Structural Selectivity of PAH Removal Processes in Soil, and the Effect of Metal Co-Contaminants
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
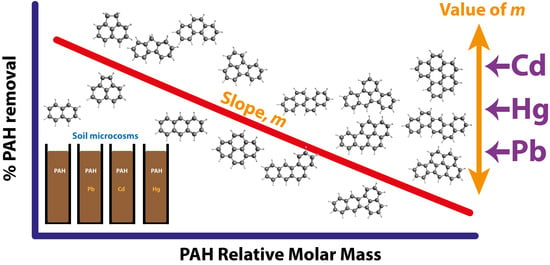
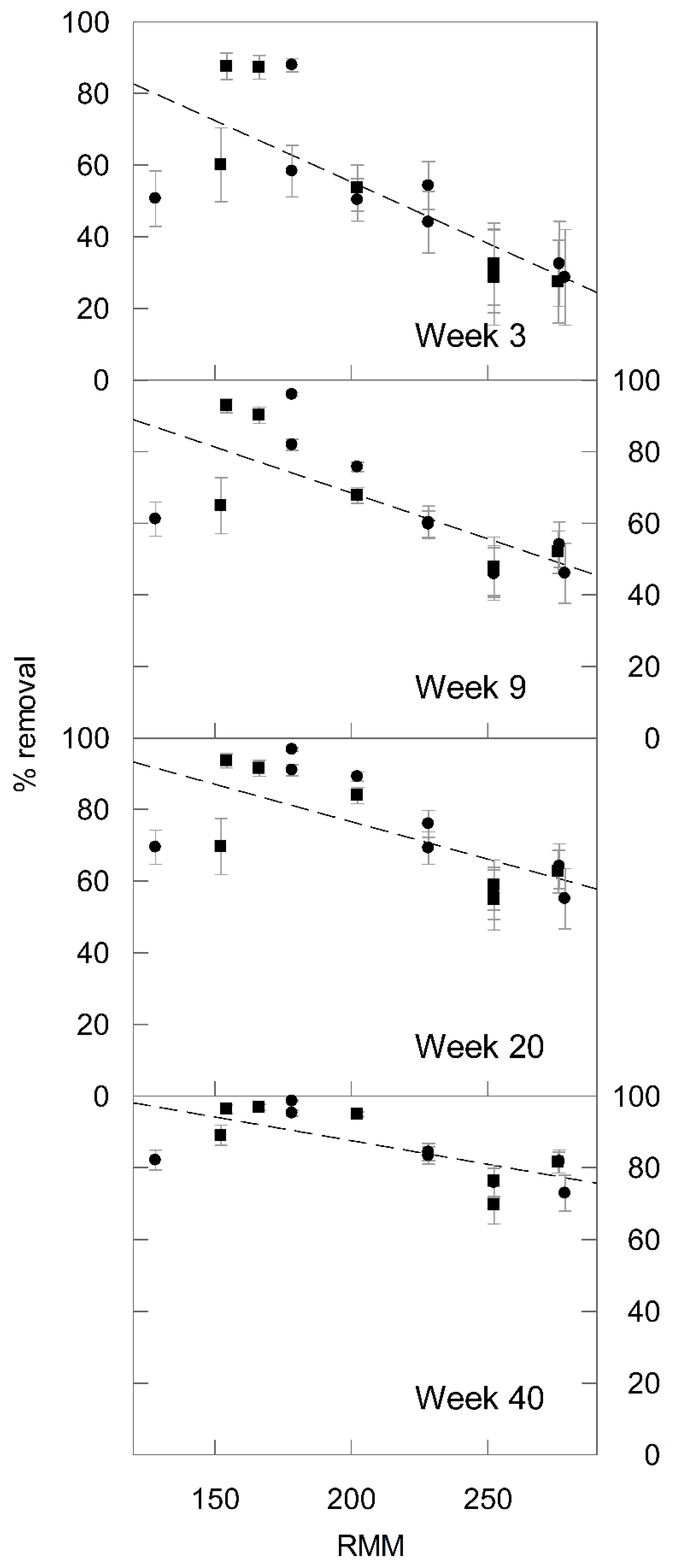
3.1. Relationship between PAH Relative Molar Mass and % Removal
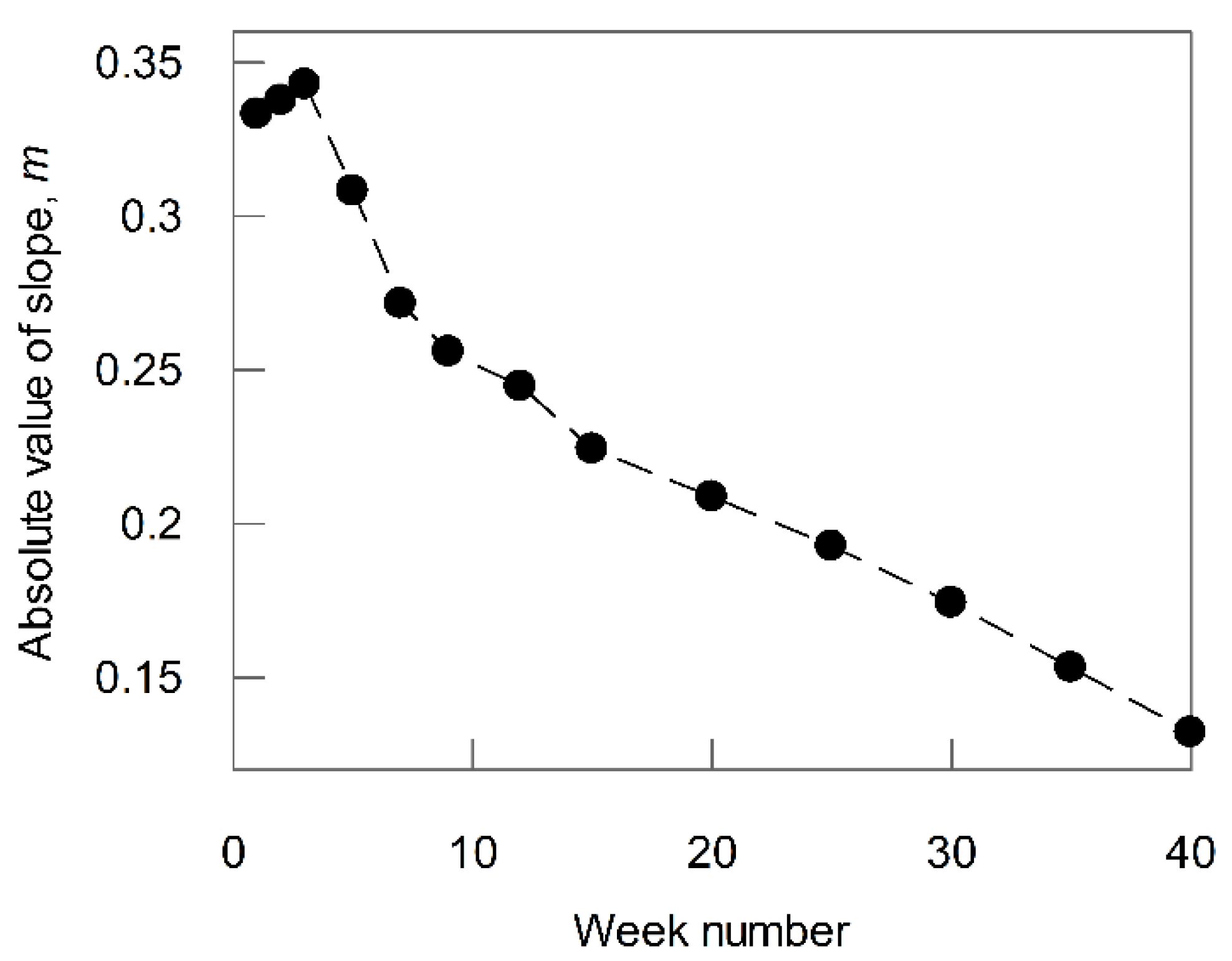
3.2. Effect of Microcosm Incubation Time on ‘m’
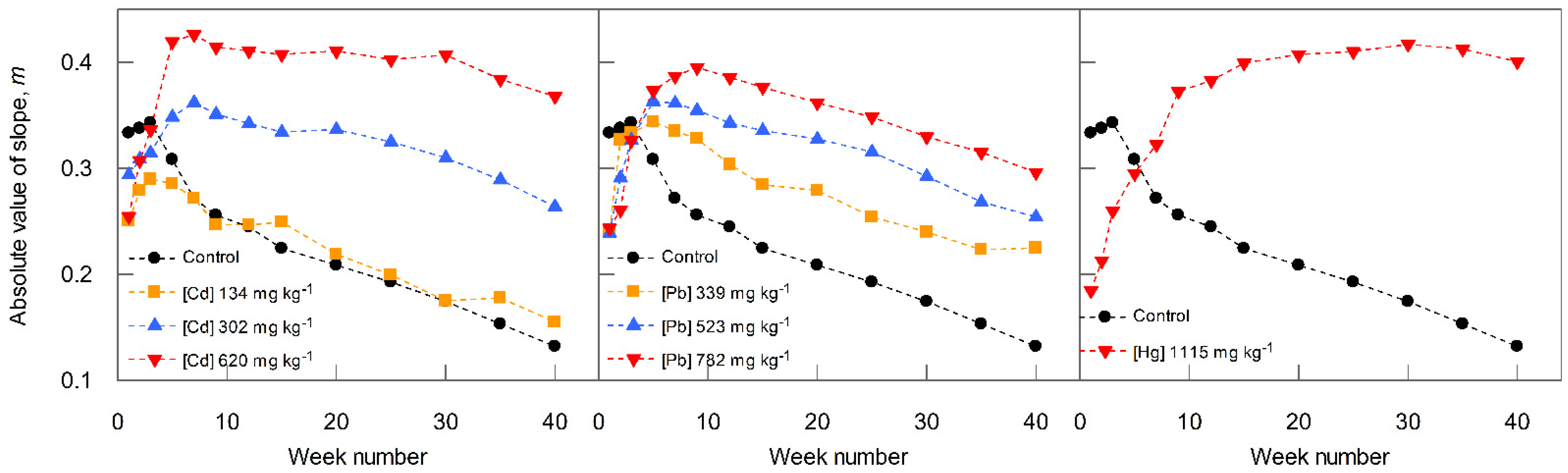
3.3. Effect of Metal Co-Contaminant Concentration on ‘m’
3.4. Significance and Application of ‘m’
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deary, M.E.; Ekumankama, C.C.; Cummings, S.P. Development of a novel kinetic model for the analysis of PAH biodegradation in the presence of lead and cadmium co-contaminants. J. Hazard Mater. 2016, 307, 240–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juhasz, A.L.; Naidu, R. Bioremediation of high molecular weight polycyclic aromatic hydrocarbons: A review of the microbial degradation of benzo [a] pyrene. Int. Biodeterior. 2000, 45, 57–88. [Google Scholar] [CrossRef]
- Park, K.S.; Sims, R.C.; Dupont, R.R.; Doucette, W.J.; Matthews, J.E. Fate of PAH compounds in two soil types: Influence of volatilization, abiotic loss and biological activity. Environ. Toxicol. Chem. 1990, 9, 187–195. [Google Scholar] [CrossRef]
- Cerniglia, C.E. Biodegradation of polycyclic aromatic hydrocarbons. Curr. Opin. Biotech. 1993, 4, 331–338. [Google Scholar] [CrossRef]
- Kanaly, R.A.; Harayama, S. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J. Bacteriol. 2000, 182, 2059–2067. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Xu, K.; Chen, J. Effect of pyrene and cadmium on microbial activity and community structure in soil. Chemosphere 2013, 91, 491–497. [Google Scholar] [CrossRef]
- Heitkamp, M.A.; Cerniglia, C.E. Effects of chemical structure and exposure on the microbial degradation of polycyclic aromatic hydrocarbons in freshwater and estuarine ecosystems. Environ. Toxicol. Chem. 1987, 6, 535–546. [Google Scholar] [CrossRef]
