerably. From an electrical point of view, there should be no arc discharge, and sound insulation properties are required to withstand transient voltages such as lightning. Robust mechanical properties are required due to weather conditions such as wind, ice, snow, and rain [4]. The porcelain insulator is not a single structure of uniform construction, but a complex structure of iron, cement, and ceramics. Deterioration of characteristics after a long period of use require diagnosis of aging [5].
If the electrical characteristics deteriorate, it leads to partial discharge or flashover, which means the insulator can no longer insulate effectively. If the mechanical characteristics deteriorate, the insulator cannot be used in the transmission tower, posing the risk of a serious accident. This is due to environmental degradation caused by various pollutants including water, and both electrical and mechanical stress [6].
Investigation has been carried out in order to analyze the degradation of cement by implementing three-dimensional computed tomography (3D-CT), which has a very high resolution and is considered a non-destructive way to analyze the deterioration due to internal defects. When applied to the analysis of porcelain insulators, 3D-CT is particularly useful in detecting degradation of the cement component. Clinker, which is the main component of cement used for porcelain insulators, consists of alite (3CaOSiO2), belite (CaOSiO2), aluminate (NaAlO2), and ferrite (Fe2O3). Alite and belite hydrate produce low-crystalline calcium silicate hydrate gel (C-S-H gel) and Ca(OH)2 [7]. The chemical composition and crystallinity of C-S-H gel varies during the hydration process. Immediately after the hydration reaction, the pH is raised to 10 or more due to the intrusion of H2O and the separation of Ca2+ ions. The low C-S layer is formed on the surface, and as time passes, the Ca2+ ion concentration increases and the hydrate layer thickens, and then the rehydration reaction occurs and the crystal growth proceeds.
When the aluminate and ferrite crystal phases react with water, rapid hydration heat occurs and they stabilize to C4AH19 and C2AH8 hydrate. After a period of time elapses, they hydrate to stable C3AH6. Therefore, gypsum is added to generate ettringite hydrate and achieve hydration using C3A hydrate [8].
2. Single-Layer Analysis of Porcelain Insulator Using Three Dimensional-Computed Tomography
The porcelain insulator for 154 kV power transmission, shown in Figure 1, has been in use since the 1950s. It is composed of an iron cap, cement, an iron pin, and porcelain material with a diameter of 250 mm [9].
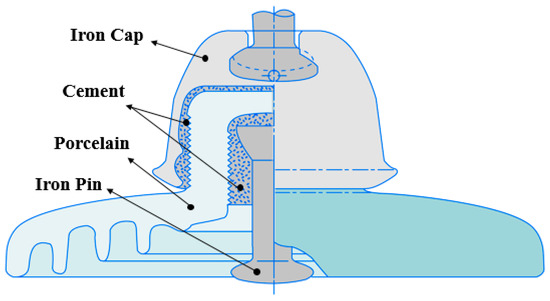
Figure 1.
Section showing the four components of the porcelain insulator.
The iron cap and iron pin are coated with zinc, which is widely used for corrosion prevention in industry. This paper focusses on the analysis of deterioration characteristics and deterioration mechanisms of aged insulators, with particular focus on the deterioration of metal and cement, as porcelain itself undergoes the least change. According to USASI (United States of America Standards Institute) 29.2 the components of porcelain insulators experience mechanical damage in the order of iron pin, iron cap, porcelain [10].
However, since the cement part acts as a bridge connecting all the constituents of the porcelain insulator, the mechanical property guarantee is very important, especially when the components are exposed to the actual service environment [11]. The risk of accidents increases as the deterioration of the cement progresses, making it difficult to maintain the characteristics of the insulator. This study mainly focuses on the reason for the corrosion and degradation of the metal and cement parts of porcelain insulators, and what happens when deterioration progresses.
