Preliminary Studies of Methylene Blue Remotion from Aqueous Solutions by Ocimum basilicum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sweet Basil (Ocimum basilicum) Herbs
2.2. Preliminary Experiments
2.3. Removal Experiments
2.4. Calculating the Percentage of Bioremoval
2.5. Analysis of Herbs Growth Rate
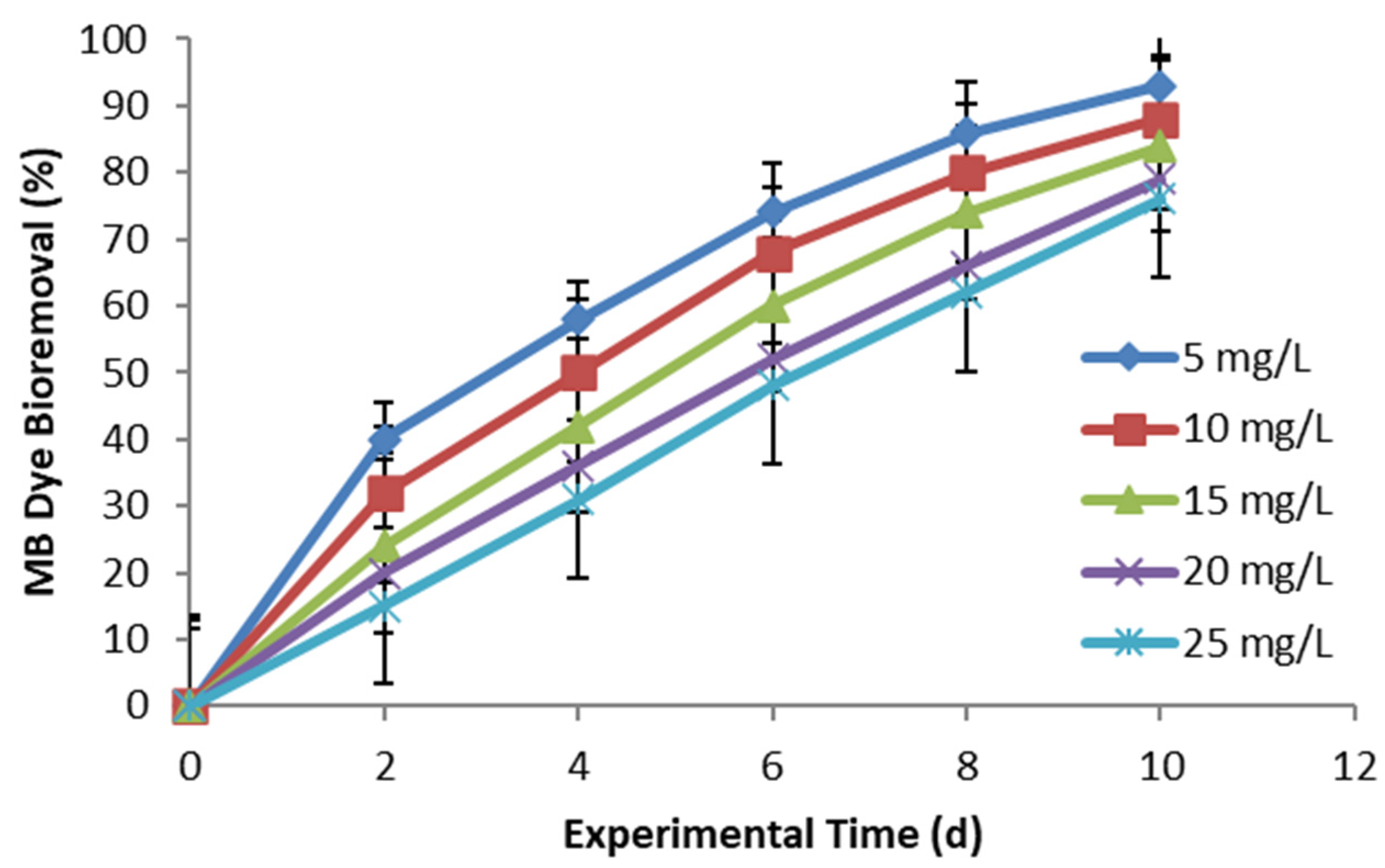
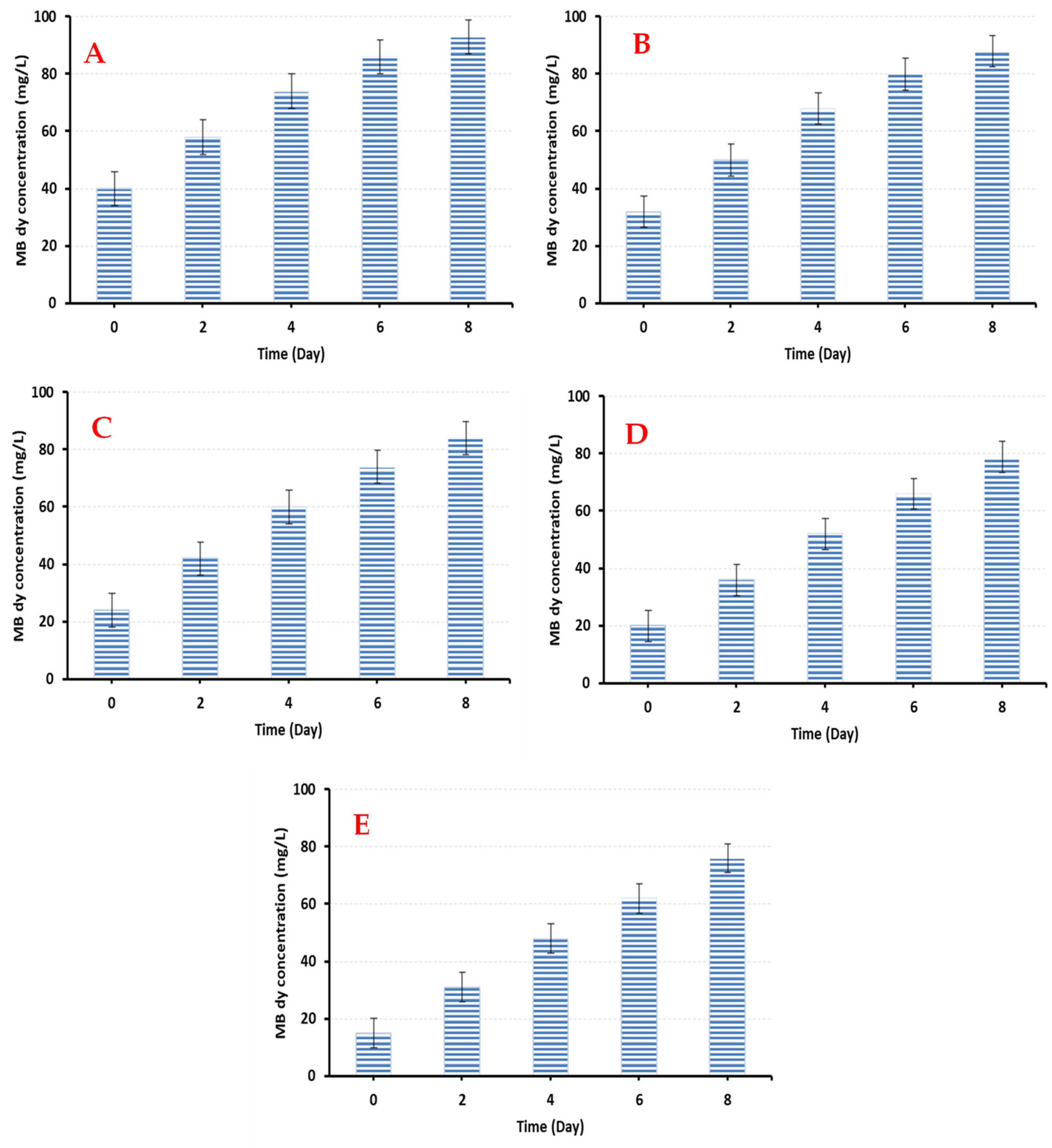
3. Results and Discussion
3.1. The Bioremoval of MB Dye by Ocimum basilicum
3.2. The Growth Profile of Ocimum basilicum
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alshekhli, A.F.; Hasan, H.A.; Muhamad, M.H.; Abdullah, S.R.S. Development of Adsorbent from Phytoremediation Plant Waste for Methylene Blue Removal. J. Ecol. Eng. 2020, 21, 207–215. [Google Scholar]
- Saba, B.; Jabeen, M.; Khalid, A.; Aziz, I.; Christy, A.D. Effectiveness of rice agricultural waste, microbes and wetland plants in the removal of reactive black-5 azo dye in microcosm constructed wetlands. Int. J. Phytoremediat. 2015, 17, 1060–1067. [Google Scholar] [CrossRef]
- Sasmaz, M.; Öbek, E.; Sasmaz, A. Bioaccumulation of cadmium and thallium in Pb-Zn tailing waste water by Lemna minor and Lemna gibba. Appl. Geochem. 2019, 100, 287–292. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Waste management by biological approach employing natural substrates and microbial agents for the remediation of dyes’ wastewater. Appl. Sci. 2020, 10, 2958. [Google Scholar] [CrossRef]
- Vikrant, K.; Giri, B.S.; Raza, N.; Roy, K.; Kim, K.-H.; Rai, B.N.; Singh, R.S. Recent advancements in bioremediation of dye: Current status and challenges. Bioresour. Technol. 2018, 253, 355–367. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Jayamuthunagai, J.; Praveenkumar, R.; Iyyappan, J. Phytoremediation techniques for the removal of dye in wastewater. In Bioremediation: Applications for Environmental Protection and Management; Springer: Berlin/Heidelberg, Germany, 2018; pp. 243–252. [Google Scholar]