- Knightes, C.D.; Peters, C.A. Aqueous phase biodegradation kinetics of 10 PAH compounds. Environ. Eng. Sci. 2003, 20, 207–218. [Google Scholar] [CrossRef]
- Deary, M.E.; Ekumankama, C.C.; Cummings, S.P. Effect of lead, cadmium, and mercury co-contaminants on biodegradation in PAH-polluted soils. Land Degrad Dev. 2018, 29, 1583–1594. [Google Scholar] [CrossRef]
- Gao, Y.; Xiong, W.; Ling, W.; Xu, J. Sorption of phenanthrene by soils contaminated with heavy metals. Chemosphere 2006, 65, 1355–1361. [Google Scholar] [CrossRef]
- Wammer, K.H.; Peters, C.A. Polycyclic aromatic hydrocarbon biodegradation rates: A structure-based study. Environ. Sci. Technol. 2005, 39, 2571–2578. [Google Scholar] [CrossRef] [PubMed]
- Lors, C.; Ryngaert, A.; Perie, F.; Diels, L.; Damidot, D. Evolution of bacterial community during bioremediation of PAHs in a coal tar contaminated soil. Chemosphere 2010, 81, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Bogan, B.W.; Lamar, R.; Burgos, W.; Tien, M. Extent of humification of anthracene, fluoranthene, and benzo [α] pyrene by Pleurotus ostreatus during growth in PAH-contaminated soils. Lett. Appl. Microbiol. 1999, 28, 250–254. [Google Scholar] [CrossRef]
- Calvo, C.; Silva-Castro, G.; Uad, I.; García Fandiño, C.; Laguna, J.; González-López, J. Efficiency of the EPS emulsifier produced by Ochrobactrum anthropi in different hydrocarbon bioremediation assays. J. Ind. Microbiol. Biot. 2008, 35, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Hino, S.; Watanabe, K.; Takahashi, N. Isolation and characterization of slime-producing bacteria capable of utilizing petroleum hydrocarbons as a sole carbon source. J. Ferment. Bioeng. 1997, 84, 528–531. [Google Scholar] [CrossRef]
- Hua, X.; Wu, Z.; Zhang, H.; Lu, D.; Wang, M.; Liu, Y.; Liu, Z. Degradation of hexadecane by Enterobacter cloacae strain TU that secretes an exopolysaccharide as a bioemulsifier. Chemosphere 2010, 80, 951–956. [Google Scholar] [CrossRef]