Figure 2a shows the 3D-CT analysis of the single layer. Figure 2b shows a new product that was manufactured in 2017 and has never been in service, a small air pocket of less than 3 mm2 appears inside, but this can occur during the curing process of the cement. The insulator shown in Figure 2c has been in use for 38 years from 1979 to 2017; the 3D-CT analysis revealed more than 20 pores with sizes of more than 3 mm2 inside the cement. In the case of porcelain insulators produced in 2017, pores smaller than 3 mm2 occur in the curing process, and in the case of porcelain insulators installed in 1979, pores larger than 14 mm2 are increased by the alkali-silica reaction (ASR) reaction. In the case of porcelain insulators that have been used for a long time, the cement degradation and the size of the pores increases.

Figure 2.
Cross-sectional composition and three-dimensional computed tomography (3D-CT) analysis photo of new and aged of porcelain insulator. (a) Cross sectional analysis; (b) 3D-CT of new porcelain insulator; (c) 3D-CT of aged porcelain insulators.
This is because the silica and alkali components in the cement react with water to form alkali-silica gel, and the gel reacts with water to cause expansion of the cement, and thereby, cracking. This reaction is called an alkali-silica reaction (ASR). In general, a pH of higher than 12, moisture, and reactive silica are required to generate an ASR reaction in the cement. When these conditions are satisfied, the mechanism of reaction in the cement is as described in the following section [12,13].
3. Mechanism of ASR and Degradation of Properties of Cement in Porcelain Insulators
ASR progresses in two distinct steps. Alkali-silica gel mainly reacts with Na+ and K+, which are high pH value cations within the cement. These two components absorb water well, causing the cement to expand, resulting in internal stresses, and thereby, cement damage. Most of the reaction occurs between the silica contained in cement and the alkali in the cement paste. The silica in solution state contained in the pores reacts with Na+ and K+ (having positive ions) to form alkaline silica gelatin (alkali-silica gel) which is unstable in volume. The gel absorbs water and expands, and the hydration reaction is accelerated by rain or melted snow [14,15]. Since the gel is restrained by the surrounding mortar, osmotic pressure is generated by the swelling. Once that pressure is larger than the tensile strength of the cement, cracks occur, leading to additional water migration or absorption and additional gel swelling [16,17]. The generation of gels lowers the rigidity of the cement and affects the electrical/mechanical properties of the porcelain insulator.
Amorphous silica in porcelain + alkali of cement + water = alkali-silica gel production,
SiO2(Solid) + Na+(aq.) + (OH)−(aq.) + H2O → Na2SiO3·2H2O,
SiO2(Solid) + Na+(aq.) + (OH)−(aq.) → NaHSiO3(Solid),
NaHSiO3(Solid) + Na+(aq.) + (OH)−(aq.) → Na2SiO3·2H2O.
The silica contained in the cement and the alkali react with water, generating the alkali-silica gel. Alkali-silica gel is combined with a solid form of SiO2 and 2NaOH in the form of a solution in the cement. When this comes into contact with water, Na2SiO3·2H2O is formed. Among these SiO2, cationic Na+ with high pH and OH− react to form NaHSiO3 in a solid state. NaHSiO3, Na+, and OH− are combined to form Na2SiO3·2H2O, resulting in cement erosion and etching. Cracking of the cement also occurs due to water expansion. This reaction is the starting point for the deterioration of the mechanical properties of the porcelain insulator [18,19].
Figure 3 shows the mechanism of the process by which ASR occurs in the porcelain insulator. Stage I is the stage where internal pores are generated by deterioration during the cement curing process, a long service period, or mechanical/electrical stress. These also include the micro-pores created mainly in the initial stage of the manufacturing process and then enlarged by water permeation. In Stage II, alkali-silica gel is formed by reaction with water. The SiO2 component within the cement reacts with high pH Na+ and K+ ions and generates Na2SiO3·2H2O. Stage III is a process in which alkali-silica gel erodes silica and alkali components in the cement causing the expansion of the cracks. Solid state SiO2 and aqueoussolutions of Na+ and OH− form NaHSiO3. Then, volume expansion occurs. Finally, Stage IV is crack propagation or growth due to water permeation.