- Gupta, V. Application of low-cost adsorbents for dye removal–a review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Karaghool, H.A.K. Biodecolorization of methylene blue using aspergillus consortium. In Proceedings of the 5th International Scientific Conference on Environment and Sustainable Development, Baghdad, Iraq, 3–6 December 2020; p. 012111. [Google Scholar]
- Kara, I.; Akar, S.T.; Akar, T.; Ozcan, A. Dithiocarbamated Symphoricarpus albus as a potential biosorbent for a reactive dye. Chem. Eng. J. 2012, 211, 442–452. [Google Scholar] [CrossRef]
- Mahmoud, M.S.; Farah, J.Y.; Farrag, T.E. Enhanced removal of Methylene Blue by electrocoagulation using iron electrodes. Egypt. J. Pet. 2013, 22, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Hashim, K.S.; Al-Saati, N.; Alquzweeni, S.S.; Zubaidi, S.L.; Kot, P.; Kraidi, L.; Hussein, A.H.; Alkhaddar, R.; Shaw, A.; Alwash, R. Decolourization of dye solutions by electrocoagulation: An investigation of the effect of operational parameters. In Proceedings of the 1st International Conference on Civil and Environmental Engineering Technologies, Najaf, Iraq, 23–24 April 2019; p. 012024. [Google Scholar] [CrossRef]
- Chollom, M.N.; Rathilal, S.; Alfa, D.; Pillay, V. The applicability of nanofiltration for the treatment and reuse of textile reactive dye effluent. Water SA 2015, 41, 398–405. [Google Scholar] [CrossRef] [Green Version]
- Zuorro, A.; Lavecchia, R. Evaluation of UV/H2O2 advanced oxidation process (AOP) for the degradation of diazo dye Reactive Green 19 in aqueous solution. Desalination Water Treat. 2014, 52, 1571–1577. [Google Scholar] [CrossRef]
- Du, W.-N.; Chen, S.-T. Photo-and chemocatalytic oxidation of dyes in water. J. Environ. Manag. 2018, 206, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Muthunarayanan, V.; Santhiya, M.; Swabna, V.; Geetha, A. Phytodegradation of textile dyes by water hyacinth (Eichhornia crassipes) from aqueous dye solutions. Int. J. Environ. Sci. 2011, 1, 1702–1717. [Google Scholar]
- Tan, K.A.; Morad, N.; Ooi, J.Q. Phytoremediation of methylene blue and methyl orange using Eichhornia crassipes. Int. J. Environ. Sci. Dev. 2016, 7, 724. [Google Scholar] [CrossRef]
- Manghabati, H.; Pazuki, G. A study on the decolorization of methylene blue by Spirodela polyrrhiza: Experimentation and modeling. RSC Adv. 2014, 4, 30137–30144. [Google Scholar] [CrossRef]