- Guarino, C.; Zuzolo, D.; Marziano, M.; Conte, B.; Baiamonte, G.; Morra, L.; Benotti, D.; Gresia, D.; Stacul, E.R.; Cicchella, D. Investigation and assessment for an effective approach to the reclamation of polycyclic aromatic hydrocarbon (PAHs) contaminated site: SIN Bagnoli, Italy. Sci. Rep. 2019, 9, 11522. [Google Scholar] [CrossRef] [Green Version]
- Kotoky, R.; Pandey, P. Difference in the rhizosphere microbiome of Melia azedarach during removal of benzo (a) pyrene from cadmium co-contaminated soil. Chemosphere 2020, 258, 127175. [Google Scholar] [CrossRef] [PubMed]
- Cristaldi, A.; Conti, G.O.; Jho, E.H.; Zuccarello, P.; Grasso, A.; Copat, C.; Ferrante, M. Phytoremediation of contaminated soils by heavy metals and PAHs. A brief review. Environ. Technol. Innov. 2017, 8, 309–326. [Google Scholar] [CrossRef]
- Gan, S.; Lau, E.V.; Ng, H.K. Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J. Hazard Mater. 2009, 172, 532–549. [Google Scholar] [CrossRef]
- Atagana, H.I. Biodegradation of PAHs by fungi in contaminated-soil containing cadmium and nickel ions. Afr. J. Biotechnol. 2009, 8, 5780–5789. [Google Scholar] [CrossRef] [Green Version]
- Czarny, J.; Staninska-Pięta, J.; Piotrowska-Cyplik, A.; Juzwa, W.; Wolniewicz, A.; Marecik, R. Acinetobacter sp. as the key player in diesel oil degrading community exposed to PAHs and heavy metals. J. Hazard Mater. 2020, 383, 121168. [Google Scholar] [CrossRef] [PubMed]
- Obuekwe, I.S.; Semple, K.T. Impact of Al and Fe on the development of phenanthrene catabolism in soil. J. Soils Sediments 2013, 13, 1589–1599. [Google Scholar] [CrossRef]
- Johnsen, A.R.; Wick, L.Y.; Harms, H. Principles of microbial PAH-degradation in soil. Environ. Pollut. 2005, 133, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Thavamani, P.; Malik, S.; Beer, M.; Megharaj, M.; Naidu, R. Microbial activity and diversity in long-term mixed contaminated soils with respect to polyaromatic hydrocarbons and heavy metals. J. Environ. Manag. 2012, 99, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Mellor, A.; Bevan, J.R. Lead in the soils and stream sediments of an urban catchment in Tyneside, UK. Water Air Soil Poll. 1999, 112, 327–348. [Google Scholar] [CrossRef]