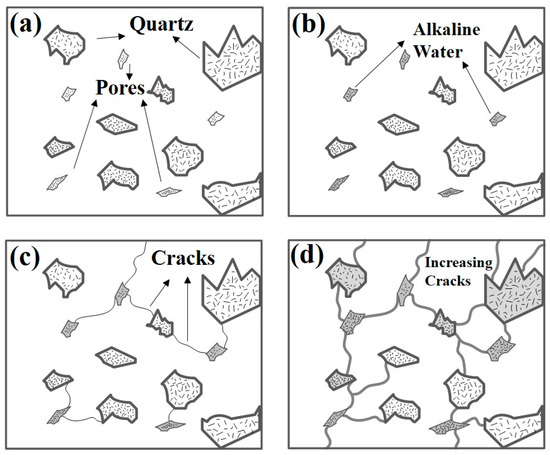
Figure 3.
A schematic diagram of the progression of the alkali-silica reaction (ASR) in the cement inside the porcelain insulator (a) Stage I, (b) Stage II, (c) Stage III and (d) Stage IV.
The high stresses inside the cement expand the volume of the cement and lead to cracks. In general, it has been reported that amorphous silica is highly susceptible to ASR. The silica that reacts in these four steps is mostly amorphous, which is the cause of accelerated deterioration. The subsequent deterioration of the cement inside the porcelain insulator has the greatest influence on the mechanical characteristics and it may cause the insulation resistance of the porcelain insulator to deteriorate [20].
4. Degradation Mechanism by Definition and Status Analysis of Corrosion of Iron Cap and Iron Pin
In Table 1, pollution grades A, B, and C were granted for the porcelain insulators that began service in 1979. In Korea, the grades of pollution are defined according to the distance from the coast. For the case of grade A, it means that the pollution rate is very low.

Table 1.
Step-by-step countermeasures of corrosion according to the level of contamination of the porcelain insulator.
In the case of a coastal region, the level of pollution depends on the distance of insulators from sea. According to the guideline in the case of the eastern coast, a site 3.5 to 5.7 km from the sea has the least polluted insulators graded as “A”. However, when distance from the sea is between 2.0 to 3.3 km, the insulators are categorized as grade “B” polluted. When the distance lowers to 1.2 to 2.0 km the insulators can be categorized as grade “C”. Sites with a distance lower than 1.2 km have the most polluted insulators, grade “D”. Considering the west coast, it can be observed that a site 9.5 to 20.2 km from the sea has the least polluted insulators, grade “A”. Whereas sites which are 4.8 to 9.5 and 2.4 to 4.8 km from the sea have been categorized as grade “B” and “C”, respectively. However, the site closest to sea i.e., less than 2.4 km away has most polluted insulators, grade “D”. The same can be observed in case of the south coast and Jeju island. In the case of the south coast, when sites are between 2.5 to 5.0 km and 1.35 to 2.5 km from the sea, the insulators are graded as “A” and “B”, respectively. Whereas when the distance between a site and the sea is 0.7 to 1.35 km and less than 0.7 km, the pollution grade is considered to be “C” and “D”, respectively. Now considering Jeju island, if the distance of a site from the sea is between 8.0 to 16.5 km and 4.1 to 8.0 km, the insulators are graded as “A” and “B”, respectively. Moreover, when the distance between a site and the sea is 2.1 to 4.1 km and less than 2.1 km, the pollution grade is considered to be “C” and “D”, respectively.
In the case of the new product (Stage I), which had never been used, no corrosion is detected by the eye. The porcelain insulator pictured in Stage II is a Pollution Level A product. Zinc plating is shown at an early stage of corrosion. The insulator does not need to be replaced immediately but must be cleaned and monitored periodically because there is some damage. The porcelain insulator pictured in Stage III is a Pollution Level B product; it has begun to degrade as corrosion progresses, and its removal from the service line should be considered. The porcelain insulator pictured in Stage IV is a Pollution Level C product; the corrosion is very severe, the metal has rust flows down to the porcelain or cement part, and the insulation resistance characteristic is poor. Rust can be seen but is not merely surface corrosion; the degradation has affected the entire structure of the insulator, which needs to be removed from the service line. Figure 4 shows the X-ray diffraction (XRD) analysis of the iron pin. Peaks of Fe2O3 were observed mainly at 36, 43, 48, 54, 65, 70, and 78 degrees, and the iron pin was also found to have the same tendency, largely due to exposure to the external environment.