- Al-Baldawi, I.A.; Abdullah, S.R.S.; Anuar, N.; Hasan, H.A. Phytotransformation of methylene blue from water using aquatic plant (Azolla pinnata). Environ. Technol. Innov. 2018, 11, 15–22. [Google Scholar] [CrossRef]
- Imron, M.F.; Kurniawan, S.B.; Soegianto, A.; Wahyudianto, F.E. Phytoremediation of methylene blue using duckweed (Lemna minor). Heliyon 2019, 5, e02206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewadh, H.M. Removal of methylene blue by coontail (Ceratophyllum demersum) using phytoremediation concept. Plant Arch. 2020, 20, 2677–2681. [Google Scholar]
- Jisha, C.; Bauddh, K.; Shukla, S.K. Phytoremediation and bioenergy production efficiency of medicinal and aromatic plants. In Phytoremediation Potential of Bioenergy Plants; Springer: Berlin/Heidelberg, Germany, 2017; pp. 287–304. [Google Scholar]
- Adiloğlu, S. Relation of Chelated Iron (EDDHA-Fe) Applications with Iron Accumulation and Some Plant Nutrient Elements in Basil (Ocimum basilicum L.). Pol. J. Environ. Stud. 2021, 30, 3471–3479. [Google Scholar] [CrossRef]
- Putwattana, N.; Kruatrachue, M.; Pokethitiyook, P.; Chaiyarat, R. Immobilization of cadmium in soil by cow manure and silicate fertilizer, and reduced accumulation of cadmium in sweet basil (Ocimum basilicum). Sci. Asia 2010, 36, 349–354. [Google Scholar] [CrossRef]
- Can-Terzi, B.; Goren, A.; Okten, H.; Sofuoglu, S. Biosorption of methylene blue from water by live Lemna minor. Environ. Technol. Innov. 2021, 22, 101432. [Google Scholar] [CrossRef]
- Al Farraj, D.A.; Elshikh, M.S.; Al Khulaifi, M.M.; Hadibarata, T.; Yuniarto, A.; Syafiuddin, A. Biotransformation and Detoxification of Antraquione Dye Green 3 using halophilic Hortaea sp. Int. Biodeterior. Biodegrad. 2019, 140, 72–77. [Google Scholar] [CrossRef]
- Reema, R.; Saravanan, P.; Kumar, M.D.; Renganathan, S. Accumulation of methylene blue dye by growing Lemna minor. Sep. Sci. Technol. 2011, 46, 1052–1058. [Google Scholar] [CrossRef]