- Knightes, C.D.; Peters, C.A. Multisubstrate biodegradation kinetics for binary and complex mixtures of polycyclic aromatic hydrocarbons. Environ. Toxicol. Chem. 2006, 25, 1746–1756. [Google Scholar] [CrossRef]
- Northcott, G.L.; Jones, K.C. Partitioning, extractability, and formation of nonextractable PAH residues in soil. 1. Compound differences in aging and sequestration. Environ. Sci. Technol. 2001, 35, 1103–1110. [Google Scholar] [CrossRef]
- Staninska-Pięta, J.; Czarny, J.; Piotrowska-Cyplik, A.; Juzwa, W.; Wolko, Ł.; Nowak, J.; Cyplik, P. Heavy metals as a factor increasing the functional genetic potential of bacterial community for polycyclic aromatic hydrocarbon biodegradation. Molecules 2020, 25, 319. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Lou, J.; Fang, J.; Cai, J.; Hu, Z.; Sun, P. Effects of heavy metals and metal (oxide) nanoparticles on enhanced biological phosphorus removal. Rev. Chem. Eng. 2020, 36, 947–970. [Google Scholar] [CrossRef]
- Müller, S.; Totsche, K.; Kögel-Knabner, I. Sorption of polycyclic aromatic hydrocarbons to mineral surfaces. Eur. J. Soil Sci. 2007, 58, 918–931. [Google Scholar] [CrossRef]
- Dougherty, D.A. The cation—π interaction. Acc. Chem. Res. 2013, 46, 885–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijay, D.; Sastry, G.N. Exploring the size dependence of cyclic and acyclic π-systems on cation–π binding. Phys. Chem. 2008, 10, 582–590. [Google Scholar] [CrossRef]
- Thavamani, P.; Megharaj, M.; Naidu, R. Bioremediation of high molecular weight polyaromatic hydrocarbons co-contaminated with metals in liquid and soil slurries by metal tolerant PAHs degrading bacterial consortium. Biodegradation 2012, 23, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Niklinska, M.; Chodak, M.; Laskowski, R. Pollution-induced community tolerance of microorganisms from forest soil organic layers polluted with Zn or Cu. Appl. Soil Ecol. 2006, 32, 265–272. [Google Scholar] [CrossRef]