Figure 4.
XRD analysis of Fe2O3 crystal phase due to corrosion of the iron cap.
The more pollution, the more various peaks in the Fe2O3. In the case of a Pollution Level C, the peak was doubled compared to other cases. Even though the iron surface is zinc-coated to prevent corrosion, depending on the degree of service and the degree of contamination, corrosion occurs on the iron cap and iron pin when iron and oxygen react to form Fe2O3.
The oxidation reactions due to environmental factors in the transmission line service environment are as follows:
with the main corrosion components of the iron cap and iron pin being
4Fe(OH)2 + O2 + 2H2O = 4Fe(OH)3,
mFeO + nFe2O3 + pH2O.
The values of m, n, and p vary depending on factors including the temperature, pH value, and oxygen content. Iron oxide, a major component of the corrosion of the metal cap, was measured by X-ray diffraction. If the oxidation of the metal in the porcelain insulator is severe, the ability to support the wires between the transmission towers may be reduced, leading to accidents. Therefore, even if zinc plating is used to protect a porcelain insulator that is in service for a long period of time, once the corrosion of iron starts, rapid replacement is needed to ameliorate risk [20].
5. Conclusions
The replacement standards for porcelain insulators are not well defined based on service period and should be defined properly to avoid accidents. In this study, the deterioration of such insulators was analyzed by service period and pollution degree, with a focus on the use of 3D-CT analysis for the study of deterioration in cement. In the case of the new product that was analyzed, very small air pockets of less than 3 mm2 were found; in the case of the porcelain insulator that had been used for a long time, there were more than 20 large and small pores of 3 mm2 or more. Pore formation occurs due to erosion by gel generated by silica and alkali in the cement due to ASR and water.
This causes cracks in the cement components of the porcelain insulator, and eventually, this expands and leads to mechanical breakage of the porcelain insulator. Results indicate that the deterioration of the cement proceeds under normal atmospheric conditions over time and that mechanical properties degrade significantly.
In addition, although the cap and pin, which are mainly composed of Fe, are coated with zinc to prevent corrosion, the longer the service period of an insulator and the higher the degree of pollution, the more advanced the progress in corrosion will be. If porcelain insulator corrosion is categorized into stages based on the degree of corrosion, as in this study, each stage can contribute to establish standard replacement criteria that can inform the appropriate and timeous replacement of damaged porcelain insulators.