- Khataee, A.; Movafeghi, A.; Torbati, S.; Lisar, S.S.; Zarei, M. Phytoremediation potential of duckweed (Lemna minor L.) in degradation of CI Acid Blue 92: Artificial neural network modeling. Ecotoxicol. Environ. Saf. 2012, 80, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Dave, B.; Sanghvi, G. Enzyme Action for Dye Degradation. In Dye Biodegradation, Mechanisms and Techniques; Springer: Berlin/Heidelberg, Germany, 2022; pp. 141–163. [Google Scholar]
- Ucisik, A.S.; Trapp, S. Uptake, removal, accumulation, and phytotoxicity of phenol in willow trees (Salix viminalis). Environ. Toxicol. Chem. Int. J. 2006, 25, 2455–2460. [Google Scholar] [CrossRef]
- Wu, H. Effect of different light qualities on growth, pigment content, chlorophyll fluorescence, and antioxidant enzyme activity in the red alga Pyropia haitanensis (Bangiales, Rhodophyta). BioMed Res. Int. 2016, 2016, 7383918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioelovich, M. Recent findings and the energetic potential of plant biomass as a renewable source of biofuels–a review. Bioresources 2015, 10, 1879–1914. [Google Scholar]
- Kadir, W.N.A.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T. Harvesting and pre-treatment of microalgae cultivated in wastewater for biodiesel production: A review. Energy Convers. Manag. 2018, 171, 1416–1429. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaghool, H.A.K.; Hashim, K.; Kot, P.; Muradov, M. Preliminary Studies of Methylene Blue Remotion from Aqueous Solutions by Ocimum basilicum. Environments 2022, 9, 17. https://doi.org/10.3390/environments9020017
Karaghool HAK, Hashim K, Kot P, Muradov M. Preliminary Studies of Methylene Blue Remotion from Aqueous Solutions by Ocimum basilicum. Environments. 2022; 9(2):17. https://doi.org/10.3390/environments9020017
Chicago/Turabian StyleKaraghool, Haneen A. K., Khalid Hashim, Patryk Kot, and Magomed Muradov. 2022. "Preliminary Studies of Methylene Blue Remotion from Aqueous Solutions by Ocimum basilicum" Environments 9, no. 2: 17. https://doi.org/10.3390/environments9020017
APA StyleKaraghool, H. A. K., Hashim, K., Kot, P., & Muradov, M. (2022). Preliminary Studies of Methylene Blue Remotion from Aqueous Solutions by Ocimum basilicum. Environments, 9(2), 17. https://doi.org/10.3390/environments9020017