- Muniz, S.; Lacarta, J.; Pata, M.P.; Jimenez, J.J.; Navarro, E. Analysis of the diversity of substrate utilisation of soil bacteria exposed to Cd and earthworm activity using generalised additive models. PLoS ONE 2014, 9, e85057. [Google Scholar] [CrossRef]
- Almås, Å.R.; Bakken, L.R.; Mulder, J. Changes in tolerance of soil microbial communities in Zn and Cd contaminated soils. Soil Biol. Biochem. 2004, 36, 805–813. [Google Scholar] [CrossRef]
- Diaz-Ravina, M.; Baath, E. Development of metal tolerance in soil bacterial communities exposed to experimentally increased metal levels. Appl. Environ. Microbiol. 1996, 62, 2970–2977. [Google Scholar] [CrossRef] [Green Version]
- Frossard, A.; Donhauser, J.; Mestrot, A.; Gygax, S.; Bååth, E.; Frey, B. Long-and short-term effects of mercury pollution on the soil microbiome. Soil Biol. Biochem. 2018, 120, 191–199. [Google Scholar] [CrossRef]
- Muhammad, A.; Xu, J.; Li, Z.; Wang, H.; Yao, H. Effects of lead and cadmium nitrate on biomass and substrate utilization pattern of soil microbial communities. Chemosphere 2005, 60, 508–514. [Google Scholar] [CrossRef]
- Bååth, E. Effects of Heavy-Metals in Soil on Microbial Processes and Populations (a Review). Water Air Soil Poll. 1989, 47, 335–379. [Google Scholar] [CrossRef]
- Giller, K.E.; Witter, E.; McGrath, S.P. Heavy metals and soil microbes. Soil Biol. Biochem. 2009, 41, 2031–2037. [Google Scholar] [CrossRef]
- Giller, K.E.; Witter, E.; Mcgrath, S.P. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biol. Biochem. 1998, 30, 1389–1414. [Google Scholar] [CrossRef]
- Kirk, J.L.; Beaudette, L.A.; Hart, M.; Moutoglis, P.; Klironomos, J.N.; Lee, H.; Trevors, J.T. Methods of studying soil microbial diversity. J. Microbiol. Meth. 2004, 58, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.K.; Westergaard, K.; Christensen, S.; Sørensen, S.J. The effect of long-term mercury pollution on the soil microbial community. FEMS Microbiol. Ecol. 2001, 36, 11–19. [Google Scholar] [CrossRef]


| Name, RMM and Initial Concentration (mg kg−1) in Microcosms | Structure | Name, RMM and Initial Concentration (mg kg−1) in Microcosms | Structure |
|---|---|---|---|