Author Contributions
T.K. contributed to this work in experiment planning, experiment measurements, data analysis and manuscript preparation. S.S. manuscript preparation. J.-B.K. contributed research sample preparation. J.-A.S. contributed research sample preparation. I.-H.C. contributed research sample preparation. J.Y. contributed in experiment planning, data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Korea Electric Power Corporation R16TA29.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baker, A.C.; Bernstorf, E.A.; Cherney, R.; Christman, R.S.; Gorur, R.J.; Hill, Z.; Lodi, S.; Marra, D.G.; Powell, A.E.; Schwalm, D.H.; et al. High Voltage Insulators Mechanical Load Limits-Part I: Overhead Line Load and Strength Requirements. IEEE Trans. Power Deliv. 2012, 27, 1106–1115. [Google Scholar] [CrossRef]
- Baker, A.C.; Bernstorf, E.A.; Cherney, R.; Christman, R.S.; Gorur, R.J.; Hill, Z.; Lodi, S.; Marra, D.G.; Powell, A.E.; Schwalm, D.H.; et al. High Voltage Insulators Mechanical Load Limits-Part I: Standards and Recommendations. IEEE Trans. Power Deliv. 2012, 27, 2342–2349. [Google Scholar] [CrossRef]
- Mishra, A.P.; Gorur, R.S.; Benkataraman, S. Evaluation of Porcelain and Toughened Glass Suspension Insulators Removed from Service. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 467–475. [Google Scholar] [CrossRef]
- Rawat, A.; Gorur, R.S. Microstructure Based Evaluation of Field Aged and New Porcelain Suspension Insulators. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 107–115. [Google Scholar] [CrossRef]
- Cherney, E.A.; Baker, A.C.; Kuffel, J.; Lodi, Z.; Phillips, A.; Powell, D.G.; Stewart, G.A. Evaluation of and Replacement Strategies for Aged High Voltage Porcelain Suspension-Type Insulators. IEEE Trans. Power Deliv. 2014, 29, 275–282. [Google Scholar] [CrossRef]
- Kaminski, J. Long-Time Mechanical and Electrical Strength of Suspension Insulators. IEEE Trans. Power Appl. Syst. 1963, 82, 446–451. [Google Scholar] [CrossRef]
- Wang, H.; Gillott, J.E. Mechanism of alkali-silica reaction and the significance of calcium hydroxide. Cem. Concr. Res. 1991, 21, 647–654. [Google Scholar] [CrossRef]
- Bogue, R.H.; Lerch, W. Hydration of Portland Cement Compounds. J. Ind. Eng. Chem. 1934, 26, 837–847. [Google Scholar]
- Mills, B. Porcelain Insulators and How They Grew; Brent Mills: Leroy, NY, USA, 1970. [Google Scholar]
- USA Standard. Wet Process Porcelain Insulators C29.2-1962; Standards Institute: New York, NY, USA, 1962. [Google Scholar]
- Cherney, E.A. Cement Growth Failure of Porcelain Suspension Insulators. IEEE Trans. Power. Appl. Syst. 1983, 102, 2765–2774. [Google Scholar] [CrossRef]
- Plusquellec, G. Determining the free alkali metal content in concrete–case study of an ASR-affected dam. Cem. Concr. Res. 2018, 105, 111–125. [Google Scholar] [CrossRef]
- Tang, S.W. Recent durability studies on concrete structure. Cem. Concr. Res. 2015, 78, 143–154. [Google Scholar] [CrossRef]
- Ramlochan, T. The effect of metakaolin on alkali-silica reaction in concrete. Cem. Concr. Res. 2000, 30, 339–344. [Google Scholar] [CrossRef]
- Beyene, M. Alkali Silica Reaction (ASR) as a root cause of distress in a concrete made from Alkali Carbonate Reaction (ACR) potentially susceptible aggregates. Cem. Concr. Res. 2013, 51, 85–95. [Google Scholar] [CrossRef]
- ACI Committee 221. State-of-the-Art Report on Alkali-Aggregate Reactivity (ACI 221.1R-98); American Concrete Institute: Farmington Hills, MI, USA, 1998. [Google Scholar]
- Shinka, H. Inhibition of Alkali-Silica Reaction in hollow porcelain insulator. Electron. Commun. Jpn. 2015, 98, 1–8. [Google Scholar] [CrossRef]
- Chatterji, S. The role of Ca(OH)2 in the breakdown of Potland Cement concrete due to alkali-silica reaction. Cem. Concr. Res. 1979, 9, 185–188. [Google Scholar] [CrossRef]
- Touma, W.; Fowler, D.W.; Carrasquillo, R.L. Alkali-Silica Reaction in Portland Cement Concrete: Testing Methods and Mitigation Alternatives; ICAR Technical Report; The University of Texas at Austin: Austin, TX, USA, 2001. [Google Scholar]
- Luo, L. Corrosion mechanism and process of hardware of disc suspension type insulator on ±800 kV transmission lines. IEEE Trans. Dielect. Electr. Insul. 2016, 23, 339–348. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