| Naphthalene 128.17 3.28 ± 0.63 | 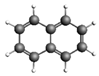 | Benzo[a]anthracene 228.29 167.9 ± 25.9 | 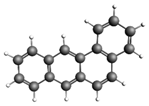 |
| Acenaphthylene 152.19 1.44 ± 0.23 | 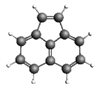 | Chrysene 228.29 196.1 ± 33.3 |  |
| Acenaphthene 154.21 7.75 ± 1.92 | 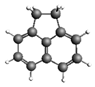 | Benzo[b]fluoranthene 252.31 324.7 ± 55.5 | 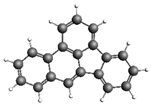 |
| Fluorene 166.22 9.53 ± 1.63 | 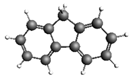 | Benzo[k]fluoranthene 252.31 138.1 ± 20.1 |  |
| Phenanthrene 178.23 93.43 ± 15.08 |  | Benzo[a]pyrene 252.31 254.9 ± 40.12 | 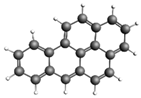 |
| Anthracene 178.23 27.56 ± 4.12 | 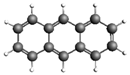 | Indeno[1,2,3-cd]pyrene 276.33 246.31 ± 43.1 | 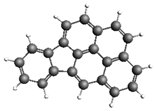 |
| Fluoranthene 202.25 217.6 ± 35.6 | 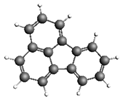 | Dibenzo[a,h]anthracene 278.34 105.4 ± 17.9 | 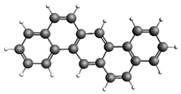 |
| Pyrene 202.25 187.8 ± 31.5 |  | Benzo[g,h,i]perylene 276.33 184.5 ± 31.8 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deary, M.E.; Ekumankama, C.C.; Cummings, S.P. Structural Selectivity of PAH Removal Processes in Soil, and the Effect of Metal Co-Contaminants. Environments 2022, 9, 23. https://doi.org/10.3390/environments9020023
Deary ME, Ekumankama CC, Cummings SP. Structural Selectivity of PAH Removal Processes in Soil, and the Effect of Metal Co-Contaminants. Environments. 2022; 9(2):23. https://doi.org/10.3390/environments9020023
Chicago/Turabian StyleDeary, Michael E., Chinedu C. Ekumankama, and Stephen P. Cummings. 2022. "Structural Selectivity of PAH Removal Processes in Soil, and the Effect of Metal Co-Contaminants" Environments 9, no. 2: 23. https://doi.org/10.3390/environments9020023
APA StyleDeary, M. E., Ekumankama, C. C., & Cummings, S. P. (2022). Structural Selectivity of PAH Removal Processes in Soil, and the Effect of Metal Co-Contaminants. Environments, 9(2), 23. https://doi.org/10.3390/environments9020023